Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
Junwen Lu ,Shunan Zhang ,Haozhi Zhou ,Chaojie Huang ,Lin Xia ,Xiaofang Liu ,Hu Luo ,Hui Wang ,
1 CAS Key Laboratory of Low-Carbon Conversion Science and Engineering,Shanghai Advanced Research Institute,Chinese Academy of Sciences,Shanghai 201210,China.
2 University of Chinese Academy of Sciences,Beijing 100049,China.
3 Institute of Carbon Neutrality,ShanghaiTech University,Shanghai 201203,China.
Abstract: The conversion of CO2 into CO via the reverse water gas shift (RWGS) reaction has recently attracted considerable attention owing to the increase in atmospheric CO2 emissions.However,metalsupported catalysts easily undergo sintering and become inactive at high temperatures.To fabricate highly active and stable catalysts,molybdenum carbide (MoxC),with properties similar to those of precious metals,has been extensively investigated.In particular,it has been demonstrated that face-centered cubic α-MoC can strongly interact with support metals,rendering it an attractive candidate as a catalyst for the RWGS reaction.Furthermore,it has been previously demonstrated that metallic Ir,with unique electronic properties and a low CO desorption barrier,is active for the RWGS at low temperatures (250–300 °C).Accordingly,in this study,a system of Ir species and α-MoC was constructed using a solvent evaporation self-assembly method.The catalytic performance of the Ir/MoC catalysts for the RWGS reaction was considerably superior to that of pure α-MoC over a wide temperature range (200–500 °C) owing to the synergistic effect of Ir and α-MoC.The optimal 0.5% Ir/MoC catalyst yielded a CO2 conversion of 48.4% at 500 °C,0.1 MPa,and 300000 mL·g-1·h-1,which was comparable to the equilibrium conversion (49.9%).The CO selectivity and space-time yield of CO over 0.5% Ir/MoC reached 94.0% and 423.1 μmol·g-1·s-1,respectively,which were higher than most of the previously reported values.Moreover,0.5% Ir/MoC retained its catalytic properties over 100 h and demonstrated excellent stability at high temperatures.Several characterization methods were used to demonstrate that the Ir species supported on α-MoC substrates were highly dispersed.The strong metal-support interaction between Ir and α-MoC,which occurred via electron transfer,considerably improved the stability of the Ir/MoC catalysts.For the Ir/MoC catalysts with Ir loadings >0.2% (mass fraction),Ir single atoms (Ir1) and clusters (Irn) coexisted to create Irn-Ir1-C-Mo synergistic sites between Ir and α-MoC.The number of Ir1 species and size of Irn species of 0.5% Ir/MoC were higher and smaller,respectively,than those of the other Ir/MoC catalysts.This conferred 0.5% Ir/MoC an optimal electron density,which contributed to the remarkable adsorption and activation of CO2 and H2 during the RWGS.In situ diffuse reflectance infrared Fourier transform spectroscopy experiments revealed that the RWGS reaction mechanism occurred via a formate pathway.Although the formation of Irn-Ir1-C-Mo synergistic sites did not affect the reaction mechanism,the generation and decomposition of formate intermediates were distinctly promoted.Therefore,the catalytic performance of Ir/MoC was effectively improved by the synergistic effect.This study provides a guide for designing efficient and stable catalysts for CO2 utilization.
Key Words: Reverse water gas shift reaction; Metal-support interaction; Synergistic effect; Molybdenum carbide;Iridium catalyst
1 Introduction
Among CO2utilization,the reverse water gas shift (RWGS)reaction (Eq.(1)) is considered to be the most attractive way to convert CO21,2.The CO produced by this reaction can serve as a feedstock for the Fisher-Tropsch process,oxo-synthesis reactions,and other syngas processes2,3that produce valuable fuels and chemicals synthesized.
Traditional supported-metal catalysts,like Cu/CeO24,Fe/Al2O35,and Ni/SBA-156,have been widely used in the RWGS reaction owing to their abundant active sites and outstanding interactions with reactants.However,due to the endothermic property of the reaction,supported metals are easily aggregated and sintered at high temperatures,severely deactivating their catalytic performance7,8.Therefore,it is very significant to resolve the conflict between high activity and high stability of RWGS reaction9.
It is generally reported that α-MoC can be used as a superior support to construct synergistic sites with single atoms or clusters22.In the meanwhile,the SMSI can be induced to resolve the issues of catalytic stability23–26.For instance,the Au/α-MoC catalyst was constructed by Maetal.27for water-gas shift reactions,and the synergistic effect between Au clusters and α-MoC remarkably improved the low-temperature activity.The dissociation of water and methanol in aqueous-phase reforming of methanol reaction was found to be synergistically promoted by Pt single atoms supported on α-MoC,which extremely increased the hydrogen production rate at low temperatures17.Therefore,using α-MoC as the support to create synergistic active sites with single atoms or clusters can not only improve the utilization efficiency of atoms but also accurately and efficiently catalyze the desired reaction28.
Ir-based catalysts are active for RWGS at low temperatures(250–300 °C) because of their unique electronic property and low CO desorption barrier29,30,such as Ir1/TiO231and Ir1/CeO232.In light of this,we decide to build Ir and α-MoC synergistic active sites for the RWGS reaction.In this work,the synergistic effect between Ir species and α-MoC was investigated by various characterization methods,including Xray diffraction (XRD),X-ray photoelectron spectroscopy (XPS),X-ray adsorption fine structure (XAFS),and aberrationcorrected scanning transmission electron microscopy (ACSTEM).The synergistic sites of Ir1-Irn-C-Mo were formed on 0.5% Ir/MoC by the SMSI between Ir species and α-MoC,which remarkably promoted the CO2and H2adsorption and activation.The dissociation of formate intermediate species to CO was also enhanced by their synergistic effect.Therefore,the 0.5% Ir/MoC achieved an exciting performance and exhibited good durability at high reaction temperatures.
2 Experimental
2.1 Catalyst preparation
The α-MoC was prepared by a temperature-programmed carburization (TPC) procedure which has been reported in the previous research33.In a typical synthesis,ammonium paramolybdate ((NH4)6Mo7O24·4H2O,≥ 99%,purchased from Sinopharm Chemical Reagent Co.,Ltd.,China) was calcined at 400 °C with a heating rate of 0.5 °C·min-1and held for 3 h to obtain MoO3.Then the oxide precursor was heated from room temperature (RT) to 700 °C (5 °C·min-1) in NH3(150 mL·min-1;pre-purified gas) and kept at 700 °C for 2 h.After the sample was cooled to RT,the gas was switched to the mixture of 20% CH4and 80% H2(150 mL·min-1) as the temperature increased to 400 °C at a rate of 5 °C·min-1,and continued from 400 °C to 700 °C at a rate of 5 °C·min-1,keeping at 700 °C for 1 h.Eventually,after the sample was cooled down to room temperature,it was passivated for 3 h using a 1% O2/Ar gas mixture.
The Ir/MoC was prepared by a novel solvent evaporation selfassembly method34.1.23 g ammonium paramolybdate and a certain amount of dihydrogen hexachloroiridate (IV) hydrate(H2IrCl6·XH2O,98%,purchased from Energy Chemical,China)were mixed in distilled water,and stirred for 4 h.Subsequently,the solution was evaporated by a rotary evaporator at 75 °C and dried at 60 °C overnight in a drying oven.The resultant light brown powder was calcined at 400 °C (0.5 °C·min-1) and hold for 3 h to obtain the IrOx-MoO3.After that,the same TPC procedure in CH4/H2mixture (150 mL·min-1) addressed above was used to prepare Ir/MoC.
2.2 Catalyst characterization
The Ir loadings of series catalysts were examined by the inductively coupled plasma optical emission spectrometer (ICPOES,Optima 8000,PerkinElmer,USA).
A X-ray powder diffractometer instrument (Rigaku Ultima IV,Japan) with a CuKαradiation (λ= 0.154056 nm,40 kV,40 mA) was used to identify the structure and crystal texture of samples (scanning angle (2θ) range: 5°–90°,scanning rate: 4(°)·min-1).
Justin was a climber. By one and a half, he had discovered the purple plum tree in the backyard, and its friendly branches became his favorite hangout.
QuadraSorb Station 3 instrument (USA) was used to perform nitrogen physical adsorption.In detail,100 mg of each sample was outgassed at 150 °C for 12 h.To calculate the specific surface area,the Brunauer-Emmett-Teller (BET)method was conducted.The Barrett-Joyner-Halenda (BJH)method was used to calculate the pore volume and the pore size of catalysts.
Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) images and EDX elemental maps were obtained on a FEI-Titan Cubed Themis G2 300 (USA).
The X-ray adsorption fine structure (XAFS) characterizations were conducted in fluorescence mode at the BL11B beamline of Shanghai Synchrotron Radiation Facility (SSRF),China,and the data was collected by a Lytle detector.Standard Ir foil and IrO2were used for comparison.Pre-edge line,and post-edge line and background calibrations were obtained in Athena (version 0.9.26).The Fourier transformed fitting,coordination number(CN),bond length (R),Debye-Waller factor (σ2),andE0shift(ΔE0) were obtained by nonlinear fitting of the EXAFS equation to the Fourier-transformed data inR-space with least-squares refinement,using Artemis software (version 0.9.26).The amplitude reduction factorS02(0.754),obtained by fitting of EXAFS of Ir foil,was used to determine the CNs in Ir-C/Ir scattering path by setting in the EXAFS analysis.
The X-ray photoelectron spectroscopy (XPS) was conducted on a Thermo Scientific K-Alpha instrument (AlKαradiation,12 kV,4 mA,hν= 1486.6 eV,USA).Prior to the test,samples were sticked on a metal plate with a conductive resin,and treated under ultrahigh vacuum conditions (5 × 10-5Pa).The raw data was calibrated by carbon contaminants (Eb= 284.8 ± 0.1 eV).
The CO2and H2temperature-programmed desorption experiments were conducted on Micromeritics Autochem II 2920 instrument (USA) with a thermal conductivity detector(TCD) and mass spectrometer (MKS Cirrus 2).Before each experiment,samples were reduced in H2at 500 °C for 30 min,and then the gas was switched to Ar cooling to 50 °C.After that,the sample was exposed to CO2or H2for 1 h,and the gas was switched back to Ar to remove the excess CO2or H2.After the baseline was stable,the desorption process was processed at the temperature range of 50 to 600 °C with a rate of 10 °C·min-1.
The data ofinsitudiffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was collected by a Thermo Scientific Nicolet 6700 spectrometer (USA).The sample was first treated in H2for 1 h and then purged with Ar at 400 °C for 1 h to remove the adsorbed H2.After recording the background spectrum,H2/CO2(3/1) was introduced into the reaction cell with an increase in the temperature from 250 to 370 °C.After holding for 30 min at each temperature point,the spectra were recorded to obtain information on adsorbates.
2.3 Testing of the catalytic activity
Conventional catalytic evaluation of the RWGS reaction was performed under atmospheric pressure on a vertical continuous fixed bed reactor with an internal diameter of 8 mm.Typically,20 mg of catalyst was mixed with 80 mg quartz sand (40–60 mesh),which was reduced in pure H2at 500 °C (50 mL·min-1)for 1 h.And then the sample was cooled to 200 °C for reacting in a mixture of 72.4% H2,24.6% CO2,and 3% N2(N2as the internal standard) with a flow rate of 100 mL·min-1.All catalysts were evaluated at a temperature range of 200 to 500 °C.The products were examined by an Agilent 8860 online gas chromatograph with a TCD connected with three packed columns.
The CO2conversion was calculated according to the equation below:
wherenCO2inandnCO2outreferred to the mole numbers of the inlet and outlet CO2,respectively.
The selectivity of CO2and CH4were calculated according to the equations below:
wherenCOoutandnCO4outis the mole numbers of CO and CH4product at the outlet gas.
The space-time yield (STY) of CO was calculated according to the equation below:
STY(CO)=X(CO2) ×nCO2in×S(CO)
3 Results and discussion
3.1 Catalytic performance
Series Ir/MoC catalysts with different Ir loadings were investigated to determine the synergistic effect between Ir and α-MoC.The catalytic performance of all catalysts was evaluated at 200–500 °C,0.1 MPa,and 300000 mL·g-1·h-1.As shown in Fig.1a,when Ir supported on α-MoC,the space-time yield of CO was markedly higher than that of pure α-MoC.With Ir loadings increased to 0.5% (wt),the CO2conversion,CO selectivity,and STY peaked at 48.4%,94.0%,and 423.1 μmol·g-1·s-1,respectively.It is worth emphasizing that the CO2conversion was close to the equilibrium conversion (49.9%) at 500 °C (Fig.S1,Table S1).The catalytic performance was 136% of pure α-MoC,outperforming most of the previous reports (Table S2)35.Additionally,it was also found to be active at low temperatures,with a CO selectivity of 100% and STY of 37.2 μmol·g-1·s-1at 250 °C.However,the STY substantially decreased to 394.45 μmol·g-1·s-1when the loading was increased to 5% (wt),which suggested the synergistic effect could only be maximum at the appropriate amount of Ir.To further verify the synergistic effect between Ir and α-MoC,Ir with identical content (0.5% (wt)) was supported on various supports (TiO2,SiO2,TiC,ZrC,and α-MoC) to compare the catalytic performance.It was evident that there was a synergistic effect between Ir and α-MoC for jointly boosting the catalytic performance since the CO STY of Ir supported on other supports was extremely lower than the Ir/MoC (Fig.S2).
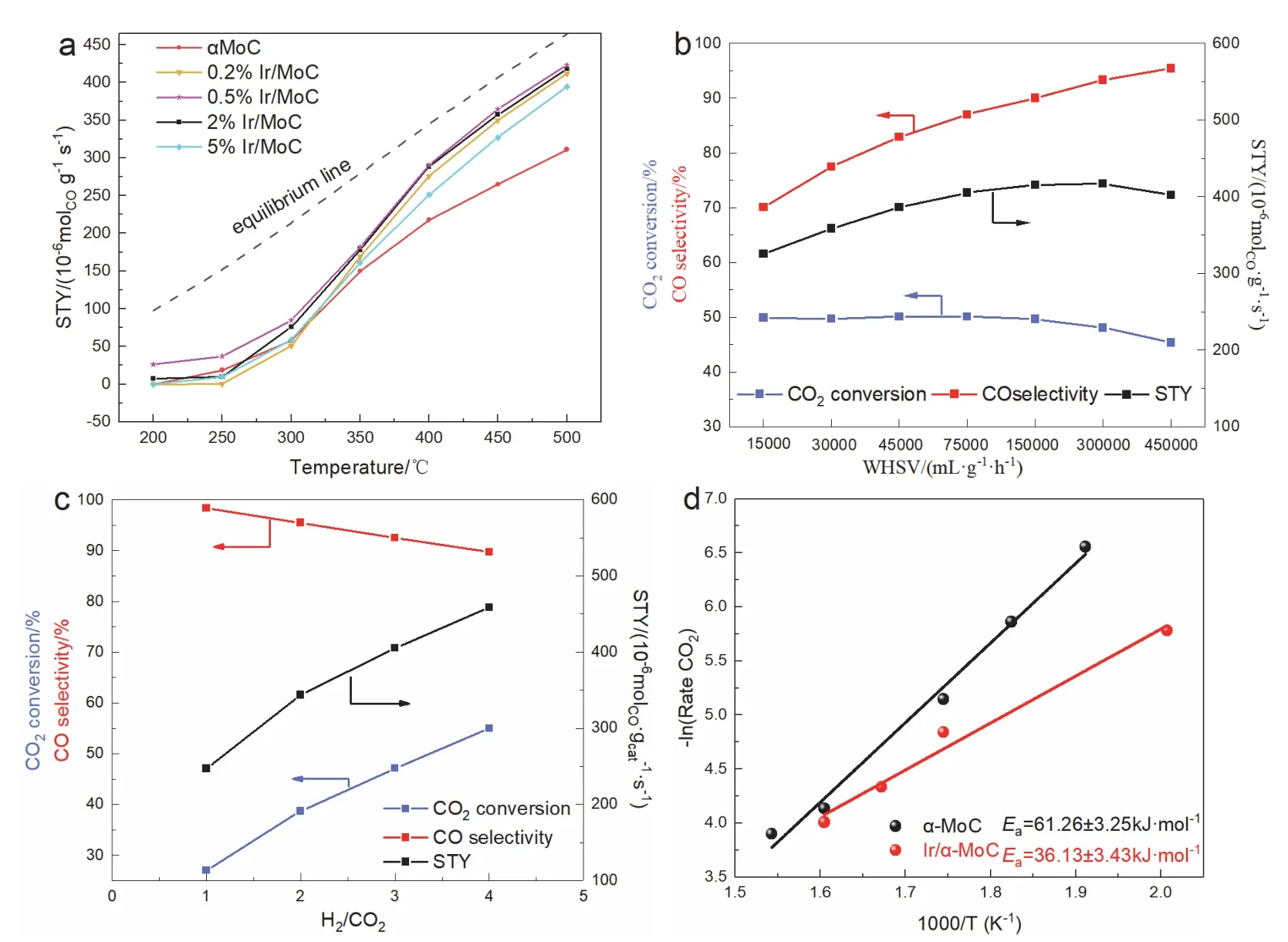
Fig.1 Catalytic performance of the Ir/MoC catalysts for RWGS reaction at different (a) temperature,(b) space velocity,(c) feed H2/CO2 ratio.(d) Arrhenius plots for CO2 conversion over Ir/MoC.
To reveal the intrinsic activity of 0.5% Ir/MoC,the weigh hour space velocity (WHSV) and H2/CO2ratio were examined.With the increase of WHSV from 15000 to 450000 mL·g-1·h-1at 500 °C,the CO selectivity dramatically increased from 70.1% to 95.4% (Fig.1b),which indicated that high WHSV shortened the residence time of intermediates to prevent the excessive hydrogenation of CO.In contrast,the CO2conversion remained relatively constant at 50%,showing that the catalyst had high intrinsic activity.The CO STY reached its maximum value of 423.1 μmol·g-1·s-1at the WHSV of 300000 mL·g-1·h-1.Increasing the H2/CO2ratio,the CO2conversion and CO STY greatly increased,in which the STY of CO can reach 458.9 μmol·g-1·s-1at the ratio of 4/1,but CO selectivity dropped to 89.7% as a result of the excessive hydrogenation (Fig.1c).According to the findings,only the appropriate H2/CO2ratio(2/1–3/1) can maintain the balance between CO2conversion and CO selectivity36,37.The calculations of the apparent activation energy (Ea) showed that theEaof 0.5% Ir/MoC (36.13 ± 3.43 kJ·mol-1) was higher than α-MoC (61.26 ± 3.25 kJ·mol-1) (Fig.1d),further suggesting that the synergistic effect of Ir extremely improved the intrinsic activity.Besides,the catalyst exhibited excellent durability under harsh conditions,with CO2conversion,CO selectivity,and STY nearly remaining unchanged at 500 °C within 100 h (Fig.S3).
3.2 Structure properties of catalysts
To reveal the structure-activity relationship,the structural properties were deeply studied.The XRD patterns showed that the peaks of fresh catalysts at 36.4°,42.3°,61.4°,73.5°,and 77.4° corresponded to the α-MoC with face-centered cubic structure (PDF#89-2868) (Fig.2a).Even though the content of Ir increased to 5% (wt) when it was supported on α-MoC,there were no peaks of metallic Ir,which suggested that Ir was highly dispersed on α-MoC38.Moreover,the peaks widened gradually as Ir content increased,implying that a strong interaction between Ir and α-MoC already existed and eventually intensified.After the reaction,the characteristic peaks of α-MoC showed no changes (Fig.2b),which could be due to the stability of Ir guaranteed induced by the SMSI.The textural properties were listed in Table 1,which showed that the practical loading of Ir was rather close to the theoretical value (within the margin of error).The BET surface area of the α-MoC with different loading of Ir decreased distinctly from 127 to 50 m2·g-1as Ir content increased.It could be explained by the Ir metal blocking these mesoporous of α-MoC39.This result confirmed the high dispersion of Ir species on α-MoC,which was in good accord with the XRD result.On the other hand,the catalytic performance of 0.5% (wt) Ir supported on supports with a higher specific surface area was inferior to that on α-MoC (Fig.S2 and Table S3).This implied that the catalytic performance was independent of the specific surface area of supports,but was attributed to the configuration of Ir species and the synergistic effect.
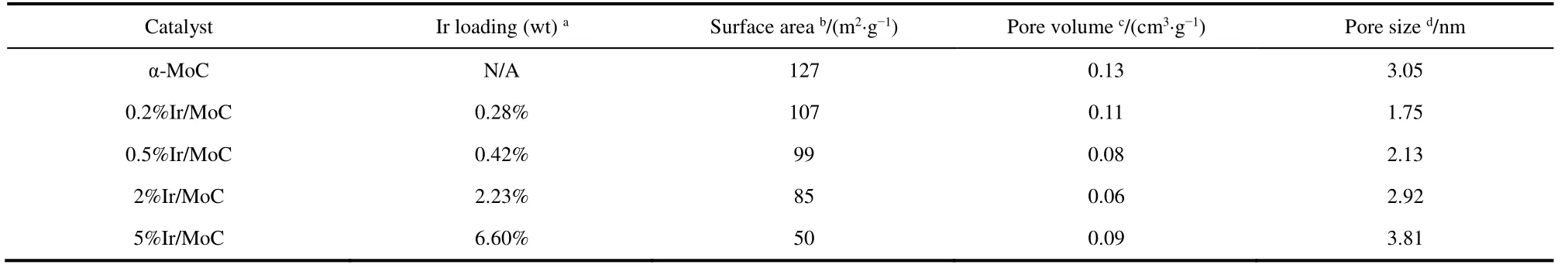
Table 1 Textural properties of the Ir/MoC catalysts.
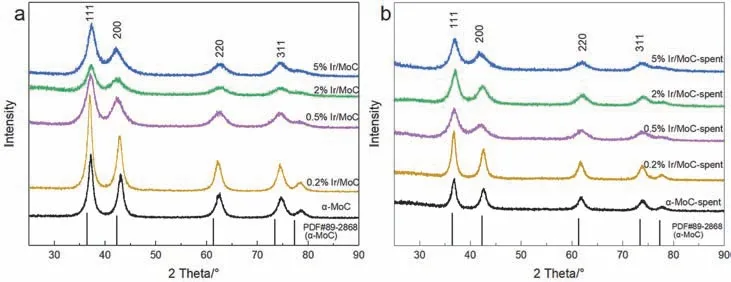
Fig.2 XRD patterns of the Ir/MoC catalysts: (a) fresh catalysts,(b) spent catalysts.
To analyze the configuration of surface Ir species,the XPS of spent catalysts was carried out.The spectra of Ir 4fshowed that the binding energies of Ir 4f7/2at around 60.80,61.3,and 62.5 eV were assigned to the Ir0,Ir4+,and Ir3+states on Ir/MoC catalysts,respectively (Fig.3a)40,41.The absence of the Ir0peaks for 0.2% Ir/MoC suggested Ir species could be single atoms in this low content.As the loadings of Ir increased,the Ir0appeared and the percentage of Irδ+(Ir3+and Ir4+) significantly decreased(Table 2),which indicated Ir clusters/particles and unsaturated Ir species co-existed.Meanwhile,the peaks of Irδ+shifted to the lower binding energy,suggesting that electron transfer occurred.On the other hand,the binding energies of Mo 3d5/2at 228.3–228.4,228.9–229.2,and 229.9–230.2 eV,assigning to the Mo2+,Mo3+,and Mo4+states,respectively,were observed in Mo 3dspectra (Fig.3b)42.With the increase in Ir loadings,the binding energy of Mo2+also migrated to the lower binding energy and the proportion of Mo2+increased substantially,reaching a peak of 59.62% at 0.5% (wt) Ir.The results strongly indicated that the SMSI,which allowed for the stable existence of Ir0and Irδ+species,was formed by the electron transfer from Ir species to α-MoC43.Combined with the catalytic performance,the coexisting Ir0and Irδ+species on the 0.5% Ir/MoC possessed the optimal electron and structure properties for the RWGS reaction.
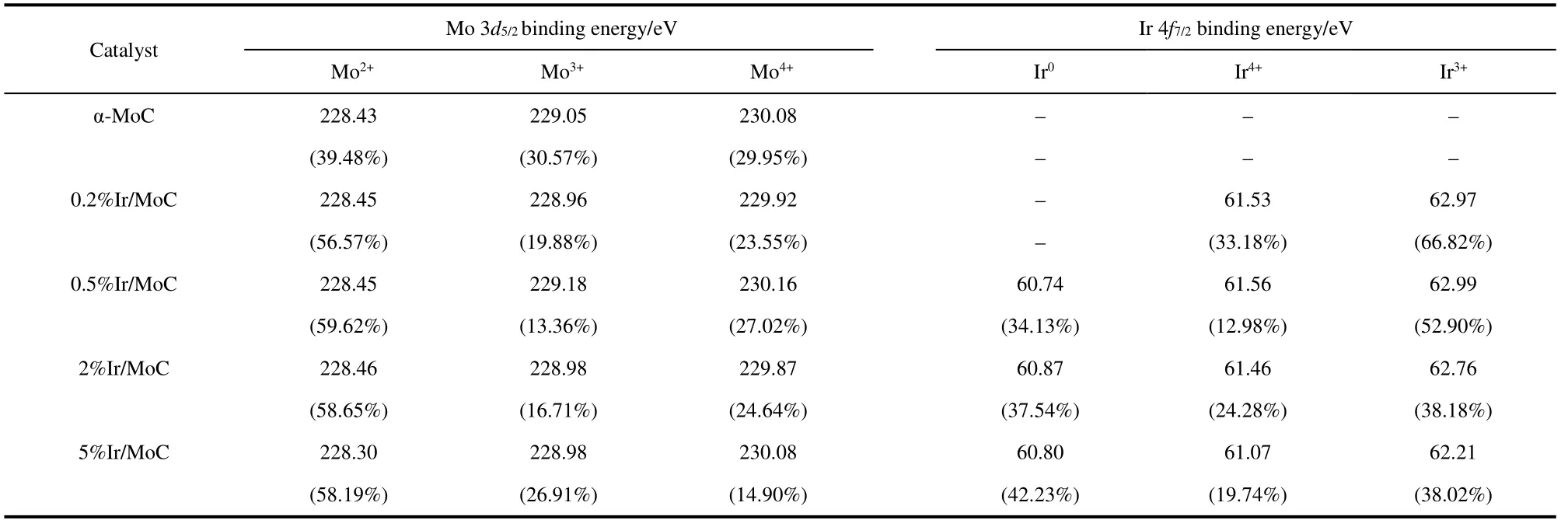
Table 2 Compositions and positions of Mo 3d5/2,Ir 4f7/2 for all samples.

Fig.3 XPS of the Ir/MoC spent catalysts: (a) Ir 4f,(b) Mo 3d.
The XAFS technique was used to further reveal the coordination environment of Ir species.The white lines of L3-edge on Ir/MoC catalysts were located between standard IrO2and Ir foil in the XANES spectra (Fig.S4),which again proved the presence of Irδ+species and SMSI.Furthermore,the EXAFS fitting results revealed that there were Ir-Mo and Ir-C coordination shells on the 0.2% Ir/MoC catalyst,while the Ir-Ir and Ir-C coordination shells were on 0.5% Ir/MoC and 5% Ir/MoC catalysts (Fig.4a).The results verified again that only Ir single atoms were present when the Ir content was 0.2%(wt),whereas Ir clusters/particles and single atoms co-existed when the Ir content was 0.5% or 5% (wt).The coordination number of Ir-Ir shell on the 0.5% Ir/MoC catalyst (3.1) and 5% Ir/MoC (5.6) was noticeably lower than that of Ir foil and IrO2,which indicated that the Ir0species were Ir clusters (Irn)44.The cluster size on the 0.5% Ir/MoC catalyst was smaller,according to the wavelet transforms results,and the majority of Ir species were present as Ir single atoms (Ir1) (Fig.4b–d)45.The above results also could be concluded that the Irn-Ir1-C-Mo synergistic sites were formed on the surface of 0.5% Ir/MoC.TEM,AC-HAADF-STEM,and element mapping images further proved that Ir single atoms and subnanometer clusters uniformly dispersed on the columnar α-MoC (220) over the 0.5% Ir/MoC catalyst (Fig.5).In general,it could be concluded that the formation of unique Irn-Ir1-C-Mo synergistic sites was the key to improve catalytic performance.
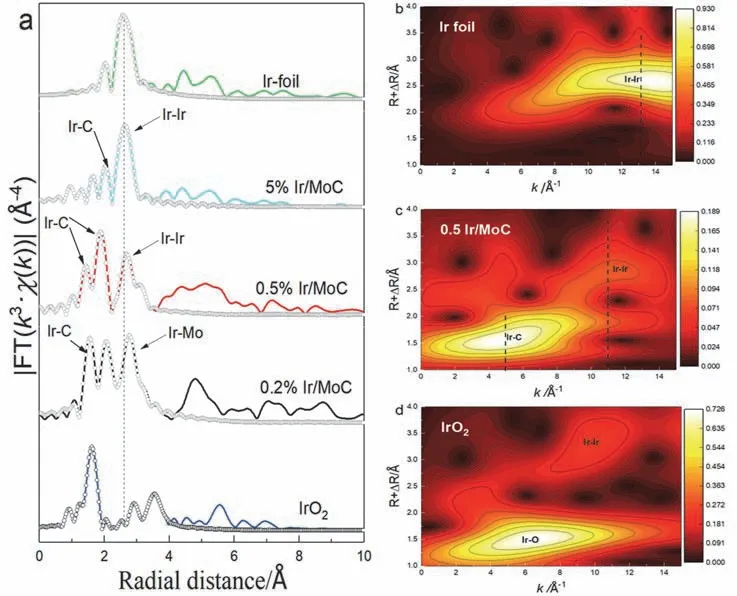
Fig.4 (a) Fourier transforms of the Ir L3-edge EXAFS spectra of Ir/MoC,Ir foil,and IrO2.(b–d) Wavelet transform of the Ir L3-edge EXAFS spectra of 0.5% Ir/MoC,Ir foil,and IrO2.1 Å = 0.1 nm

Fig.5 (a) TEM images,(b) AC-HAADF-STEM images (the Ir atoms were highlighted by the red circles,and Ir clusters were highlighted by the white circles),(c) EDS mapping images of Ir/MoC.Color online.
3.3 Adsorption properties and reaction mechanism
To determine the effect of synergistic sites on the reaction process,CO2and H2-TPD experiments were performed.As shown in Fig.6a,there were almost no CO2desorption peaks on pure α-MoC,demonstrating that it had a weak adsorption ability for CO2.Contrarily,a desorption peak of CO2at 200–450 °C appeared with the increase of Ir content,which suggests that the synergistic sites of Ir species and α-MoC promoted CO2adsorption38–47.The largest peak area was on the 0.5% Ir/MoC catalyst,corresponding to the maximum of CO2adsorption,which could be attributed to its smaller Ir cluster size.In terms of the H2-TPD data,additional peaks at 50–150 and 300–600 °C that corresponded to the weak and strong adsorption of H2,respectively,appeared with the addition of Ir when compared to α-MoC (Fig.6b)48.It implied that the H2adsorption was enhanced by Ir species.Additionally,the strong desorption peak of the 0.5% Ir/MoC catalyst distinctly shifted to the lower temperature region,illustrating that the catalyst was more favorable to the adsorption and activation of H2at low temperatures.Therefore,the optimal RWGS performance was displayed on the 0.5% Ir/MoC catalyst due to the strong adsorption and activation ability of reactants.
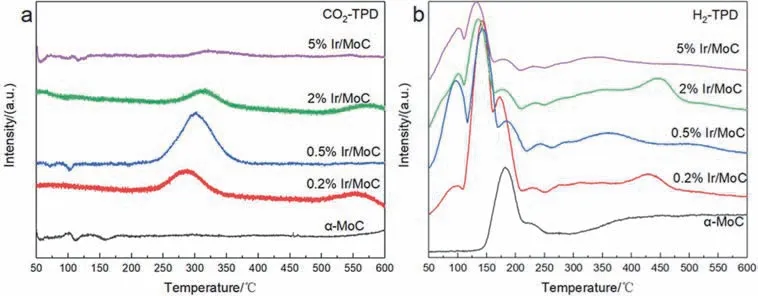
Fig.6 TPD profiles of the Ir/MoC catalysts: (a) CO2-TPD,(b) H2-TPD.
The reaction mechanism of RWGS was first investigated by the temperature-programmed surface reaction (TPSR)experiment.The sample was exposed to pure CO2and the signals of CO2and CO were recorded after being pretreated with H2at 500 °C for 1 h and then cooled down to 50 °C in Ar.As shown in Fig.7a,there was no CO2consumption or CO generation below 600 °C over 0.5% Ir/MoC,which elucidated that it was difficult for the RWGS reaction to proceed without the assistance of H2.However,when H2was introduced into the system,the signal of CO2decreased and a CO signal began to emerge around 200 °C,indicating the reaction mechanism of RWGS involved the formate or carboxylate route with the assistance of H2(Fig.7b)9.The TPSR results of pure α-MoC were similar to those of 0.5% Ir/MoC (Fig.S5),which proved that the formation of Irn-Ir1-C-Mo synergistic sites had no influence on the reaction mechanism.

Fig.7 (a) The CO2 dissociation experiment of 0.5% Ir/MoC,(b) TPSR results of the 0.5% Ir/MoC.
To gain insight into the effects of synergistic sites on the reaction process,theinsituDRIFTS was conducted.The adsorption of formate species with peaks at 2847 and 2915 cm-1and gaseous CO with peaks at 2110 and 2177 cm-1were observed at temperatures between 250 and 370 °C on both pure α-MoC and 0.5% Ir/MoC catalysts (Fig.8)49–51.Since no peaks of carboxylate species were observed,it can be determined that the reaction mechanism was the formate route.Furthermore,the peak intensity ratio of formate species above 250 °C to that at 250 °C was calculated (denoted asIT/I250) to determine the effects of Ir species on the reaction intermediates.It was obvious that the ratio ofIT/I250on 0.5% Ir/MoC increased considerably more quickly than that of pure α-MoC with a steep slope (Fig.S6).The results indicated that the formation rate of formate intermediates was accelerated by Ir species52,53.This provided evidence that the formation of Irn-Ir1-C-Mo synergistic sites promoted the generation and decomposition of formate intermediate species,which stepwise hydrogenated to CO,and thus the catalytic performance was enhanced.
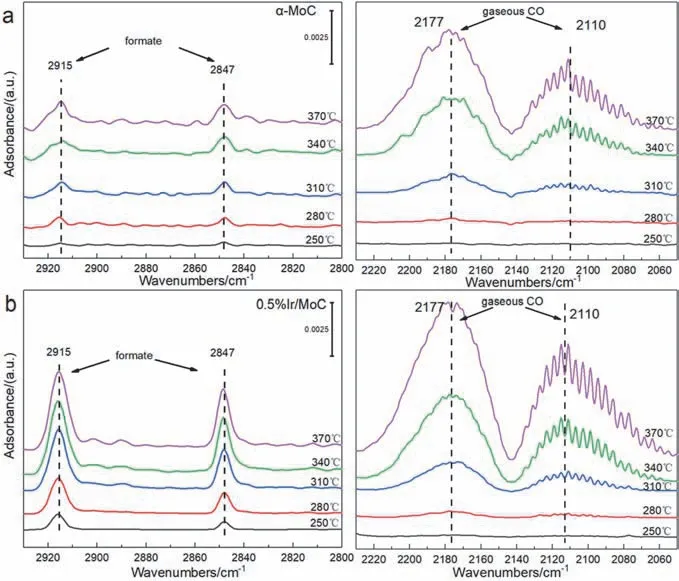
Fig.8 DRIFTS spectra of (a) α-MoC and (b) Ir/MoC catalysts in RWGS at 250–370 °C.
4 Conclusions
In this work,a synergistic Ir/MoC system was constructed for the RWGS reaction.The SMSI between Ir and α-MoC was formed by the electron transformation,which further created Irn-Ir1-C-Mo synergistic sites.The optimal electron properties and the smaller Ir cluster size of 0.5% Ir/MoC catalyst significantly promoted the adsorption and activation of CO2and H2,while boosting the generation and decomposition of formate intermediate species.A CO2conversion of 48.9% close to the conversion equilibrium (49.9%) at 500 °C and a STY of CO of 423.1 μmol·g-1·s-1were achieved.Meanwhile,the catalytic performance can be stabilized within 100 h under harsh conditions.This study provides a rational idea for the design of catalysts with both high performance and stability in the utilization of CO2.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
- Holey Graphene for Sodium-Ion Battery Anode Material
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution
- Methylene Blue Incorporated Donor-Acceptor g-C3N4 Nanosheet Photocatalyst for H2 Production

