Holey Graphene for Sodium-Ion Battery Anode Material
Yanan Hao ,Ziming Cai
1 State Key Laboratory of Information Photonics and Optical Communications,School of Science,Beijing University of Posts and Telecommunications,Beijing 100876,China.
2 School of Material Science and Physics,China University of Mining and Technology,Xuzhou 221116,Jiangsu Province,China.
Electrochemical energy storage becomes indispensable due to the wide-scale implementation of intermittent renewable energy and the growing market for portable electronic devices.Nowadays,lithium-ion batteries are prevalent and mature.However,there remain some issues such as the high cost of lithium,questioned battery safety,and poor low-temperature performance1,2.Thus,sodium-ion batteries are conceived as promising alternatives.The anode and cathode materials have been extensively explored.Graphene-based nanomaterials stand out as anode materials due to their electrochemical stability and ease of hybridization with transition metal compounds3,4.Nevertheless,their rate performance and initial Coulombic efficiency are still unsatisfactory.This is ascribed to the limited diffusion of sodium ions across the two-dimensional sheets5.Hence,the generation of in-plane nanoholes results in the construction of holey graphene (HG),benefiting sodium ion intercalation and diffusion6.
Inspired by the merits of the above-mentioned design,recently,Liuetal.adopt an easy thermal etching andinsituconversion method to prepare Co3Se4/HG hybrid (Fig.1a)7.The introduction of cobalt chalcogenides,with a narrow bandgap,can provide extra capacity from conversion reactions8.The synthetic protocol is composed of a simple chemical bath deposition (CBD),thermal etching,and selenylation treatment.Obviously,an overall similar structure can be observed from the low-magnification image.

Fig.1 (a) Schematic illustration of the synthetic process,(b,c) TEM images,(d,e) HR-TEM images,(f) HAADF-STEM image and corresponding EDS mappings of Co3Se4/HG.
Besides,the microstructure of Co3Se4/HG is examined by TEM (Fig.1b).Of note,Co3Se4nanoparticles (NPs),are distributed evenly.Particularly,the NPs are closely adjacent to nanoholes (NHs) (Fig.1c).The lattice spacing of 0.267 nm (Fig.1e) is obvious at a glance.The existence of Co3Se4is further authenticated.The homogeneous distribution of Co and Se as the NPs and C as the scaffold is displayed in Fig.1f.Except for the merits of ion diffusion,this construction offers extra cushion space for the volume change of Co3Se4during cycling.Additionally,rapid electron transport is furnished by the HG.Moreover,theinsitugrowth of Co3Se4NPs restrains their aggregation.
The experimental results validate the expectations.Fig.2a manifests the first CV scan for the composite.to the sodium of sodium into the active material contributes to the reduction peak.Besides,a solid electrolyte interfacial layer (SEI) is also built.The Co is further reduced into zero valence,as evidenced by another wide peak.Correspondingly,the oxidation from Co to the active material appears during discharging.Moreover,the next three oxidation peaks are almost the same as the first one.Although the reactions are the same,the reduction peaks experience some deviations.At 100 mA·g-1,the first four GCD curves of the sample are recorded.And superb initial charge capacity of 622.2 mAh·g-1is delivered.The initial Coulombic efficiency of 69% also validates its superiority.The irreversible capacity in the first cycle is caused by the irreversible decomposition of electrolytes and the formation of the SEI layer.In addition,the GCD curves overlap in successive cycles,indicating the great stability of Co3Se4/HG for Na ion storage.All the plateaus of the GCD curve are in concert with the peaks of the CV curve.
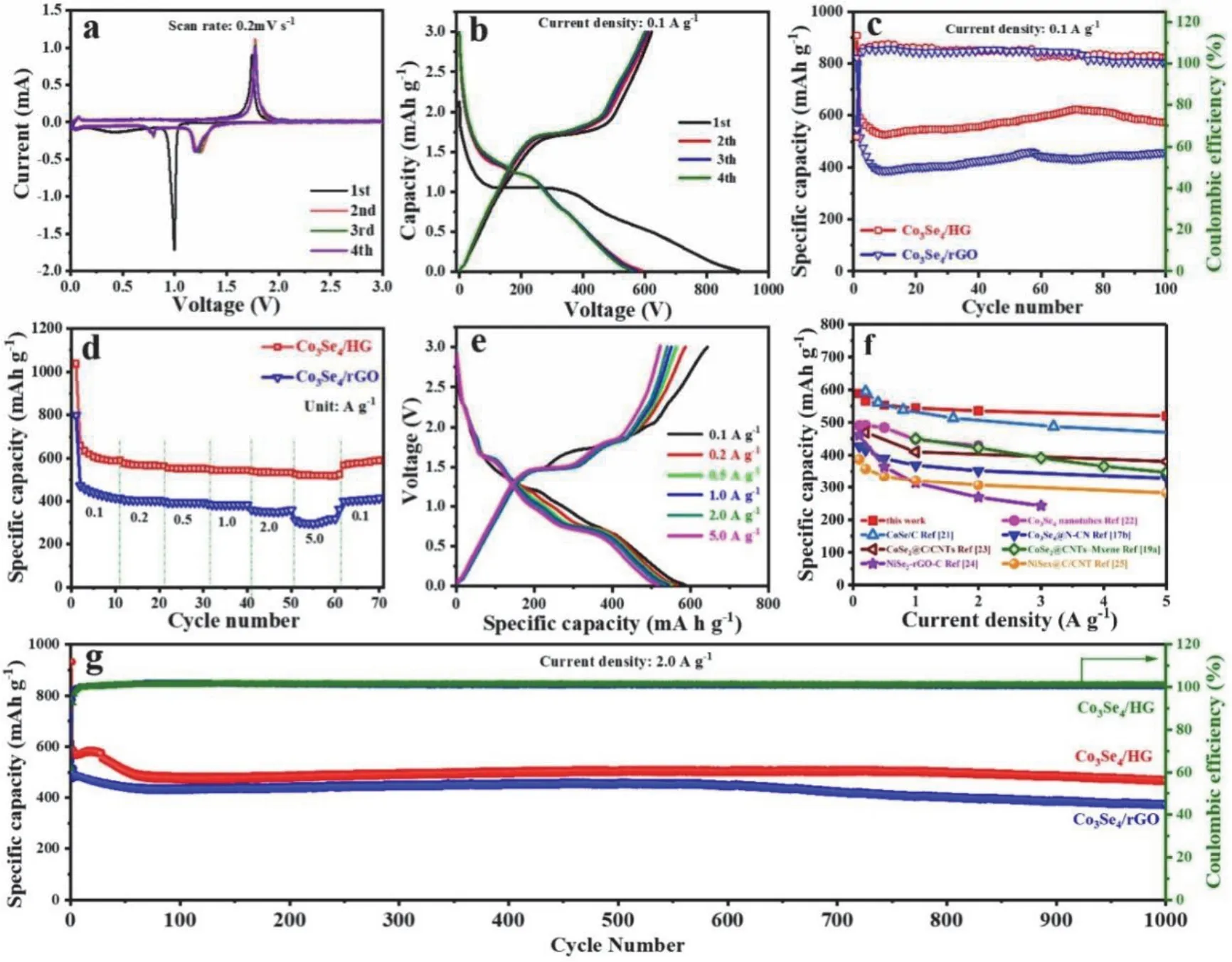
Fig.2 (a) CV curves at 0.2 mV·s–1 and (b) GCD profiles of Co3Se4/HG.(c) Cycling performance and(d) rate performance of Co3Se4/HG and Co3Se4/rGO.(e) GCD profiles at various current densities of Co3Se4/HG.(f) Comparison diagram.(g) Long cycling performances of Co3Se4/HG and Co3Se4/rGO electrodes.
The effectiveness of constructing holey graphene is confirmed by comparing the Co3Se4/HG and Co3Se4/rGO.After 100 cycles,the specific capacity of Co3Se4/HG reaches 575.2 mAh·g-1(Fig.2c).It far exceeds that of Co3Se4/rGO (456.7 mAh·g-1).The rate capability of Co3Se4/HG is also superior to the rGO-based counterpart (Fig.2d,e).Co3Se4/HG has a specific capacity of 588.8 mAh·g-1at 0.1 A·g-1,and can maintain a high capacity of 519.5 mAh·g-1even at 5.0 A·g-1,far superior to Co3Se4/rGO(417.8 and 318 mAh·g-1),indicating the accelerated diffusion of electrolyte ions because of the formation of in-plane pores in the graphene nanosheets.
The rate capability of Co3Se4/HG exceeds that of most reported conventional metal chalcogenides (Fig.2f).The reasons are that the conductivity of HG is unprecedented.And the NPs offer captivating diffusion.Also,the Na+storage mechanism of the optimal sample is tested byinsituXRD.The reversible phase transition of the chalcogenide has been disclosed.The full cell performance is also evaluated by coupling with sodium vanadium phosphate.After consecutive cycles,a high discharging capacity of 473.7 mAh·g–1is realized at 0.1 A·g–1.Moreover,it also possesses a good rate performance.And 94% of the original capacity is maintained,demonstrating its fascinating cycling life.
To sum up,this work highlights pathbreaking holey graphene design with decent performance.The presence of holey graphene serves as a mechanical backbone and an electrical highway,accommodating the volume expansion of Co3Se4.The novel synthesis endows the hybrid with exceptional characteristics such as rapid ion diffusion and intercalation,and superb electron conductivity.The competent performances,including high rate performance,appreciable initial Columbic efficiency,and goodish cycling stability,make the Co3Se4/HG a unique candidate as the anode for sodium-ion batteries.This new strategy will enlighten the development of viable anode materials for sodium-ion batteries.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
- Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution
- Methylene Blue Incorporated Donor-Acceptor g-C3N4 Nanosheet Photocatalyst for H2 Production

