Interstitial Carbon in Ni Enables High-Efficiency Hydrogenation of 1,3-Butadiene
Shaoming Dong ,Yinghui Pu ,Yiming Niu ,Lei Zhang ,Yongzhao Wang ,2,Bingsen Zhang
1 Shenyang National Laboratory for Materials Science,Institute of Metal Research,Chinese Academy of Sciences,Shenyang 110016,China.
2 School of Materials Science and Engineering,University of Science and Technology of China,Shenyang 110016,China.
Abstract: Selective hydrogenation is an essential catalytic reaction in modern industrial chemistry.For instance,butene can be used to produce many important organic chemical products,but the catalytic cracking of naphtha to produce olefins also produces some diolefins,which contain approximately 0.2%–2.0% 1,3-butadiene.The selective hydrogenation of 1,3-butadiene is a crucial step in purifying single olefins and prevents poisoning of the catalysts used in polymerization.Currently,the most common industrially employed catalysts in the reaction are palladium-based catalysts,but drawbacks associated with these include high cost and low abundance.Transition metal Ni-based catalysts have the advantages of being low cost and having high hydrogenation activity,but they are prone to excessive hydrogenation in butadiene hydrogenation reactions.This leads to reduced selectivity and the loss of monoolefins in the feed gas.In addition,Ni-based catalysts tend to accumulate carbon on the surface,which results in catalyst deactivation.Therefore,designing Ni-based catalysts with excellent catalytic performance has been an industrial research priority.Herein,we synthesized Ni3Zn/Al2O3 catalysts by impregnation and achieved the alumina-supported Ni3ZnC0.7 structure by acetylene atmosphere treatment.Interstitial sites of the Ni3Zn intermetallic catalyst were modified by introducing interstitial carbon atoms.This enhances the catalytic performance of the 1,3-butadiene hydrogenation reaction.X-ray diffraction and transmission electron microscopy revealed that the catalyst presents a typical Ni3ZnC0.7 phase.The interstitial carbon structure can suppress excessive hydrogenation,exhibiting up to 93% butene selectivity at a 98% conversion of 1,3-butadiene,which renders it superior to the Ni3Zn/Al2O3 catalyst.More importantly,the selectivity to 1-butene is improved by approximately 40% compared to the Ni3Zn/Al2O3 intermetallic catalyst.In addition,the Ni3ZnC0.7/Al2O3 catalyst exhibits superior and stable selectivity within a wide H2/1,3-butadiene ratio range and can operate reliably under fluctuating conditions.CO-diffuse reflectance infrared Fourier transformed spectroscopy (CO-DRIFTS) demonstrated that coordinating the carbon atom in the interstitial site with the neighboring Ni atoms alters the electron structure of the Ni sites in the Ni3ZnC0.7 structure.The electrons at the surface Ni sites are transferred to the carbon atoms at the interstitial sites rendering Ni more electron-deficient and decreasing the adsorption strength of 1-butene,which inhibits the excessive hydrogenation reaction pathway.It is also noteworthy that the interstitial carbon structure can inhibit carbonaceous species formation and accumulation significantly improving the Ni3ZnC0.7/Al2O3 catalyst’s stability.This work is significant for understanding the structure-performance relationship at the interstitial sites in transition metal catalysts.Furthermore,it provides new insights into the design of hydrogenation catalysts.
Key Words: Ni-based catalyst; Interstitial site; Ni3ZnC0.7; Selective hydrogenation; Subsurface carbon
1 Introduction
Unsaturated hydrocarbons,especially butene,are widely used as raw materials for the production of organic chemicals1.Raw alkene generated by catalytic cracking generally contain several alkadiene impurities,which has to be removed or controlled to below 10 × 10-6to avoid the polymerization catalysts poisoning2,3.Catalytic hydrogenation is the most widely route to remove residual 1,3-butadiene from butene-rich streams4,5.Due to the low H2dissociation barrier,palladium-based catalysts are widely used in modern industrial production for the selective hydrogenation of alkynes,but its selectivity to olefins is usually low at high conversion6–8.Furthermore,the high metal cost and low abundance of Pd-based catalysts inevitably limit its widespread application.Therefore,the development of nonnoble metal catalysts has always been the focus of industrial catalysis research.
Transition metal Ni is also widely used in the selective hydrogenation of unsaturated hydrocarbons due to its low cost and comparable hydrogenation activity9–12.However,metal Ni is peculiarly prone to over-hydrogenation in butadiene hydrogenation reaction,which reduces the catalytic selectivity of alkenes.In addition,the three neighboring Ni sites could trigger oligomerization that causes carbon deposition on the catalyst surface and rapid deactivation.In contrast,the isolated Ni sites can effectively suppress the oligomerization and enhance the stability of the catalyst.Introducing a second metal to alloy with Ni could regulate the geometric and electronic structures of surface atoms and thus form the completely isolated Ni active sites.For instance,Pt-Ni13and Ni-In14have been studied for improving the selectivity and activity of catalysts by introducing a second metal to modify Ni surface properties.However,bimetallic catalysts still face various problems,including complicated preparation processes,poor reaction stability,and surface segregation15,16.
In the selective hydrogenation of unsaturated hydrocarbons,the modulation of catalyst surface and subsurface structures could tailor the electronic and geometric structures of active sites,providing substantial opportunities for further optimization of catalyst performance17.The related investigations have indicated that the carbon atoms located in the interstitial site of metal catalysts could regulate the selectivity of unsaturated hydrocarbons.As early as 1978,Frackiewiczetal.18reported that carbon atoms penetrate into the Pd lattice during the hydrogenation of acetylene at 373 K.The work by Ziemeckiet al.19demonstrated that carbon entry inhibits the formation of β-PdH.Teschneretal.20employedinsituX-ray photoelectron spectroscopy (XPS) technology to explore that the interstitial carbon can inhibit hydrogen in bulk phase from participating in reaction and thus improve the selectivity of acetylene hydrogenation.Subsequent studies attempted to introduce carbon atoms into the interstitial space of Pd under drastic conditions to prepare stable interstitial carbide structures and enhance the catalysis selectivity21.It seemed to Ni-based catalysts that the introduction of a second metal increased the distance between Ni atoms and expanded the octahedral interstitial space.The light elements with small atomic radii could easily penetrated into the interstitial sites of the intermetallic compound while modulating the surface adsorption of the active sites.For example,our group synthesized Ni3ZnC0.7viathe adsorption dissociation of acetylene on the surface of Ni3Zn alloy particles and the introduction of carbon atoms,which possess excellent selectivity and stability in the semihydrogenation of acetylene22.Leeetal.23also demonstrated the reversible phase transition between the Ni3Ga intermetallic compounds and Ni3GaCxand the presence of interstitial carbon during the dry reforming of methane byinsitucharacterization.Thus,tailoring the interstitial atoms in transition metal catalysts is of great importance for tuning the catalytic performance.
Herein,we synthesized supported Ni3Zn/Al2O3catalysts by impregnation and further obtained the Ni3ZnC0.7structure by acetylene atmosphere treatment.The various characterization techniques were used to investigate the effects of interstitial carbon atoms on the electronic and geometric structures of Ni3ZnC0.7/Al2O3catalysts.Further,the selective hydrogenation reaction of 1,3-butadiene was correlated with the catalyst structure to establish the structure-performance relationship,which is significantly for the design and preparation of efficient non-noble metal catalysts.
2 Experimental and computational section
2.1 Preparation of Al2O3 nanosheets
Hydrothermal method was used to synthesize alumina nanosheets.In 75 mL of deionized water,3 g of NaOH (≥ 97.0%)has been fully dissolved to prepare a 1 mol·L-1NaOH solution.Approximately 3 g sodium dodecyl benzene sulfonate (AR,mixture) was dissolved in 90 mL deionized water (18.25 MΩ·cm).Then,11.25 g of Al(NO3)3·9H2O (98%) was added to the solution,and magnetic stirring was performed for 20 min in order to obtain a uniform distribution of the solution.Dropwise additions of the prepared NaOH solution were made to the mixed solution and the mixture was magnetically stirred for 20 min.Following,the suspension was transferred to an autoclave lined with Teflon and kept at 180 °C for 24 h.This was followed by the collection of precipitate by filtration and thorough washing with ethanol and deionized water.The precipitate was dried overnight at 80 °C and then calcined at 800 °C in air for 2 h to obtain Al2O3nanosheets.
2.2 Preparation of Ni-based catalyst
15% (mass fraction) Ni/Al2O3,Ni3Zn/Al2O3,and Ni3ZnC0.7/Al2O3catalysts were prepared by the impregnation method.303 mg Ni(NO3)2·6H2O (99%) and/or 101.1 mg Zn(NO3)2·6H2O (99.99%) were dissolved in 40 mL methanol,then 338 mg Al2O3nanosheets were added to the solution,and the suspension was ultrasonically dispersed for 1 h to obtain a uniform distribution.Methanol was evaporated at 50 °C using a rotary evaporator.Following drying in static air for 2 h,under 100 mL·min-150% (vol.,volume fraction) H2/Ar,the temperature was increased to 500 °C at a constant rate of 5 °C·min-1for 2 h,and then cooled to 20 °C to obtain Ni3Zn/Al2O3catalyst.Ni3ZnC0.7/Al2O3catalyst was prepared by switching to 1.0% (vol.) C2H2/Ar at 200 °C for 1 h following the reduction of Ni3Zn/Al2O3.Similarly,monometallic Ni catalysts were prepared as reference samples.
2.3 Catalyst characterizations
A D/MAX-2400 (Riguka,Japan) diffractionmeter was used to characterize the X-ray diffraction (XRD) patterns of the catalysts,which collected the patterns using a CuKαsource at a scanning rate of 5 (°)·min-1in the 2θrange of 10°–80°.Transmission electron microscopy (TEM),high-resolution TEM(HRTEM),high-angle annular dark-field scanning TEM(HAADF-STEM) and energy dispersive X-ray spectroscopy(EDX) elemental maps of the catalysts were acquired using an FEI Tecnai G2 F20 (FEI,USA) transmission electron microscope.XPS spectra were conducted by ESCALAB 250(Thermo Fisher Scientific,USA) instrument with radiation of AlKα.Based on the C 1speak (284.6 eV),the binding energies of the samples were corrected.The fitting and deconvolution of peak spectra were conducted by the casaXPS software.The in situdiffuse reflectance infrared Fourier transformed spectroscopy (DRIFTS) data were collected using a NICOLET iZ10 (Thermo Fisher Scientific,USA) spectrometer equipped with a DRIFTS cell (Harrick Praying Mantis,USA).We ground and mixed 20 mg of the sample with 80 mg of KBr (FTIR Grade)and loaded them into the infrared device'sinsitudiffuse reflectance sample cell.After treatment with flowing hydrogen at 500 °C for 2 h,the samples were cooled to 200 °C,and the gas subsequently changed to 20 mL·min-11% (vol.) C2H2/He for 1 h.After cooling to 20 °C and purging with helium for 30 min,the background spectrum was collected.Thereafter,the CO gas was switched for 5 min before being purged with 20 mL·min-1of helium and simultaneously collecting the CO adsorption spectrum of the sample.The acquisition resolution was 4 cm-1,and the scanning range was 400 to 4000 cm-1.All parameters of the Ni3Zn/Al2O3samples were the same,except that they were not exposed to an acetylene atmosphere at 200 °C.
2.4 Procedure for the hydrogenation of 1,3-butadiene
The catalytic performance of the samples for 1,3-butadiene was measured in a quartz tube microreactor at fixed atmospheric pressure.Adjusting the sample mass in the quartz tube,ranging from 1 to 6 mg.Ni3ZnC0.7samples were reducedinsituat 500 °C at 10 mL·min-1with 50% (vol.) H2/He gas for 2 h before being cooled to 200 °C and treated with 20 mL·min-11.0% (vol.)C2H2/He gas for 1 h before the test.All other samples were reducedinsituat 500 °C at 10 mL·min-1,50% (vol.) H2/He for 2 h before the reaction test.
Conversion and selectivity are calculated as follows:
3 Results and discussion
The phase structures of Ni3Zn and Ni3ZnC0.7catalysts were identified through XRD.As shown in Fig.1,the diffraction peaks at 31.9°,37.6°,39.5°,45.9°,60.9° and 67.0° for these two samples correspond to the typicalγ-Al2O3phase.Ni3Zn intermetallic phase with FCC structure shows diffraction peaks at 43.9°,51.2°,and 75.3° indexed to the (111),(200),and (220)planes.After reduction and treatment with 1.0% (vol.) C2H2/He,the diffraction peaks shifted to the smaller diffraction angles,indicating an increased lattice spacing.The diffraction peaks appearing at 42.8°,49.8° and 73.1° attributed to the (111),(200)and (220) planes of Ni3ZnC0.7structure,suggesting the formation of interstitial carbon structures.
The microstructural features of the obtained catalysts were further investigated by using TEM characterization.Figs.2a,b and Fig.S1 (Supporting Information) display typical TEM images and the corresponding particle size distribution (PSD)histograms of Ni3Zn/Al2O3and Ni3ZnC0.7/Al2O3.Ni3Zn and Ni3ZnC0.7nanoparticles (NPs) were uniformly distributed on Al2O3supports.According to the PSD analysis,the size distribution of Ni3Zn NPs ranged from 1 to 15 nm,with an average particle size of 6.4 ± 1.9 nm.A small increase in particle size (7.4 ± 2.2 nm) was observed after Ni3Zn was treated under acetylene atmosphere to form Ni3ZnC0.7.A detailed microstructural analysis was further done by HRTEM.As shown in Figs.2c and S2,the Ni3Zn NP show lattice spacing of 0.205 and 0.177 nm with an included angle of 54.7°,which corresponds to the (111) and (200) crystal planes of FCC structure.The crystal plane spacing is slightly greater compared to that of Ni,indicating the alloy structure has been formed and the crystal form remains unchanged.After the introduction of carbon by acetylene atmosphere treatment,the same angle of 54.7° with crystal lattice spacing of 0.212 and 0.184 nm were observed in Fig.2d and Fig.S2,which is consistent with the(111) and (200) crystal planes of Ni3ZnC0.7structure and demonstrates the presence of C atoms located in the Ni3Zn crystal lattice.The EDX elemental maps further evidenced the incorporation of interstitial C atoms into Ni3Zn alloys.All NPs in Fig.2e and Fig.S3 contain uniformly distributed Ni and Zn signals,confirming that the Ni3Zn alloy structure was synthesized24.As can be seen in Fig.2f and Fig.S4,the NPs are composed of Ni,Zn,and C elements.The three elements are uniformly distributed throughout the NPs,supporting the existence of interstitial carbon structure in Ni3ZnC0.7.

Fig.2 TEM images and PSD histograms of Ni3Zn/Al2O3 (a) and Ni3ZnC0.7/Al2O3 (b).HRTEM images of Ni3Zn (c) and Ni3ZnC0.7 (d).The top-right insets are the corresponding FFTs.STEM images of Ni3Zn (e) and Ni3ZnC0.7 (f) with the corresponding EDX elemental maps of C (white),Al (red),Ni (green),and Zn (yellow).Color online.
The butadiene hydrogenation reaction was selected to evaluate the prepared Ni-based catalysts.Fig.3a shows the conversion of butene and the product selectivity over Ni3Zn/Al2O3and Ni3ZnC0.7/Al2O3catalysts at the temperature range from 50 to 150 °C.Ni3ZnC0.7/Al2O3catalyst exhibits excellent selectivity for butene,which could be maintained at~90% for the target butene product at different temperatures(Fig.S5).In addition,Fig.3b shows that Ni3ZnC0.7/Al2O3catalyst exhibits an extraordinary butene selectivity of 93.6% even at complete conversion of butadiene.In contrast,the Ni/Al2O3and Ni3Zn/Al2O3catalysts show much lower butene selectivity of 32.1% and 38.6%,respectively.Moreover,the selectivity of 1-butene was significantly increased for Ni3ZnC0.7/Al2O3compared to that of Ni3Zn/Al2O3,and the ratio oftrans-2-butene tocis-2-butene decreased from 1.99 to 1.64.It was suggested that the interstitial carbon affected the electronic structure of Ni22,25,thus promoting the desorption of 1-butene and hindering its complete hydrogenation and isomerization.The pure Al2O3support did not exhibit catalytic activity under the same conditions.

Fig.3 (a) Conversion and selectivity as a function of temperature on Ni3Zn/Al2O3 and Ni3ZnC0.7/Al2O3.(b) Selectivity of total butene,1-butene,trans-2-butene,and cis-2-butene in flow reactor at conversion of 98% (7.41% (vol.) H2,0.92% (vol.) 1,3-BD,helium as balance).(c) Conversion and selectivity of Ni3Zn/Al2O3 (1.0 mg) and Ni3ZnC0.7/Al2O3 (6.0 mg) catalysts under various H2 to 1,3-BD ratios.(d) Stability test of Ni3ZnC0.7/Al2O3 catalyst (6.0 mg,150 °C,7.41% (vol.) H2,0.92% (vol.) 1,3-BD,helium as balance).
As shown in Fig.3c,testing the obtained catalysts in different H2/1,3-butadiene ratios revealed that the activity of both catalysts increased with increasing hydrogen ratios.When H2/1,3-BD was increased from 2 : 1 to 10 : 1,the conversion of Ni3ZnC0.7/Al2O3catalyst increased from 92.1% to 97.7% while the selectivity decreased from 97.5% to 92.5%.The high selectivity within a wide H2/1,3-butadiene ration range indicates that Ni3ZnC0.7/Al2O3catalyst can operate reliably under fluctuating conditions.During this process,the butene selectivity of Ni3Zn/Al2O3catalyst decreased significantly,which may be attributed to the subsurface hydrogen.The stability tests of Ni3ZnC0.7/Al2O3catalyst was further carried out in 1,3-butadiene hydrogenation (Fig.3d).The conversion on Ni3ZnC0.7/Al2O3catalyst remains at 97.6% for 14 h without any deactivation.All the above results demonstrate that Ni3ZnC0.7/Al2O3catalyst exhibits excellent selectivity and stability in 1,3-butadiene hydrogenation.
The electronic structures of Ni3Zn/Al2O3and Ni3ZnC0.7/Al2O3catalysts were analyzed by XPS.For Ni3Zn/Al2O3catalyst in Fig.4a,the binding energies of 873.1 and 855.0 eV correspond to Ni2+2p1/2and Ni2+2p3/2,respectively,while the binding energies of 868.7 and 851.5 eV are attributed to Ni02p1/2and Ni02p3/2.The satellite peaks of Ni are located in the green area26.In contrast,the Ni02p1/2and Ni02p3/2peaks of Ni3ZnC0.7/Al2O3shift to higher binding energies compared with those of Ni3Zn/Al2O3,resulting in binding energy (BE) values of 869.4 and 852.2 eV,respectively.Because of the higher electronegativity of C (2.5) than that of Ni (1.9),the Ni 2ppeaks of Ni3ZnC0.7/Al2O3shift towards higher BE with 0.7 eV in comparison with those of Ni3Zn/Al2O3,demonstrating the electrons are transferred from Ni to C in Ni3ZnC0.7structure.The formation of interstitial carbon structures results in the variation of the electronic structure of Ni,making it more electrondeficient.The change in the electronic structure of the catalysts will affect the adsorption heat and activation barrier,which in turn altering the catalytic activity and selectivity27.
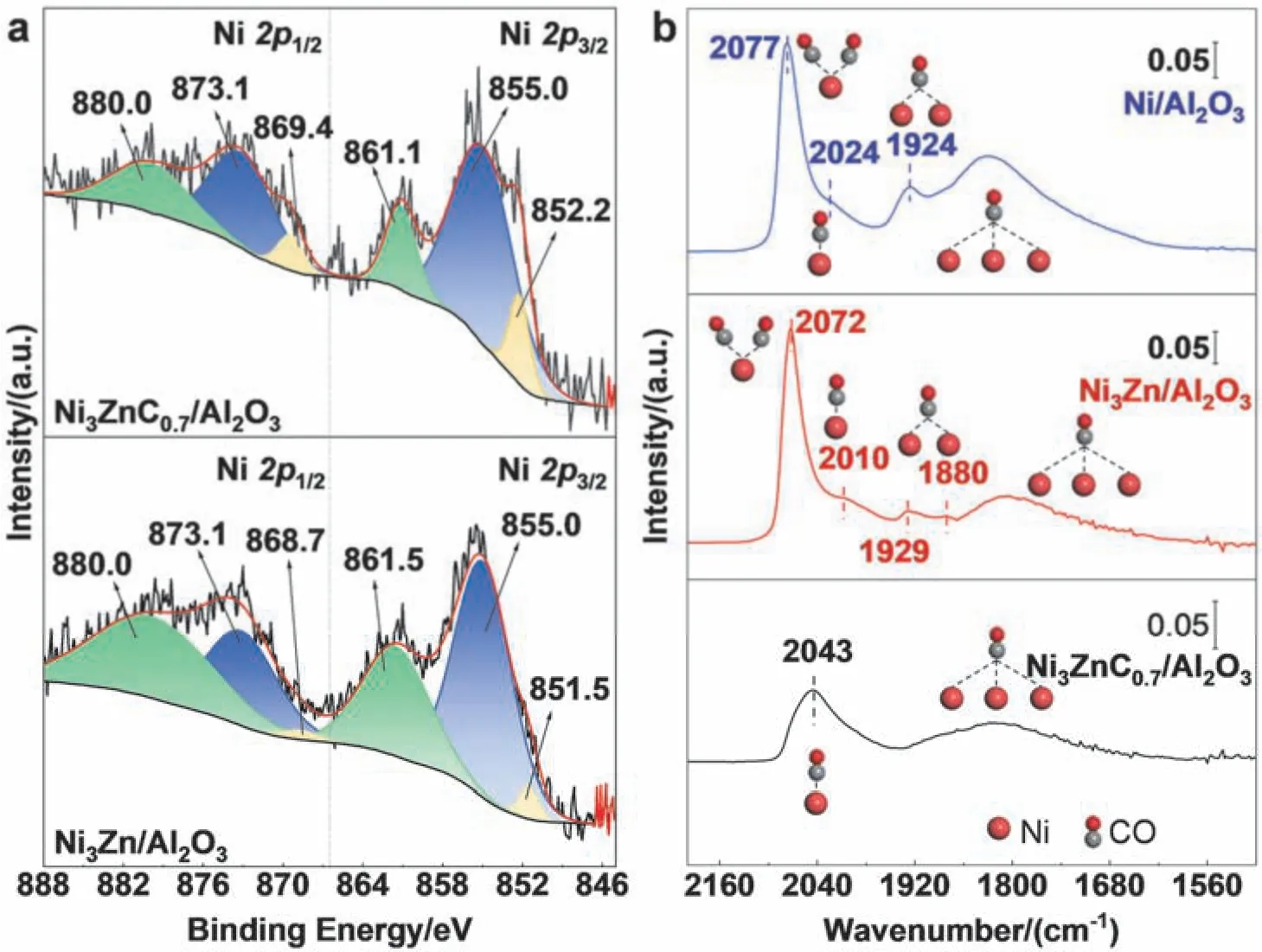
Fig.4 (a) XPS spectra of Ni3Zn/Al2O3 and Ni3ZnC0.7/Al2O3 catalysts.(b) In situ CO-DRIFTS spectra of Ni/Al2O3,Ni3Zn/Al2O3 and Ni3ZnC0.7/Al2O3 catalysts.
CO-DRIFTS spectrum was employed to reveal the CO chemisorption properties of the surface of Ni-based catalysts.As shown in Fig.4b,four main bands of surface-bonded CO can be observed beside the gas-phase CO absorption bands between 2211 and 2100 cm-128.The CO adsorption band centered at 2078 cm-1can be attributed to the CO molecules adsorbed on the surface defect sites of Ni-based catalysts,which exist in the form of subcarbonyls compounds28,29.The one at 2024 cm-1can be assigned to the linearly adsorbed CO on the surface of metallic Ni,while the bands at 1924 and 1833 cm-1can be interpreted as bridge and threefold coordinated30,31.The addition of Zn to form Ni3Zn intermetallic structure disrupts the continuous Ni sites,causing the bridge adsorption peak and the triple adsorption peak to be weaker.For Ni3ZnC0.7/Al2O3catalyst,the formation of interstitial carbon structure resulted in a smooth catalyst surface,leading to the disappearance of the subcarbonyls adsorption peak as well as the weakening of the overall CO adsorption bond.The bridge adsorption peak cannot be found in the spectrum due to the weak adsorption.The CO adsorption peaks in Ni3ZnC0.7/Al2O3show an evident shift to higher wavenumbers as compared with that in Ni3Zn.It is attributed to the charge transfer between Ni and C after introducing C atoms into the interstitial site of Ni3Zn structure.
The hydrogenation process of 1,3-butadiene on the surface of some transition metals is usually divided into two stages.The first stage only produces monoolefin,while the monoolefin is hydrogenated into butane and isomerized in the second stage9.The surface electrons and geometric structure of metal catalysts play a significant role in this reaction10,13.For the Ni-based catalysts,the adsorption strength of 1,3-butadiene and hydrogenation products on the catalyst surface is affected by the electronic structure27,32,33,and the change in the relative adsorption strength will alter the selectivity of the products.The Ni3ZnC0.7/Al2O3catalyst exhibits more extraordinary butene selectivity and long-term stability in 1,3-butadiene hydrogenation,which was mainly attributed to the charge transfer between surface Ni species and interstitial C atoms favoring the monoolefin desorption and inhibiting further hydrogenation and isomerization of 1-butene33.It has been demonstrated through XPS and CO-DRIFTS,and a reduction in thetrans/cis2-butene ratio can also indicate a change in the electronic structure25.
Other effects could also be invoked to explain the differences between Ni/Al2O3and Ni3ZnC0.7/Al2O3: the subsurface hydrogen in Ni-based catalysts tends to be more active than surface hydrogen34,and the formation of interstitial carbon structures can prevent subsurface hydrogen from participating in the reaction and thus inhibit over-hydrogenation reaction pathway20.Additionally,previous studies have shown that the formation of interstitial carbon structures can inhibit the deposition of surface carbon species,thus improving the stability of catalysts26.The active-site isolation effect has been considered as an effective solution to improve the selectivity in selective hydrogenation reaction35.The introduction of Zn increased the distance between Ni atoms,expanded the octahedral interstitial space22,and destroyed the continuous Ni site,improving the selectivity.And the isolated Ni sites can effectively suppress the oligomerization and enhance the strength of the catalyst.
4 Conclusions
The Ni-based catalysts with different structures have been successfully synthesized,exhibiting that the interstitial carbon into Ni3Zn can improve 1,3-butadiene hydrogenation performance with butene selectivity up to 93% at 98% conversion of butadiene.CO-DRIFTS indicates that the introduction of interstitial carbon atoms coordinated with neighboring Ni atoms and change the electronic/geometry structure of Ni sites in Ni3ZnC0.7.The electron transfer from the surface Ni active sites to interstitial carbon atoms,resulting in an increase in BE value.It weakens the adsorption strength of 1-butene on the catalyst surface and inhibits the overhydrogenation reaction pathway.More importantly,due to the suppressed deposition of carbon species on the catalyst surface,the stability of Ni3ZnC0.7/Al2O3catalyst was also significantly improved.This work is of great significance for studying the structure-performance relationship on the interstitial sites in transition metal catalysts.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
- Holey Graphene for Sodium-Ion Battery Anode Material
- Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution

