Semiconducting Polymers for Photosynthesis of H2O2: Spatial Separation and Synergistic Utilization of Photoredox Centers
Yao Xie ,Qitao Zhang ,*,Hongli Sun ,Zhenyuan Teng ,Chenliang Su ,*
1 International Collaborative Laboratory of 2D Materials for Optoelectronic Science & Technology,Engineering Technology Research Center for 2D Materials Information Functional Devices and Systems of Guangdong Province,Institute of Microscale Optoeletronics,Shenzhen University,Shenzhen 518060,Guangdong Province,China.
2 School of Chemistry,Chemical Engineering and Biotechnology,Nanyang Technological University,Singapore 637459,Singapore.
3 Department of Applied Chemistry,Faculty of Engineering,Kyushu Institute of Technology,Kitakyushu 804-8550,Japan.
Abstract: The photocatalytic synthesis of hydrogen peroxide using earth-abundant water and/or O2 as raw materials and solar energy as the sole energy input is an attractive route to achieving a carbon-neutral future.In particular,semiconducting polymer photocatalysts have piqued the interest of researchers working on the photocatalytic synthesis of H2O2 because their bandgap structures,reactivation sites,and components are easily tunable at the molecular level.However,there are two major challenges: 1) the photoredox centers are difficult to separate and recombine easily,resulting in low reactivity in the photocatalytic production of H2O2,and 2) the low utilization rate of the redox centers.In several cases,only one side of the redox center is used for the photocatalytic synthesis of H2O2,while the other side typically reacts with a sacrificial agent.In this review,we provide a timely survey of recent advances in the spatial separation and synergistic utilization of photoredox centers for photocatalytic H2O2 production.The key aspect for achieving spatial separation of the redox centers is to engineer electron donoracceptor (D-A) units on a single photocatalyst,such as by incorporating atomically dispersed metals into the polymer frameworks to build metal-organic D-A units or constructing all-organic D-A units.Depending on the photocatalytic behavior of the redox centers,the synergistic utilization of photoredox centers can be classified into three major reaction models: 1)the oxygen reduction reaction (ORR) combined with the oxidative production of chemicals; 2) the water oxidation reaction(WOR) combined with the reductive production of chemicals; and 3) the ORR combined with the WOR.Based on this,the regulation modes,characteristics,catalytic mechanisms,and reaction pathways to overcome the two challenges of efficient H2O2 production are summarized and discussed.Finally,we demonstrate efficient photocatalytic H2O2 production and provide prospects and challenges for the photocatalytic production of H2O2 using photoredox centers.
Key Words: H2O2 synthesis; Photo-redox center; Spatial separation; Synergistic utilization; Polymer photocatalyst
1 Introduction
Hydrogen peroxide (H2O2) was first discovered by Thenard in 1818; since then,it has become one of the most valuable chemicals1,2.Globally,with the widespread application of H2O2(organic synthesis,wastewater treatment,disinfection,and paper bleaching) and environmentally friendly processes (the major products are clean and nontoxic),the global demand for H2O2is expected to increase to 5.7 million tons by 20273–5.Furthermore,H2O2is a potential green energy alternative to H2in the fuel cell field due to its comparable energy density to H2and more convenient storage and transportation6.Thus,the synthesis method of H2O2has attracted increasing attention.The oxidation of anthraquinone (AQ) was first developed in 1953 and has dominated the industrial production of H2O2ever since4,7.Nevertheless,it involves multiple catalytic reactions of oxygen and hydrogen,leading to a variety of side reactions,which produce a large amount of waste.This results in the waste of resources,which makes the AQ process nonenvironmentally friendly.Furthermore,the oxidation of AQ is characterized by a high energy input and explosion risks; therefore,for a carbonneutral future,ecofriendly and safe methods for the sustainable production of H2O2are highly desired.
Photocatalysis has become a unique route that can simultaneously meet the requirements of environmental protection,energy saving,and safety since it uses water and oxygen as reactants and solar energy as the energy input,leading to zero or even negative carbon emissions.Interestingly,the photocatalytic synthesis of H2O2can be traced back to 1927,when H2O2was producedviaphotocatalytic oxidation on ZnO particles8.Recently,research on the photocatalytic technology has become a hot spot,and many efforts have been devoted to studying the related field of photocatalysis9.In particular,the study of polymer-based photocatalysts has become increasingly popular,making the research on the photocatalytic synthesis of H2O2a hot topic (as shown in Fig.1)3,10,11.

Fig.1 Timeline showing the critical polymer photocatalysts in H2O2 production.
Notably,the classification,modification method,reaction pathway,and activity enhancement mechanism of polymerbased photocatalysts used in the photocatalytic production of H2O2have been summarized in previous reviews1–3,10,11.However,there are still two major challenges that should be faced in this aspect,which lead to a low utilization of solar energy and a poor activity.One is the poor separation of redox centers,which leads to the easy recombination of the photogenerated carriers and the low selectivity of the photocatalytic production of H2O2; the other is the low utilization rate of redox centers.In many cases,only one side of the redox centers is utilized for the photocatalytic H2O2synthesis,with the other side being sacrificed.For the reaction to proceed,sacrificial agents are usually added to consume the unreactive photoelectrons or holes,leading to a waste of resources and energy12,13.Accordingly,in order to enhance the utilization of solar energy and activity,the spatial separation and synergistic utilization of the redox centers are vital for an efficient H2O2production,and extensive research efforts have been devoted to the advancement of this technology.Furthermore,compared with traditional inorganic metal compound photocatalysts (such as TiO214,WO315,ZnO16,CdS17,MoS218,and BiVO419),polymer-based photocatalysts mostly consist of C,H,and N,which provide rich site structures (central atoms,coordination atoms,surface functional groups,and coordination number),a variety of bond levels (hydrogen bonds,covalent bonds,coordination bonds,and ionic bonds),intrinsic hybrid performance,highly tunable molecular and band structures20.In the photocatalytic production of H2O2,polymerbased photocatalysts are superior over conventional inorganic semiconductor photocatalysts upon rational design; as a result,polymer-based photocatalysts are more easily designed and expanded and thus exhibit wide application prospects.Consequently,they are promising materials for low-cost and easy-to-process photocatalytic H2O2production21.Over the past decade,numerous polymer-based photocatalysts have been investigated for achieving the photocatalytic production of H2O2(Fig.1),including polymeric carbon nitride (denoted as C3N4or PCN)12,22–24,covalent organic frameworks (COFs)25–28,metalorganic frameworks (MOFs)10,24,29,linear conjugated polymers(LCPs)30,conjugated microporous polymers (CMPs)31,32,resorcinol-formaldehyde (RF) resins33–37,and covalent triazine frameworks (CTFs)38,39.Therefore,this review focuses primarily on polymer-based photocatalysts for the spatial separation and synergistic utilization of the redox centers for efficient H2O2production.
The spatial separation of the photoexcited redox centers is an effective way to achieve efficient H2O2production,and a promising strategy is to modify the redox centers,developing a type of photocatalyst with photoredox centers working independently.Based on this strategy,two approaches have been developed,which afford superior efficiency.Atomic metal cocatalysts and polymer frameworks40could be utilized to engineer electron donors and acceptors on different moieties of a single catalyst,thus achieving the spatial separation of the redox centers.These were proved to be effective methods for improving the catalytic efficiency and have thus received considerable research attention41.For example,Co and Sb were incorporated as atomic active sites into polymer-based photocatalysts to drive the spatial separation of the redox centers42–44.Moreover,organic moieties,such as AQ45,46,acetylene47,diacetylene38,48,diarylamine26,49,triazine39,50,thiourea51,52,and porphyrin53,54,which serve as reduction centers or oxidation centers,were introduced in some polymer frameworks,so as to achieve effective spatial separation of the redox centers.
The synergistic utilization of photoexcited redox centers is also challenging,but it is effective in increasing the solar energy utilization rate and avoiding the use of sacrificial agents.Therefore,depending on the photocatalytic behavior of the redox centers,polymer-based photocatalysis are here classified into three reaction models,as illustrated in Table 1.Type I: Oxygen reduction reaction (ORR) combined with the oxidative production of chemicals.The photoexcited electrons reduce O2to H2O2,while the photoexcited holes oxidize the substrates to produce valuable chemicals.Type II: Water oxidation reaction(WOR) combined with the reductive production of chemicals.The photoexcited holes oxidize water to H2O2and hydrogen species,while the photoexcited electrons reduce the substrates to produce value-added chemicals with the hydrogen species.Type III: ORR combined with the WOR.The photoexcited electrons reduce O2through the 2e-route,while the photoexcited holes oxidize water,and eventually the redox centers couple to generate H2O2without the utilization of sacrificial agents nor the generation of any by-products.Based on this,the regulation modes,characteristics,catalytic mechanisms,and reaction pathways for achieving the separation and full utilization of the redox centers for efficient H2O2production are summarized and discussed.Finally,we demonstrate an efficient photocatalytic production of H2O2and prospect the opportunities and challenges that are involved in the spatial separation and synergistic utilization of the redox centers for the photocatalytic production of H2O2.

Table 1 Three types of synergistic utilization of photo-redox centers.
2 Spatial separation of photoexcited redox centers
Generally,multiphase photocatalytic processes can be divided into four independent steps: (I) photoexciton generation;(II) photoexciton separation; (III) carrier migration to the surface catalytic center; and (IV) surface catalytic reaction55.The four steps determine the photocatalytic efficiency.After absorbing photons,the polymer-based photocatalyst generates ‘hot’electron (e-) and hole (h+) pairs,and the bound excitons form through energy relaxation,triggering the formation of photoexcited redox centers56.Accordingly,when the organic semiconductor polymer absorbs photons with an energy higher than its forbidden band,the electrons will be excited and will migrate to the surface of the catalyst; they will then overcome the overpotentials for the subsequent redox reaction (Fig.2)1.However,the redox centers are hard to separate due to the intrinsic limitations of the photocatalysts.Furthermore,the overlapping of the redox centers often increases the recombination of the photogenerated carriers and decreases the selectivity of the photocatalytic production of H2O257.Thus,to achieve an efficient photocatalytic production of H2O2,the spatial separation of the redox centers is an issue that must be addressed.Notably,natural organisms can perform efficient photosynthesis and continuously convert solar energy into biological energy.This is mainly because the redox centers in organisms are physically separated,which is quite challenging to achieve in artificial photosynthesis.Generally,the simultaneous loading of redox centers often causes them to act as recombination centers for the photogenerated electrons and holes,which hinders the photocatalytic synthesis.Anchoring single-atom cocatalysts on polymer photocatalysts is a superior strategy that can enable the mutual independence of the photoexcited redox centers since the single-atom cocatalysts incorporated into the polymer frameworks usually serve only as reduction (oxidation) centers accepting electrons (holes)58.Additionally,single atoms have the potential to trigger an increase in the number of photogenerated holes (electrons) in neighboring organic units to form oxidation (reduction) centers,realizing an efficient spatial separation of the redox centers.For example,we introduced atomically dispersed Sb atoms in C3N4;the oxidation state of the Sb atom is around 3+ with a 4d105s2electron configuration.It is found that such Sb single sites,which act as centers for the accumulation of electrons,adsorb oxygenviaPauling-type adsorption and accumulate electrons,acting as catalytic sites for O2reductionviathe 2e-ORR pathway43.Simultaneously,the accumulated holes at the N atoms of the melem units neighboring the Sb single sites act as catalytic oxidation centers,accelerating the water oxidation kinetics.As a result,a record-high solar-to-chemical conversion(SCC) efficiency of 0.61% for H2O2production was realized.

Fig.2 Schematic of the processes involved in the photocatalytic production of H2O2.
In addition,introducing Co single atoms above C3N4associated with loading AQ at its edge has been shown to result in the successful spatial separation of the photoexcited redox centers,as shown in Fig.3a42.The photooxidation deposition of MnOxand the photoreduction deposition of noble metals provide a visual confirmation of the obtained spatial separation of the photoexcited redox centers.As shown in Fig.3b,the formation of MnOxon the Co atoms suggests that the Co atoms act as oxidation centers.Additionally,Au and Pt nanoparticles are formed on AQ,which is anchored at the edge of the C3N4nanosheets (Fig.3c),confirming that AQ serves as a reduction center.Thus,the artificial photogenerated redox centers can be used to achieve spatial separation leading to a superior separation of the photogenerated carriers (Fig.3d).This is key for achieving efficient H2O2selectivity,resulting in an enhanced H2O2photosynthesis (Fig.3e).

Fig.3 (a) Schematic of the spatial separation of Co single-atom cocatalysts (as oxidation centers) and AQ cocatalysts (as reduction centers) on a two-dimensional (2D) ultrathin C3N4.(b) Photooxidative deposition of Mn on Co1/C3N4.(c) Photoreductive deposition of Au on AQ/C3N4.(d) Steady-state photoluminescence (PL) emission spectra (excitation at 375 nm).(e) Time course of the H2O2 production.Adapted from Ref.42.Copyright 2020,PNAS.
To avoid the use of metal compounds,metal-free strategies have also been developed to achieve spatially separated redox centers through the rational design and construction of polymer frameworks.For example,we developed a type of PCN with 2,5,8-triamino-tri-s-triazine and barbituric acid; the copolymerization suppressed the charge recombination,resulting in a nonsacrificial photocatalytic production of H2O259.Furthermore,the copolymerization of electron acceptors and donor moieties has been proved to be an effective strategy for spatially separating of redox centers.Based on this,RF resins were developed as high-activity donor (D) and acceptor (A) polymer photocatalysts,as shown in Fig.4a33.The RF resins consist of benzenoid (acting as the donor) and quinoid(acting as the acceptor),which are beneficial to the formation of benzenoid-quinoid D-A couplesviaπ-conjugation between them.Furthermore,the RF resins with D-A couples cause energy level hybridization due to theπ-conjugation andπ-stacking of the benzenoid-quinoid D-A couples,endowing the RF resins with a low bandgap (~2.0 eV) and a high conductivity (Fig.4b),which promote an effective separation of the photogenerated carriers and redox centers and broaden the light response range to ~700 nm.Benefiting from this,the RF resin in pure water with O2exhibited a high SCC efficiency (more than 0.5%) for the photocatalytic production of H2O2.This could enable the widespread use of D-A polymers in the photocatalytic production of H2O2and could provide novel pathways for the subsequent rational design and construction of polymer-based photocatalyst frameworks.

Fig.4 (a) π-conjugated and π-stacked D–A structure.(b) Electronic structures of the π-conjugated and π-stacked (RF resins)D-A couples.Ref.33,adapted from Nature Materials.Copyright 2019,Springer Nature.(c) Schematic illustrating the mechanism of the photocatalytic production of H2O2 for AQTEE-COP (left) and NATEE-COP (right).Adapted with permission from Ref.45.Copyright 2022,American Chemical Society.
AQ was also introduced into organic polymer frameworks as a photocatalytic reduction center for the ORR due to its electronacceptor properties45.The Sonogashira cross-coupling reaction between AQ and 1,1,2,2-tetrakis(4-ethynylphenyl) ethene (TEE)was used to form AQTEE-COP.Compared with NATEE-COP(without the introduction of AQ),AQTEE-COP possesses a wider light absorption range and a weaker PL intensity,indicating that the introduction of electron-withdrawing AQ moieties effectively broadens the light absorption range,hinders the recombination of the photogenerated carriers,and accelerates the migration of the optical carriers.Furthermore,the AQ moieties could act as reduction centers due to their electronwithdrawing properties.In the process of the photocatalytic production of H2O2,the AQ moieties absorb the photogenerated electrons with the carbonyl carbon leaving the photoinduced holes at the carbonyl oxygen,which promotes the separation of the photogenerated carriers and the reduction of O2to produce H2O2.Thus,the photoexcited redox centers are spatially separated,and the photocatalytic synthesis of H2O2occurs through the binding of endoperoxide intermediates (AQTEECOP-•O2-) with H+(Fig.4c).This work provides molecularlevel insights into the mechanistic understanding of the efficient photocatalytic production of H2O2from H2O and O2.
Very recently,the copolymerization of acetylene or diacetylene with the heptazine ring was found to yield a type of CHF,achieving the spatial separation of the photoredox centers at the molecular level (Fig.5a) and showing high activities in the synergistic utilization of the photoredox centers for the photocatalytic production of H2O232.The primary idea behind the construction of these CHFs is that during the photocatalytic reaction,the generated electrons accumulate on the heptazine ring due to the electron-withdrawing properties,while the generated holes accumulate on the acetylene or diacetylene groups due to the hole-absorbing properties,thus realizing the effective spatial separation of the photoredox centers,as shown in Fig.5b.In the excited state,the electron density around the heptazine ring increased significantly,indicating that the photogenerated electrons were stabilized and attracted to the ring.In addition,by comparing the behavior of the C atoms in the heptazine ring moieties with that in the acetylene or diacetylene monomers,the Gibbs free energy change (ΔG) of the C atoms in the heptazine ring moieties was found to be favorable to the formation of the *H and *OO* intermediates.On the other hand,the ΔGof the C atoms in the acetylene or diacetylene monomers was found to be more beneficial to the formation of OH*.These findings suggest that the heptazine ring monomers may serve as reduction centers to drive oxygen reduction,while the acetylene or diacetylene monomers act as oxidation centers to drive water oxidation (Fig.5c,d).Benefiting from these phenomena,the CHFs achieved an H2O2production rate of 69 μmol·h-1with a measured SCC efficiency of 0.78% under visible-light irradiation.This work represents a significant step toward the rational design of semiconducting polymer photocatalysts at the molecular level enabling spatially separated photoredox centers,which is of crucial importance in the development of highly efficient photocatalysts.
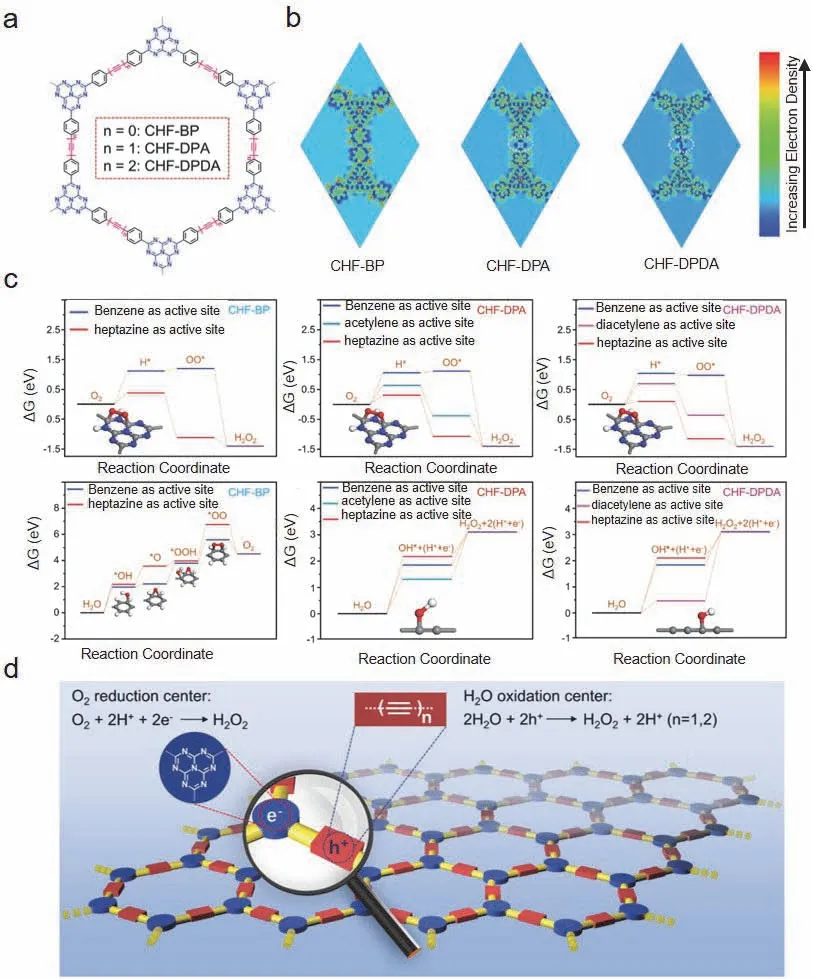
Fig.5 (a) Synthetic scheme of CHFs.(b) Calculated electron distributions in CHFs under photoexcitation.(c) Calculated free energy diagrams of oxygen reduction and water oxidation pathways toward H2O2 production on different active sites in CHF-BP.(d) Schematic illustration of photocatalytic reaction pathways toward H2O2 production on the spatially separated O2 reduction and water oxidation centers under visible-light irradiation.Adapted from Ref.32.Copyright 2021,Wiley-VCH.
Furthermore,covalent triazine framework (CTFs) constructedviatriazine ring polymerization similarly provide a model for the rational design and construction of polymer frameworks to achieve spatially separated photoredox centers.Grafting thiourea functional groups onto CTFs (named Bpt-CTF; see Fig.6a) was proved to be an efficient strategy51.As shown in Fig.6b,the polar thiourea moieties introduced into the CTF exhibited a longer charge carrier lifetime,namely 5.05 and 1.09 ns for Bpt-CTF and unfunctionalized CTF (Dc-CTF),respectively,indicating that the thiourea moieties can considerably enhance charge separation.Additionally,as shown in Fig.6c,Bpt-CTF exhibits a significantly increased attenuation time,indicating that the addition of the thiourea monomer can effectively trap the photogenerated electrons,thus inhibiting the recombination of the photogenerated carriers,accelerating their migration,and realizing the separation of the photoredox centers.As a result,the yield of the photocatalytic production of H2O2for Bpt-CTF was found to be an order of magnitude higher than that for Dc-CTF.Mechanistic studies reported that the introduction of the thiourea monomer generated a strong dipole moment due to the polarization between the thiourea and the triazine ring monomers,leading to the formation of an in-built electric field.This field enhanced the electron trapping ability of the C2 atoms of Bpt-CTF and promoted the separation and migration of the photogenerated electrons and holes (Fig.6d,e).More importantly,due to the electron-withdrawing capability,the C2 atoms could act as electron acceptors and thus serve as reduction centers,leading to the accumulation of the photogenerated holes at the thiourea sites.Thus,the thiourea could act as oxidation centers for the photocatalytic production of H2O2,and the redox centers were well spatially separated.
Similarly,a new COF was developed and denoted as TA-Porsp2-COF (Fig.6f); this COF was constructedviathe polymerization between the porphyrin moieties and the triazine ring.It was proved that the porphyrin moieties act as electron donors,while the triazine moieties act as electron acceptors20,53.Therefore,in the process of the photocatalytic production of H2O2,a pair of spatially separated redox centers were obtained,with the triazine moieties serving as reduction centers and the porphyrin moieties as oxidation centers.The spatial separation of the photoredox centers resulted in the generated carriers being able to move along specific pathways,leading to a high separation efficiency.Thus,TA-Por-sp2-COF showed a significant enhancement in the activity of the photocatalytic production of H2O2(Fig.6g,h).
In summary,the key aspect for achieving spatial separation of the redox centers is to engineer electron donors and acceptors on different moieties of a single catalyst.The spatially separated redox centers can promote the separation of the photogenerated carriers,inhibit their recombination,and increase their utilization probability.More importantly,if the donor-acceptor moieties could act as both carrier separation sites and catalytic redox sites for activation of the absorbed reagents (e.g.,H2O and O2),the efficiency of the solar energy conversion for the synthesis of H2O2would be remarkably improved.The carrier separation,absorption of the reagents (e.g.,H2O and O2),and carrier transfer processes should be carefully explored through the use of advanced time- and space-resolved techniques.
3 Synergistic utilization of the photoexcited redox centers
After spatially separating the photoexcited redox centers,their synergistic utilization for synthesizing H2O2should be addressed.For ideal photocatalysts,all electrons and holes in the excited states can participate in surface chemical reactions.However,only the electrons and holes that migrate to the surface of the redox centers can be actually utilized; thus,the utilization of the photocarriers is the limiting factor.Furthermore,most studies have only focused on one side of the photoexcited redox centers for photocatalytic reactions,which results in an inadequate utilization of the solar energy.The unused electrons or holes could be consumed by the catalysts themselves due to the charge balance,resulting in a reduction in the activity of the catalysts or even their inactivation,which is not beneficial to the stability of the photocatalyst.However,the sacrificial agent can take the role of the photocatalysts in consuming the unused electrons or holes,thereby improving the stability of the photocatalyst but increasing the resource waste and environmental pollution.The synergistic utilization of the photoexcited redox centers has been proved to be an effective strategy to improve the solar energy utilization rate and avoid the waste of the sacrificial agent.Interestingly,H2O2can be synthesizedviathe ORR or WOR half reaction,involving diverse reaction pathways and overpotentials (Fig.2),thereby making the H2O2synthesisviathe synergistic utilization of the photoexcited redox a reality.Based on this,the polymer-based photocatalysis can be classified into: (i) Type I,i.e.,ORR combined with the oxidative production of chemicals; (ii) Type II,i.e.,WOR combined with the reductive production of chemicals; (iii) Type III,i.e.,ORR combined with the WOR.Accordingly,considerable work has been devoted to the preparation of H2O2for the synergistic utilization of the photoexcited redox centers over nearly a decade.
3.1 ORR combined with the oxidative production of chemicals
Generally,the disadvantages of most photocatalysts are a low selectivity and a high reaction potential of the WOR,which lead to a low activity in the photocatalytic production of H2O2.Therefore,sacrificial agents have been often used to consume the photoexcited holes and provide protons for H2O2production60–63.Although the activity of the photocatalytic production of H2O2has clearly increased (Table 2),vast amounts of resources and energy are wasted,and the process is not ecofriendly.Accordingly,it is urgent to find a low-cost method that can achieve a high activity for the photocatalytic production of H2O264–66; for example,replacing the oxidation of sacrificial agents with the oxidative production of value-added chemicals(Fig.7a).

Table 2 Summary of the reported high-efficiency photocatalysts for the photosynthesis of H2O2 with and without sacrificial agent.

Fig.7 (a) Schematic of the ORR combined with chemical production.(b) Digital photographs of MIL-125-NH2 (left) and MIL-125-R7 (right) dispersed in the two-phase reaction system.(c) Photographs of water droplets on tablets of MIL-125-Rn.(d) Time courses of the H2O2 production under photoirradiation.(e) Reaction mechanism of the H2O2 production in the two-phase system.Adapted from Ref.68.Copyright 2019,Wiley-VCH.(f) Reaction mechanism of the H2O2 production as well as the benzylamine and thioanisole selective oxidation.(g) Time courses of the H2O2 production as well as the benzylamine and thioanisole selective oxidation under photoirradiation.Adapted with permission from Ref.53.Copyright 2022,American Chemical Society.
Harnessing biomass conversion can enable the production of fuels and high-value chemicals with a low-carbon footprint;thus,the biomass conversion reaction has become a research hotspot with the aim of attaining future carbon neutrality67.On this basis,ORR combined with the oxidization of biomass to synthesize H2O2and high-value chemicals is an important topic.A photoactive MOF,namely MIL-125-Rn,was developed and used in a benzyl alcohol/water two-phase system.Benzaldehyde and H2O2were obtained from the redox centers,realizing the synergistic utilization of the photoredox centers68.As shown in Fig.7b,in this system,MIL-125-NH2was dissolved in an aqueous phase,while MIL-125-Rn was dissolved in a benzyl alcohol phase due to its superior hydrophobic nature (Fig.7c).Interestingly,MIL-125-Rn exhibited higher photoactivity in the H2O2production (Fig.7d).As shown in Fig.7e,in the benzyl alcohol phase,the benzyl alcohol was oxidized to benzaldehyde by the photoexcited oxidation centers,while O2was reduced to O2•-,which moved to the aqueous phase to form H2O2.Thus,this work was able to achieve the synergistic utilization of the photoexcited redox centers and avoided the waste of the sacrificial agent.Furthermore,insituseparation of H2O2was achieved,which provided a reference for the utilization of photocatalytically synthesized H2O2.In addition,a new COF denoted as TA-Por-sp2-COF was developed through the copolymerization of the porphyrin moieties and the triazine rings(Fig.7f).The triazine moieties acted as reduction centers to reduce O2to H2O2,while the porphyrin moieties acted as oxidation centers to drive the aerobic coupling reaction of benzylamine and the thioanisole selective oxidation,thereby achieving the synergistic utilization of the photoexcited redox centers (Fig.7g).
The synergistic coupling of the redox reactions can facilitate the production of high-value-added chemicals (Fig.8a).For example,formate was synthesized by combining the photocatalytic redox reaction with a binary Pdots photocatalyst69.The Pdots photocatalyst consists of PFBT (as electron donor)and PCBM (as electron acceptor).As shown in Fig.8b,the photoexcited reduction center (PCBM) drives the photocatalytic formation of H2O2,while the photoexcited oxidation center(PFBT) drives the formation of formate with the assistance of the disproportionation reaction of H2O2.This work was able to achieve the synergistic utilization of the photoexcited redox centers and avoid the waste of the sacrificial agent.Importantly,it demonstrated a new strategy for theinsituutilization of photocatalytically synthesized H2O2.
In summary,the ORR combined with the oxidative production of chemicals is an efficient approach to achieve the synergistic utilization of the photoredox centers since not only the high yield of the photocatalytic synthesis of H2O2can be guaranteed,but also another high-value chemical can be obtained simultaneously.However,limited by the oxidative capacity of the photoexcited oxidation centers,only a few reactions for the production of high-value chemicals were developed.More efforts should be devoted to developing new reactions.Furthermore,the active species produced by the coupling of the oxidation centers with the H2O2generated by the reduction centers to synthesize high-value chemicals should provide a promising direction for the synergistic utilization of the photoredox centers.This is due to theinsituutilization of H2O2,which permits skipping the complicated purification and enrichment processes of H2O2.
3.2 WOR combined with the reductive production of chemicals
Generally,the synthesis of H2O2dominated by the reduction centers is attributed to the advantages of the ORR photocatalytic thermodynamics.A question worth asking is whether it is possible to synthesize H2O2viathe oxidation centers.Generally,the WOR is much more difficult than the ORR due to its high reaction potential,and the WOR is also more demanding on the catalysts.Nevertheless,the photocatalytic water splitting makes it a real possibility,and the reaction of water splitting into H2and O2(i.e.,2H2O → O2↑ + 2H2↑) has drawn significant attention70.However,so far,insufficient attention has been paid to the H2O2and H2pathway (i.e.,2H2O → H2O2+ H2↑)71.In such a pathway,the photoexcited oxidation centers oxidize water to H2O2and hydrogen species,while the photoexcited reduction centers reduce the hydrogen species to H2(Fig.9a).Two highvalue chemicals were synthesized simultaneously for the synergistic utilization of the photoexcited redox centers.

Fig.9 (a) Schematic of the WOR combined with the chemical production.(b) Diagram of the photoelectrode system for the production and recovery of H2O2 and H2 using a WO3/BiVO4 photoanode under solar-light irradiation.Ref.72,adapted from Chemical Communications.Copyright 2016,The Royal Society of Chemistry.(c) Transmission electron microscopy (TEM) image of the PCN-10-CP-10 photocatalyst.(d) Evolution of the H2 and H2O2 amounts after a 5-h photocatalytic pure water-splitting reaction determined by the RDE current.(e) Reaction mechanism of the H2 and H2O2 production with a detailed presentation of the electron transfer via the formed P-P transmission channel.Ref.73,adapted from Nano Energy.Copyright 2022,Elsevier Ltd.
In order to drive the H2O2and H2reaction pathway toward water splitting,a photoelectric cell was used.As shown in Fig.9b,WO3/BiVO4acted as the photoanode,while Pt acted as the cathode72.In this system,the photoanode served as the oxidation center to oxidize water to H2O2,while the photocathode served as the reduction center to reduce water to H2.Due to the heterojunction formed by the photoanode,photogenerated carriers are produced under irradiation,leading to a lower applied voltage than the theoretical value.As a result,the yield of the H2O2and H2production was significantly enhanced.This work was able to realize the H2O2and H2pathway for water splitting,which is of great significance for energy development.
However,the H2O2and H2pathway for water splitting remains challenging in a photocatalytic system.Thus,the coincorporation of red phosphorus and CoxP into the surface of g-C3N4was proposed as a novel heterojunction to split waterviathe H2O2and H2pathway,and the synergistic utilization of the photoredox centers was realized,as shown in Fig.9c73.It was found that the introduction of red phosphorus formed a red phosphorus bridge between the CoxP unit and the PCN framework,providing a pathway for the migration of the photocarriers.It was proved that the CoxP unit and PCN served as the reduction center and the oxidation center in the reaction,respectively,thus enabling the rapid vector migration of the photoactive electrons from the oxidation center to the reduction center.As a result,the electrons could rapidly migrate to the CoxP cocatalysts through the P–P bridges,and then CoxP served as the reduction center to drive the H2evolution with the absorbed protons on the active sites.Additionally,the holes on the valence band (VB) of PCN were utilized for 2e-water oxidization for H2O2production (Fig.9d,e).This work reported the fabrication of a new heterojunction,which provided new ideas for the design of materials,valuable experience for the utilization of the photoredox centers,and increased understanding of the heterojunctions.
Another strategy for g-C3N4-based photocatalytic systems used for water splittingviathe H2O2and H2pathway is the construction of an inorganic-biological hybrid photocatalytic system74.Chlorella and carbon particles were connected to the surface of g-C3N4to construct C-N-g-C3N4,which exhibited a superior performance in water splittingviathe H2O2and H2pathway,as shown in Fig.10a.According to the experiments(Fig.10b),the needle coke served as the photoexcited reduction center for H2evolution,while the living Chlorella vulgaris combined with g-C3N4played a key role as the photoexcited oxidation center for H2O2production (Fig.10c).This work was able to obtain the H2O2and H2pathway for water splitting in a photocatalytic system and provided a new strategy for catalyst modification.
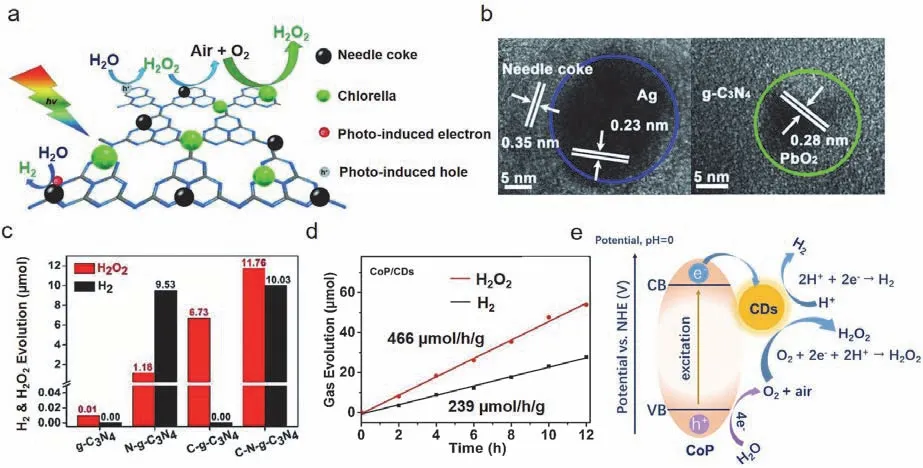
Fig.10 (a) Proposed schematic mechanism of the H2 and H2O2 evolution in the hybrid system.(b) Reduction sites and oxidation sites with C-N-g-C3N4 (the blue and green circles represent Ag and PbO2 particles,respectively).(c) Comparison of the H2O2 and H2 evolutions under different atmospheres over 12 h with C-N-g-C3N4.Adapted from Ref.74.Copyright 2018,WILEY-VCH.(d) H2 and H2O2 evolutions from photocatalytic water splitting with the CoP/CD composite (CD: 8.3%,wt) as the photocatalyst.(e) Photocatalytic mechanism for the overall water splitting with the CoP/CDs photocatalyst via the 4e--2e- cascaded pathway.Ref.75,adapted from Applied Catalysis B: Environmental.Copyright 2022,Elsevier.
In addition,the introduction of CDs into CoP was also found to result in water splittingviathe H2O2and H2pathway with excellent H2and H2O2yield rates of 239 and 466 μmol·h-1·g-1,respectively,as shown in Fig.10d75.CoP served as the photoexcited oxidation center to oxidize H2O to O2and the CDs then acted as photoexcited reduction centers to drive the H2and H2O2evolution; H2O2was evolved from the reaction between O2and the hydrogen species generated by the photoexcited oxidation centers (Fig.10e).This work offered a new route regarding the H2O2and H2pathway for water splittingviathe synergistic utilization of the photoexcited redox centers.
Recently,a notable coupling of the WOR with the CO2reduction reaction (CO2RR) was achieved.A large number of mesopores were formed in MOFs,which provided not only active sites and gas mass transfer channels for the photocatalytic reaction but also space for the redox centers.The tungsten oxides and their hydrates were successfully introduced into the mesopores of MOF MIL-100-Fe76.As shown in Fig.11a,the tungsten oxides acted as the photoexcited oxidation centers to oxidize water to H2O2and hydrogen species,while the MOF moieties acted as photoexcited reduction centers to convert the CO2and hydrogen species into CO and CH4.It exhibited a superior performance in both CO2reduction and H2O2production,realizing the WOR combined with the CO2RR (Fig.11b).This work opened a route for the WOR combined with the CO2RR,broadening the application range of the synergistic utilization of the photocatalytic redox centers.

Fig.11 (a) Illustration of the steps involved in the photocatalytic process in molecular compartments.(b) Production rates of CO and CH4 for WO3·H2O-in-MIL-100-Fe with different WO3·H2O contents and 24%-WO3-in-MIL-100-Fe.Adapted with permission from Ref.76.Copyright 2022,American Chemical Society.
In summary,WOR combined with the reductive production of chemicals is also an efficient way to achieve the synergistic utilization of the redox centers.Although several models have been proposed,more efforts should be devoted to innovating the cooperative systems.Furthermore,for the WOR combined with the hydrogen evolution reaction (HER),theinsituharnessing of the hydrogen species to obtain more value-added chemicals instead of H2is a promising strategy.
3.3 ORR combined with the WOR
The most potentially viable route for achieving a carbonneutral future is ORR combined with the WOR to synthesize H2O2,enabling the direct production of H2O2from H2O and O2without using any sacrificial agent.This approach improves the solar energy utilization rate and avoids the waste of sacrificial agents.In theory,the maximum efficiency of the photocatalytic production of H2O2should be obtained.For the ORR,improving the 2e-ORR selectivity can increase the H2O2yield.Thus,many efforts have been devoted to this field12,and the most effective way is to adjust the absorption model of O2by regulating the moiety structure or electronic structure of the active sites since Pauling-type O2adsorption is more favorable than Yeager-type and Griffiths-type to the 2e-ORR43; the related works were summarized by previous reviews1–3,10,11.Notably,the WOR is the key factor for this reaction mode,since the 2e-ORR possesses a lower reaction potential (+0.68 Vvs.NHE) than the 2e-WOR (+1.76 Vvs.NHE) and the 4e-WOR (+1.23 Vvs.NHE),indicating that the 2e-ORR process can occur more easily (Fig.2).Consequently,the WOR pathway requires a higher reaction energy than the ORR.Additionally,it can proceedviaeither the 2e-or the 4e-WOR pathway,which increases the challenges of the photocatalytic oxidation centers.From a thermodynamic perspective,the reaction potential of the 4e-WOR pathway to produce O2is lower than that of the 2e-WOR pathway to produce H2O2,which has led to an increased number of investigations on oxidation centers for the 4e-WOR pathway (Fig.12a).

Fig.12 (a) Schematic diagram of the ORR combined with the 4e- WOR.(b) Schematic illustration of Nv―C≡N―CN.(c) Comparison of the photocatalytic H2O2 generation activity of different samples from pure water.(d) Time course of photocatalytic O2 evolution measured over PCN and Nv―C≡N―CN.Ref.63,adapted from Energy & Environmental Science.Copyright 2022,The Royal Society of Chemistry.(e) Mechanism for photocatalytic H2O2 production.(f) Photocatalytic H2O2 generation activity and SCC efficiency.Adapted with permission from Ref.64.Copyright 2016,American Chemical Society.(g) Photocatalytic properties of mesoporous polymer spheres with different mesopore sizes.(h) Schematic illustration of mesoporous phenolic resin for high efficiency photocatalytic production of H2O2.Adapted from Ref.35.Copyright 2021,Wiley-VCH.
Defect engineering has been widely used in systems for the photocatalytic synthesis of H2O2since the multiple defect sites can act as photoexcited redox centers12,63.The cyanogen group and nitrogen vacancies were successfully introduced into the framework of g-C3N4(Nv―C≡N―CN)viadefect engineering(Fig.12b).Both experiments and DFT calculations proved that the cyanogen group acted as the oxidation center,driving the 4eoxidation of water to generate oxygen,while the N vacancies acted as the reduction center,driving the reduction of oxygen to H2O2.Thus,Nv―C≡N―CN displayed a superior performance in the photocatalytic production of H2O2(Fig.12c).Furthermore,it displayed a superior photocatalytic production of O2(Fig.12d),suggesting a trend of the 4e-WOR pathway.Furthermore,the introduction of pyromellitic diimide (PDI) into the frameworks of g-C3N4also facilitated the 4e-oxidation of water,leading to an enhancement of the photocatalytic production of H2O2.As shown in Fig.12e,the photogenerated electrons transferred to graphene from the PDI moieties,leading to an enhancement of the charge separation.Additionally,the holes in the PDI moieties were engaged in oxidizing waterviathe 4e-WOR pathway,and as a result,this system exhibited an SCC efficiency of 0.20% (Fig.12f)64.Surprisingly,pore size and structure engineering can also be used to regulate the photocatalytic WOR ability35.As shown in Fig.12g,constructing mesopores on the polymer spheres and regulating the pore size were proved to promote the 4e-oxidation of water.Notably,the rate of the H2O2evolution was determined by the pore size and structure since the mesopores with a suitable pore size provided reaction active sites for the 4e-water oxidation,and the distribution of the photoredox centers was adjusted accordingly to enable their spatial separation,which accelerated charge migration (Fig.12h).Thus,the monodispersed mesoporous structure offered an excellent platform for the photocatalytic generation of H2O2,endowing the catalyst with a high SCC (1.1%) for H2O2production.This represents a new step for photocatalyst regulation in the photocatalytic production of H2O2.
The quantum yield in green plants is near unity due to the redox species (plastoquinones and NADP),which is crucial for minimizing hole-electron recombination and back reactions31.The AQ moieties acting as electron acceptors were combined with the tetraphenylethylene (TPE) moieties,which acted as electron donors to mimic the NADP-mediated photosynthetic processes in green plants,as shown in Fig.13a.Similar to the natural photosynthesis,due to the electron withdrawing and storage properties of the AQ moieties,H2O2was still generated even without illumination,indicating that the artificial photosynthesis of H2O2through TPE-AQ involves light and dark processes (Fig.13b).Accordingly,the WOR occurredvia4e-to generate O2,similar to the photosynthesis in green plants.As a result,TPE-AQ exhibited an H2O2production rate of 909 μmol·h-1·g-1and an SCC efficiency of 0.26% under ambient conditions (Fig.13c).
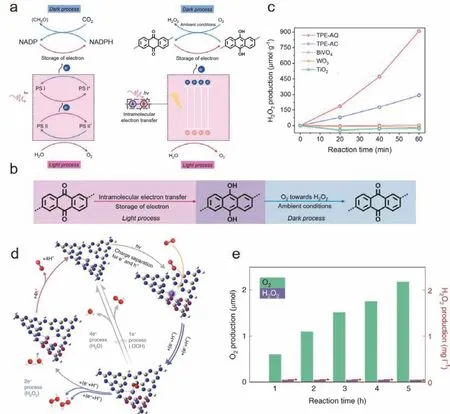
Fig.13 (a) Mechanism of the photosynthesis in green plants and the TPE-AQ system.(b) Photocatalytic pathway for the H2O2 production via TPE-AQ.(c) Photocatalytic performance of the frequently studied photocatalysts for H2O2 production under ambient conditions.Adapted from Ref.35.Copyright 2021,PNAS.(d) Mechanism of the photocatalytic production of H2O2.(e) Amounts of O2 and H2O2 produced with Sb-SAPC15 in a 0.1 mol·L-1NaIO3 solution (acting as the electron acceptor).Adapted from Ref.43.Copyright 2021,Springer Nature.
Notably,as mentioned above,in the photocatalytic generation of H2O2,the oxidation centers produce O2viathe 4e-WOR.It would then be necessary to know where the O2goes and whether it is possible to use itinsitufor the 2e-ORR to produce H2O2.Herein,we provide an answer to this issue.A photocatalyst with atomically dispersed Sb sites was designed for the artificial photosynthesis of H2O2in a simple water and oxygen mixture under visible-light irradiation (Fig.13d)43.The single Sb sites accumulated electrons for the selective 2e-ORR and induced a high concentration of holes at the neighboring melem units,thus accelerating the 4e-WOR.In such a system,a considerable amount of O2was detected after capturing the photoelectronsviaNaIO3,which confirms the 4e-WOR mechanism of this system.Furthermore,when H216O and18O2were used as the raw materials to produce H2O2,which was subsequently decomposed by Fe3+to release O2,the produced oxygen was found to contain both16O2and18O2using isotopic tracking methods,indicating that the oxygen generated by the system participated in the ORR process and improved the atomic utilization rate and the solar energy conversion efficiency.Benefiting from this simultaneous utilization of the photoexcited redox centers,the catalyst displayed an AQE of 17.6% at 420 nm for H2O2synthesis (Fig.13e).This work developed an organic-polymer-based singleatom catalyst in the photocatalytic synthesis of H2O2.Similarly,regarding the grafting of thiourea functional groups onto CTFs,it was proved that the generated oxygen participated in the ORR process and improved the atomic utilization rate and the solar energy conversion efficiency51.
Although the 4e-WOR pathway to produce O2is beneficial for making the photoexcited redox centers cooperate to synthesize H2O2,combining the 2e-WOR pathway with the 2e-ORR (Fig.14a) to directly produce H2O2is more efficient,as shown in Table 2.For example,very recently,Lan’s group realized the coupling of the 2e-ORR with the 2e-WOR by constructing an oxidation-reduction molecular junction77.In this oxidation-reduction molecular junction,the 2e-WOR and the 2e-ORR occurred at the oxidation-reduction centers respectively,leading to an effective electron-hole separation efficiency and photoredox sites that enable a full reaction for the generation of H2O2.From a theoretical point of view,the highest efficiency of the photocatalytic production of H2O2can be expected in the mode where the ORR is combined with the 2e-WOR due to the increased atomic utilization and solar energy conversion efficiency.However,due to its high reaction potential,the 2e-WOR pathway often leads to a low selectivity and activity,which greatly reduce the efficiency of the photocatalytic synthesis of H2O2; thus,it is crucial to overcome its reaction potential and improve its selectivity and activity.Actually,the WOR pathway can be regulated by adjusting the modules in covalent polymer frameworks.Acetylene or diacetylene was introduced into the polymeric triazine ring framework,leading to a 2e-WOR pathway for the photocatalytic production of H2O238.As can be seen in Fig.14b,in an anaerobic environment,when the photoelectrons of the system were captured by NaIO3,O2could be detected in the CTF-BPDCN system but not in the CTF-EDDBN (with acetylene moieties) nor CTF-BDDBN (with diacetylene moieties) system,indicating that the CTF-BPDCN system favors the 4e-WOR pathway.By contrast,an amount of H2O2was detected in the CTF-EDDBN and CTF-BDDBN systems but not in the CTF-BPDCN system,indicating that the CTF-EDDBN and CTF-BDDBN systems favor the 2e-WOR pathway.Furthermore,DFT calculations indicated that the acetylene and diacetylene groups could reduce the OH* formation energy and favor the 2e-water oxidation to directly generate H2O2.As a result,the 2e-WOR pathway resulted in a higher activity for the photocatalytic production of H2O2(Fig.14c).
However,a new challenge emerges with the realization of the 2e-WOR in the photocatalytic production of H2O2,since the 2e-WOR involves two pathways,namely the 2e-one-step process(2H2O + 2h+→ H2O2+ 2H+) and the 2e-two-step process (H2O +h+→ •OH + H+,•OH + •OH → H2O2),as shown in Fig.14d.Thus,it is important to understand which process is most beneficial to the photocatalytic production of H2O2and how the selectivity of the two processes can be regulated.Based on this challenge,a bipyridine-based COF photocatalyst (COF-TfpBpy)was established,and the corresponding amorphous polymer(AP-TfpBpy) was prepared for comparison66.It was found that the bipyridine monomer enhanced the Yeager-type oxygen adsorption,which resulted in the acceleration of the 2e-one-step WOR process,while the corresponding amorphous polymer without the bipyridine monomer favored to the 2e-two-step WOR process.Furthermore,the system with the 2e-one-step WOR process exhibited a higher activity for the photocatalytic production of H2O2and a higher SCC than the 2e-two-step WOR process (Fig.14e),suggesting that the 2e-one-step WOR process is more beneficial to the photocatalytic production of H2O2(Fig.14f).This work realized the regulation of the selectivity of the 2e-one-step WOR process,which is important for maximizing the atom utilization efficiency of the photocatalysts.45Nevertheless,the redox potential of the 2eone-step WOR is rather positive (1.76 Vvs.NHE),leading to a self-oxidation of the surface of the catalyst and the decomposition of H2O2; thus,the stability of the photocatalyst should be considered further.Theinsituutilization is an effective strategy to avoid the decomposition of H2O2,as will be discussed in detail in the next section.
In summary,the ORR combined with the WOR process is the most efficient strategy to achieve the synergistic utilization of the redox centers for the photocatalytic production of H2O2.For the system in which the ORR is combined with the 2e-WOR,a maximum efficiency of the photocatalytic production of H2O2can be expected.The stability of the produced H2O2with highly oxidative holes should be considered.Furthermore,the purification,enrichment,and application of the H2O2produced by photocatalysis also need to be considered.
4 Efficient photocatalytic application of H2O2
The ultimate goal for the photocatalytic production of H2O2is its use in practical applications; however,the low yield of H2O2synthesized by photocatalysis restricts considerably the development of its applications.In this regard,purification,enrichment,andinsituutilization (i.e.,the Fenton reaction) are continuously developed.
Purification and enrichment are the most direct means to increase the concentration of H2O2to practical application levels.Notably,the sluggish O2diffusion and low solubility in water also limit the H2O2production in photocatalytic systems.Thus,the realization of a gas-liquid-solid interface with an excellent mass transfer is an effective approach for overcoming this problem27.This is because at the triphasic interface,O2remains a gas instead of dissolving in water,leading to a high O2concentration and diffusion rate of the photocatalysts78.Additionally,the increased O2mass transfer can facilitate the separation of the photogenerated carriers since oxygen can capture photoelectrons79.Inspired by this idea,Zhang and our group developed an efficient triphasic system,which exhibited an extremely high H2O2production efficiency and an outstanding water disinfection performance80.As shown in Fig.15a,Au/TiO2NPs were developed to construct a triphasic system,which exhibited a superior activity in the photocatalytic production of H2O2(Fig.15c).More importantly,a fluid triphasic system was developed based on this triphasic system for the enrichment of H2O2(Fig.15b),which was already demonstrated to achieve more than a 100-fold enrichment of the H2O2synthesized by photocatalysis.The enriched H2O2exhibited a high bacterial killing rate (Fig.15d).

Fig.15 (a) Structural characterization of Au/TiO2 on a porous hydrophobic carbon substrate.(b) Schematic of the photosynthesis-concentration tandem system for H2O2 production.(c) Time-dependent H2O2 yield for Au/TiO2 NPs under UV irradiation.The insets show the schematic of the diphase (bottom) and triphase (top) photocatalytic systems.(d) Photographs of overnight-cultured plates with spread droplets taken in different time slots during H2O2 (10 mmol·L-1) oxidation.Adapted with permission from Ref.80.Copyright 2020,Elsevier.
Insituutilization is an efficient strategy to improve the application efficiency of H2O2synthesized by photocatalysis since it skips the complicated purification and enrichment processes.Carbon nitride nanorod were employed to produce H2O2,which was usedinsituto photocatalytically degrade tetracycline under visible-light irradiation81.Furthermore,thein situutilization of the photocatalytically generated H2O2for methane conversion is also a superior strategy,which can promote the utilization efficiency of H2O2up to 93.3%.
Generally,the most efficientinsituutilization of H2O2is provided by the Fenton oxidation method due to the strong oxidation characteristics of •OH.Based on this,Cu atoms were atomically dispersed in PCN (Fig.16a,b) and used to degrade organic contaminants in wastewater82.The Cu single-atom catalysts could catalytically activate H2O2to generate •OH under visible-light irradiation without any other energy input.As a result,they displayed a superior degradation capacity for organic pollutants (Fig.16c).Based on this,an electrolysis reactor for producing H2O2was combined with this catalyst,forming a sustainable wastewater treatment system (Fig.16d) with a low cost (Fig.16e).Additionally,the photocatalytic Fenton reaction has also been successfully implemented in ballast water sterilization83,hypoxic tumor therapy84,photo-oxidation of methane to methanol85,etc.,and a superior efficiency was achieved.
5 Conclusion and outlook
The photocatalytic clean production of H2O2from earthabundant resources (H2O,O2,CO2,etc.) offers a promising pathway to convert solar energy into value-added chemicals.Semiconducting polymer-based photocatalysts,which exhibit molecular-level flexibility,a tunable bandgap,and numerous different active moieties have recently emerged as attractive candidates for the highly efficient photocatalytic production of H2O2.An efficient spatial separation of the photoredox centers accompanied by their rational full utilization would ensure a high SCC efficiency.The spatially separated redox centers are beneficial to the migration of photogenerated carriers and act as sites for the absorption-desorption of H2O and O2,which avoids the energy loss of the carrier migration to the absorptiondesorption sites,resulting in a high charge carrier utilization and a high H2O2production activity.Herein,we overviewed the recent advances on the photocatalytic production of H2O2from semiconducting polymersviathe ORR or/and WOR;specifically,the systems that avoid the use of sacrificial reagents.The design principles in promoting spatial separation,the advanced models of the synergistic utilization of the photoredox centers,the strategies to incorporate high-reactive organic moieties,and the origin of their catalytic behavior and mechanism were discussed in detail.
Regarding the challenges,the artificial photosynthesis of H2O2is still far from the possibility of being used in practical applications due to the low H2O2yield (generally in the range from a few to hundreds of µmol/h without sacrificial agents)3,10,11which in turn is caused by the photodecomposition of H2O2,and there is a large gap between the H2O2yield of photocatalysis and the electrocatalysis as well as the heterogeneous catalysis (generally in the range from a few to dozens of mmol·h-1)6.As a result,employing a photoelectric cell might be an effective solution72,86.In this case,the autoseparation of the redox centers can be easily achieved,as oxidations and reductions occur at different photoelectrodes.Nevertheless,the relatively poor conductivity of semiconducting polymers restricts their efficiency.Constructing micro-nanoscale photocells on semiconducting polymers might be desirable,whereas the redox centers could be linked with specifically designed hole or electron transfer units,thus facilitating the spatial separation of the photoredox centers.Secondly,the main models of the synergistic utilization of the photoredox centers for the clean production of H2O2include the ORR combined with the oxidation of organics,the WOR combined with the HER,the WOR combined with the CO2RR,and the WOR combined with the ORR.The development of new systems is highly desired,for instance,the WOR combined with the reduction of organics,the 1e-WOR for producing highly reactive hydroxyl radicals combined with the selective ORR,the ORR combined with new types of oxidations of organics (i.e.,C―H activation)etc.87.Importantly,in these aforementioned multi-models,a wide range of active species could be rationally designed and generated.Theinsituharnessing of these active species toward different applications,such as high-value-added chemicals production and water purification,could represent promising research directions.Next,regarding the H2O2production,the ORR combined with the WOR,especially the 2e-WOR,is the most ideal approach to achieve a superior efficiency.In this respect,the stability of the photocatalysts as well as the produced H2O2should be considered as it is extremely important for industrial applications.To provide valuable information for understanding the reaction mechanism,more efforts are thus necessary to study the reaction pathways,kinetics and thermodynamics,and micro-nano-scale identification of the active domains.Additionally,isotopic tracking experiments should also be conducted.Lastly,to further improve the efficiency,other fields,such as the electric and thermal fields,should be considered for the H2O2production.AI chemists might also be helpful88.We believe that with continuous research inputs,the artificial photosynthesis of H2O2from clean sources and solar energy will definitely contribute to achieving a carbon-neutral future.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
- Holey Graphene for Sodium-Ion Battery Anode Material
- Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution

