Isolation and purification of diketopiperazines with antialgal activity from marine macroalgae
ZHUANG Liwen,ZHOU Wenjing,MAO Yilin,GUO Ganlin,2,3,WANG Changhai,SUN Yingying,2,3
1.Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University, Lianyungang 222005,China;
2.Co-Innovation Center of Jiangsu Marine Bio-industry Technology,Lianyungang 222005,China;
3.Jiangsu Key Laboratory of Marine Bioresources and Eco-environment, Jiangsu Ocean University,Lianyungang 222005,China;
4.Resources and environment science institute,Nanjing Agricultural University,Nanjing 210095,China
Abstract: The cyclic peptides with antialgal activity were screened from Gracilaria lemaneiformis, Neoporphyra yezoensis, Silvetia siliguosa and Undaria pinnatifida, by thin-layer in-situ chemical reaction with ninhydrin reagent and antialgal activity test.The cyclic peptides with antialgal activity were found in the extracts of Gracilaria lemaneiformisandNeoporphyrayezoensis,respectively.Cyclo(Pro-Gly),cyclo(Ser-Pro), cyclo(Ala-Trp) were finally isolated from Gracilaria lemaneiformis, and cyclo(Leu-Pro) and cyclo(Val-Tyr) were purified from Neoporphyra yezoensis by repeated silica gel column chromatography and(or)silica gel thin layer chromatography.Except for cyclo(Ser-Pro), the other four cyclic dipeptides were obtained from corresponding marine macroalgae for the first time.Cyclo(Pro-Gly),cyclo(Leu-Pro)and cyclo(Val-Tyr) significantly inhibited the growth of Amphidinium carterae, Heterosigma akashiwo, and Phaeocystis globosa; they showed selective growth inhibition for Karenia mikimotoi and Skeletonema costatum. At the concentration of 50 μg/mL, the growth inhibition of cyclo(Pro-Gly), cyclo(Leu-Pro) and cyclo(Val-Tyr) for Amphidinium carterae, Heterosigma akashiwo and Phaeocystis globosa was ≥50%(on the 4th day);the growth inhibition of cyclo(Pro-Gly) for Karenia mikimotoi and that of cyclo(Leu-Pro)for Skeletonema costatum were more than or close to 50%. Further, EC50-96h of cyclic dipeptide for red tide microalgae was obtained. EC50-96h of cyclo(Pro-Gly) for Karenia mikimotoi and Phaeocystis globosa, EC50-96h of cyclo(Leu-Pro) and cyclo (Val-Tyr) for Heterosigma akashiwo were less than that of potassium dichromate for corresponding red tide microalgae. This suggested that cyclo(Pro-Gly), cyclo(Leu-Pro) and cyclo(Val-Tyr) have more advantages in inhibiting Karenia mikimotoi, Phaeocystis globosa or Heterosigma akashiwo, and are expected to be developed into an environment-friendly algal inhibitor against red tide microalgae.
Keywords: Gracilaria lemaneiformi, Neoporphyra yezoensis, antialgal activity,diketopiperazines,isolation
In recent years, the study on the interaction between macroalgae and red tide microalgae is increasing day by day. And it has become a new research direction to prevent and control red tide by using the inhibitory effect between them[1].Currently,certain metabolites in macroalgae have been confirmed to be the main cause of growth inhibition effects against red tide microalgae[2-10], such as polyphenols[3], bromoform[4], fatty acid[5],unsaturated fatty acid[6],glycerol glycolipids[7]and other compounds[8-10]. More importantly,they are produced by macroalgae, which can be degraded under natural conditions and will not accumulate for a long time.They have good ecological safety and are expected to be developed as environmentally friendly algal inhibitor against red tide microalgae.
Cyclic dipeptides, also known as diketopiperazines(Diketopiperazines, DKPs), are formed by condensation of 2 amino acids and are found in yeast,fungi,plants and animals,and marine organisms[13-15].Cyclic dipeptides have attracted close attention of researchers due to their diverse structures and activities[12,16-18]. The research of marine cyclic dipeptides at home and abroad is mainly focuses on the field of marine microorganisms,and the research on macroalgae cyclic dipeptides is not much. So far, cyclic dipeptides have only been found inUlva prolifera[9],Gracilaria lemaneiformis[10],Ulva lactuca[18]andSymphyocladia latiuscula. Meanwhile, in terms of biological activity research, there are very few studies on the antialgal activity of cyclic dipeptides[22-25].No more than 10 kinds of cyclic dipeptides with antialgal activity have been reported, including cyclo(Ser-Pro)[22],cyclo(Pro-Gly)[9,23,24],cyclo(Ala-Leu)[23,24],cyclo(Pro-Val)[23,24],cyclo(Phe-Ala)[25]and cyclo(Ala-Try)[25], et al.Among them, cyclo(Ser-Pro) and cyclo(Pro-Gly) have obvious antialgal activity onKarenia mikimotoi[10,22], but no other cyclic dipeptides were studied on antialgal activity.
Gracilaria lemaneiformis, Neoporphyra yezoensis, Silvetia siliguosaandUndaria pinnatifidaare common macroalgae in China. In this paper, the thin-layer in-situ chemical reaction with ninhydrin reagent combing with bioactivity-guided isolation method were used to find thatGracilaria lemaneiformisandNeoporphyra yezoensiscontain cyclic peptides with antialgal activity among these above four macroalgae. On this basis, five cyclic dipeptides were isolated and purified fromGracilaria lemaneiformisandNeoporphyra yezoensisusing silica gel column chromatography and(or) preparative thin layer chromatography. Further, the inhibitory effects of three cyclic dipeptides on the growth ofAmphidinium carterae,Heterosigma akashiwo,Karenia mikimotoi,Phaeocystis globosaandSkeletonema costatumwere evaluated, and the median effect concentration(EC50-96h)of each cyclic dipeptide for tested red tide microalgae were obtained.This paper lays an experimental foundation and provides a theoretical basis for the isolation,purification and activity study of cyclic dipeptides from macroalgae.
1 Materials and methods
1.1 Experimental materials
Amphidinium carterae,Heterosigma akashiwo,Karenia mikimotoi,Phaeocystis globosaandSkeletonema costatumwere provided by the Ocean University of China and cultured aseptically in f/2 medium at 23±1 ºC with illuminance of 3 000 lx and a 16/8 dark/light cycle.
Gracilaria lemaneiformis, Neoporphyra yezoensis, Silvetia siliguosaandUndaria pinnatifidawere provided by Jiangsu Bilan Marine Biotechnology Co. LTD, washed, dried and sieved(<0.5 mm),and stored at 4ºC.
Aged natural seawater was filtered through absorbent cotton and 300 mesh screen silk sieved, boiled, cooled, and the pH and salinity of the seawater were adjusted to 8.0 and 30, respectively. Unless otherwise specified, the seawater used in the experiments was treated as above in this paper.
1.2 Preparation and liquid-liquid separation of macroalgae extracts
Macroalgae powder and ethanol were mixed and extracted for 24 h by ultrasonic heating extraction concentrator. The residue was removed by filter paper, and then the particulate matter was removed by 0.22 μm organic filter membrane to obtain leach liquor.The leach liquor was rotated and evaporated at 40 ℃,and obtained the extract,which was the ethanol extract of macroalgae. This extract was added to an appropriate amount of distilled water, shaken thoroughly and allowed to stand at 4 ℃for 12 h. And then, this turbid liquid was centrifuged at 3500 r/min for 15 min and the supernatants were collected.Ethyl acetate was added to the supernatants and extracted 3-4 times. And then upper layer(ethyl acetate phase)was combined and evaporated under reduced pressure at 40 ℃to obtain fraction A.Subsequently,the lower layer was evaporated under reduced pressure to remove ethyl acetate, and n-butanol was added for extraction 3 to 4 times. The n-butanol phase and the raffinate phase were evaporated to dryness under reduced pressure to obtain fractions B and C. These three fractions were re-dissolved in ethanol and prepared at a concentration of 5 mg/L.Then,the cyclic peptide and antialgal activity of these three fractions were detected.
1.3 Cyclic peptide detection
The fraction to be measured was spotted on two silica gel G plates(25 mm×50 mm,plates 1 and 2), and chloroform/methanol(9:l, v:v) was used as the unfolding agent. After the development, the solvent was volatilized and the plate 1 was placed. The plate 2 was suspended in a closed grinding glass cylinder(acid and high temperature resistant) with about 1.5 mL concentrated hydrochloric acid at the bottom and placed in an oven heated to 110 ºC, heated for hydrolysis for 1-2 h. After cooling, the plate 2 was taken out and the hydrochloric acid was volatilized in the fume hood. Plate 1 and plate 2(after heating and acid hydrolysis treatment) were sprayed with 0.2% ninhydrin-acetone reagent and heated by electric blowers for 2-5 min to develop color. According to the results of color development, if plate 2 shows purplish red or yellow spots, while plate 1 has no color development spots at the corresponding position, it indicates that the fraction may contain cyclic peptide or peptide amide.
The fraction showing cyclic peptides or peptide amides as described above were spotted on silica gel G plates(50 mm × 50 mm, plate 3) using a bidirectional thin layer chromatography plate spotting method, and unfolded in the first direction with chloroform/methanol(9:1,v:v). After evaporation of the solvent, plate 3 was subjected to high-temperature acid hydrolysis as described above. After evaporating the hydrochloric acid, the second direction of unfolding was performed, and the unfolding agent was chloroform/methanol/glacial acetic acid(8:2:2 drops, v:v:v).At the end of the unfolding, the solvent was evaporated, 0.2% ninhydrin-acetone reagent was sprayed, and the color development was heated by electric blowers. If plate 3 shows multiple magenta or yellow spots on the same axis in the second dimension, it indicates that the c fraction contains cyclic peptides[26].
1.4 Antialgal activity
1.4.1 Antialgal activity of the component in the separation process
The component(fraction extracted by liquid-liquid extraction,or sub-fraction obtained by silica gel column chromatography, or sample prepared by preparative thin-layer chromatography) was added to the f/2 medium, mixed well and inoculated with red tide microalgae, and the final concentration of the component was 100 μg/mL. Meanwhile, the control group with the same volume of ethanol added(the volume of ethanol added should not exceed 1% of the total volume of culture system) was set, and three repetitions were set for each culture flask.All culture flasks were placed in GXZ-260B intelligent type light incubator. The red tide microalgae was cultured under 62 μmol photons m-2s-1and a 12/12 dark/light cycle. The culture flasks were regularly shaken twice a day to prevent the growth of microalgae attached to the walls.Every other day,1 mL of culture was taken from the culture flask, fixed with Lugol's reagent, and the number of red tide microalgae cells was counted.
1.4.2 Antialgal activity of cyclic dipeptide
The inhibition activity of cyclic dipeptide against red tide microalgae was tested with Costar 96 micro-well plate.The final volume of the culture system was 200 μL,including 1 μL of cyclic dipeptide solution and 199 μL of red tide microalgae culture.The final concentration of ethanol in the culture system was 0.5% and the final concentration of cyclic dipeptide(dissolved in ethanol) was 0.4, 2.0, 10 and 50 μg/mL. And the negative control group with the same volume of ethanol was added, and potassium dichromate was used as the positive control group. Three repetitions were set for each experiment, and the culture conditions were the same as above.
1.5 Isolation and preparation by silica gel column chromatography and preparative thin-layer chromatography
1.5.1 Isolation and preparation of cyclic peptide with antialgal activity fromGracilaria lemaneiformis
According to the method mentioned above, 166 g ethanol extract was obtained from 1200 g dried powder ofGracilaria lemaneiformis. After separation by liquid-liquid extraction, fraction A(22.2 g), fraction B(12.8 g) and fraction C(10.2 g) were obtained. The assay showed the presence of cyclic peptide in the fraction C fromGracilaria lemaneiformis. Subsequently, 5.0 g of fraction C was loaded into silica gel column chromatography(3.0 cm × 60 mm, 100-200 mesh), and chloroform/methanol(8:1,v/v) was used as eluent. After eluting 2.5 times of the column volume, each elution was concentrated and carried out cyclic peptide detection. The above silica gel column chromatographic isolated was performed again, and fraction C also was 5.0 g. The concentrated elutions with the same TLC development behavior were combined and obtained sub-fractions C1(0.97 g) and C2(1.25 g). Sub-fractions C1and C2were separated again by silica gel column chromatography(2.0 cm×50 mm, 200-300 mesh), respectively,and eluted with chloroform/methanol(8:1)for 2.5 times of the column volume to obtain the samples C13(0.17 g), C21(0.96 g) and C22(0.21 g). Among them, the sample C21was purified by silica gel column chromatography and obtained to the target component C211(0.15 g).
1.5.2 Isolation and preparation of cyclic peptide with antialgal activity fromNeoporphyra yezoensis
200 g ethanol extract was prepared from 1 500 gNeoporphyra yezoensispowder.After liquid-liquid extraction, 18.3 g of fraction A, 20.4 g of fraction B and 10.1 g of fraction C were obtained. The results showed that fraction B had the cyclic peptide. 10.0 g of fraction B was loaded on silica gel column chromatography(5.0 cm × 60 mm, 100-200 mesh)with dichloromethane/methanol(6:1,v/v)as elution,and after eluting 2.5 times of the column volume, the elutions were concentrated and the cyclic peptide assay was performed. And then, the elutions with the same TLC development behavior were combined. Also, another 10.0 g of fraction B was isolated by silica gel column chromatography. After two isolations, the elutions were combined to obtain four sub-fractions B1(1.061 g), B2(2.179 g), B3(0.636 g) and B4(1.885 g). After the assay for antialgal activity, the sub-fractions B2and B4were again isolated by silica gel column chromatography(3.0 cm×50 mm, 100-200 mesh), respectively, and eluted with dichloromethane/methanol(6:1) for 2.5 times of the column volume to obtain samples B21(0.373 g), B22(0.224 g) and B23(0.163 g), and samples B41( 0.127 g), B42(0.146 g) and B43(0.275 g). The assay showed that these samples B41and fraction B42contained the cyclic peptide. Finally, two target components B411(0.042 g) and B421(0.057 g) were obtained by preparative thin layer chromatography on silica gel using chloroform/methanol(8:1, v/v) as the unfolding agent.
1.6 Structure identification of target components
The purified target components were subjected by HRESI-MS,1H-NMR, and13C-NMR with spectral data. NMR spectra were obtained with a Bruker AV III 600 NMR spectrometer (chemical shift values are presented as δ values with TMS as the internal standard).HR-ESI-MS spectra were performed on a LTQ-Obitrap XL spectrometer.
1.7 Data process
The experimental data were analyzed statistically using the SPSS 11.5 software package with independent sample test.p<0.05 is considered a significant difference andp<0.01 is considered a highly significant difference.
Growth inhibition of microalgae,I=(1-N/N0)×100%,where N is the number of algal cells in the treatment group(×104/mL);N0is the number of algal cells in the negative control group(×104/mL).
2 Results and analysis
2.1 Screening,isolation and purification of cyclic peptides from four macroalgae
From Tab. 1 and Fig. 1, it can be seen that the fraction C ofGracilaria lemaneiformisand the fraction B ofNeoporphyra yezoensiscontained cyclic peptides or peptide amides,and these two fractions had antialgal activity; the fractions A ofSilvetia siliguosaandUndaria pinnatifidacontained cyclic peptides or peptide amides, however, they did not show a significant inhibitory effect on the growth ofKarenia mikimotoi.Subsequently,after repeated isolations such as silica gel column chromatography and preparative thin-layer chromatography, the five target fractions were finally obtained fromGracilaria lemaneiformisandNeoporphyra yezoensis, and their thin-layer chromatographic expansions are shown in Fig.2.

Fig.1 Cyclic peptide or peptide amide detection of fractions by liquid-liquid extraction from macroalgae

Fig.2 Thin-layer chromatography of target components(A.Cyclo(Val-Tyr),B.Cyclo(Leu-Pro),C.Cyclo(Pro-Gly))
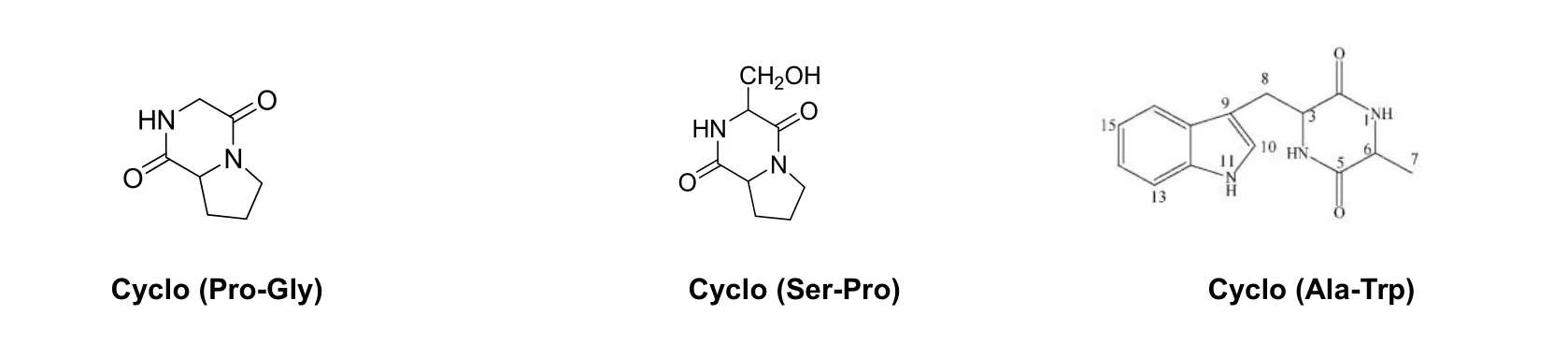
Fig.3 Structure of cyclic dipeptides with antialgal activity from Gracilaria lemaneiformis
2.2 Structural identification of cyclic peptides with antialgal activity
2.2.1 Cyclic peptides with antialgal activity inGracilaria lemaneiformis
The mass spectrometry and nuclear magnetic resonance spectrum data of fraction C13,C22and C211are shown in Tab.2.Compared with the reported cyclic dipeptide data[27-29],they were identified as cyclo (Pro-Gly), cyclo (Ser-Pro) and cyclo (Ala-Trp). Except for cyclo (Ser-Pro) which has been isolated fromGracilaria lemaneiformis[10], the other two cyclic dipeptides were obtained fromGracilaria lemaneiformisfor the first time.
2.2.2 Cyclic peptide with antialgal activity inNeoporphyra yezoensis
Two thin-layer pure target components B411and B421were purified fromNeoporphyra yezoensis.Their mass spectrometry and nuclear magnetic resonance spectrum data were shown in Tab.3. Compared with the corresponding data of existing cyclic dipeptides[28,30],the two components B411and B421were identified as cyclo(Leu-Pro)and cyclo(Val-Tyr)(Fig.4). This is the first time that cyclic dipeptide has been obtained fromNeoporphyra yezoensis.

Fig.4 Structure of cyclic dipeptide with antialgal activity in Neoporphyra yezoensis
2.3 Inhibitory effects of three cyclic dipeptides on the growth of 5 red tide microalgae
Among the five cyclic dipeptides, our previous study reported the antialgal activity of cyclo(Ser-Pro)[10], and no studies on the inhibitory effect of the remaining 4 cyclic dipeptides on the growth of red tide microalgae have been seen. Since cyclo(Pro-Gly),cyclo(Leu-Pro)and cyclo(Val-Tyr)are commercially available,the inhibitory effects of these three cyclic dipeptides on the growth ofAmphidinium carterae,Heterosigma akashiwo,Karenia mikimotoi,Phaeocystis globosaandSkeletonema costatumwere analyzed(Fig.5).
These three cyclic dipeptides strongly(p<0.05) inhibited the growth ofAmphidinium carterae,Heterosigma akashiwo and Phaeocystis globosa, and their growth inhibition of the tested red tide microalgae was significantly(p<0.05) enhanced with increasing concentration of the cyclic dipeptide(Fig. 5).At a concentration of 50 μg/mL, they inhibited the growth of three red tide microalgae by more than or very close to 50%(on the 4th day);only cyclo(Pro-Gly) showed a significant(p<0.05) inhibitory effect onKarenia mikimotoi,with a growth inhibition rate of 55.4%at 50 μg/mL(on the 4th day);the cyclo(Leu-Pro)was significant(p<0.05)inhibited the growth ofSkeletonema costatum,and the growth inhibition was close to 50% at 50 μg/mL(on the 4th day); cyclo(Pro-Gly), cyclo(Leu-Pro) and cyclo(Val-Tyr)showed selective inhibition on the growth of 5 red tide microalgae.
EC50-96hvalues of the three cyclic dipeptides on the growth of each red tide microalgae were calculated using literature methods[31](Tab. 4). Compared with the reported results[10], EC50-96hvalues of cyclo(Pro-Gly) on the growth ofKarenia mikimotoi,andPhaeocystis globosa, and EC50-96hvalues of cyclo(Leu-Pro) and cyclo(Val-Tyr) on the growth ofHeterosigma akashiwowere significantly lower than that of potassium dichromate on the growth of corresponding red tide microalgae.
In addition, we found some significant changes in the cell morphology ofKarenia mikimotoi,Heterosigma akashiwoandPhaeocystis globosaunder the action of cyclo(Pro-Gly),cyclo(Leu-Pro)and cyclo(Val-Tyr)(Fig.6).In Fig.6,it can be clearly seen that the cells ofHeterocurvium akashiwoswell and appear hollow; the cell membrane ofKarenia mikimotoiis blurred and the contents are detached; the cells ofPhaeocystis globosabecome elongated and accompanied by the detachment of the contents. Under the effect of the three cyclic dipeptides, the cell morphology ofAmphidinium carteraeandSkeletonema costatumdid not change significantly.

Fig.6 Morphological changes of tested red tide microalgae cells under the action of three cycli c dipeptides(Optical microscope(×400))
3 Discussion
There are abundant species of marine macroalgae in China,and reports indicate that there are at least 1300 species of marine macroalgae in China[32]. However, the lack of research on cyclic dipeptides from marine macroalgae at home and abroad has greatly restricted the application of cyclic dipeptides from marine macroalgae. In this paper, five cyclic dipeptides(Fig. 1-4,Tab. 1-3) were successfully isolated and purified fromGracilaria lemaneiformisandNeoporphyra yezoensisby thin-layer in-situ chemical reaction with ninhydrin reagent combing with bioactivity-guided isolation method.In addition to the cyclo(Pro-Gly) and cyclo (Ser-Pro) that have been obtained fromUlva prolifera[9], Ulva lactuca[18],andGracilaria lemaneiformis[10], cyclo (Ala-Try), cyclo (Leu-Pro), and cyclo (Val-Tyr)were obtained from macroalgae for the first time.The low yields of the five cyclic dipeptides prepared in this paper limit their applications. In the future work, we should try to improve the extraction and isolation process,and replace the separation methods such as silica gel column chromatography and preparative thin layer chromatography with other isolation methods with relatively low loss rate, so as to increase the yield of cyclic dipeptide in the extraction and isolation process. In addition, we found the presence of cyclic peptides inSilvetia siliguosaandUndaria pinnatifida, which can be carried out in the follow-up work(Tab.1).

Tab.1 The yield of four kinds of macroalgae extracts fractioned by liquid-liquid extraction(g),test results of cyclic peptide and antialgal activity
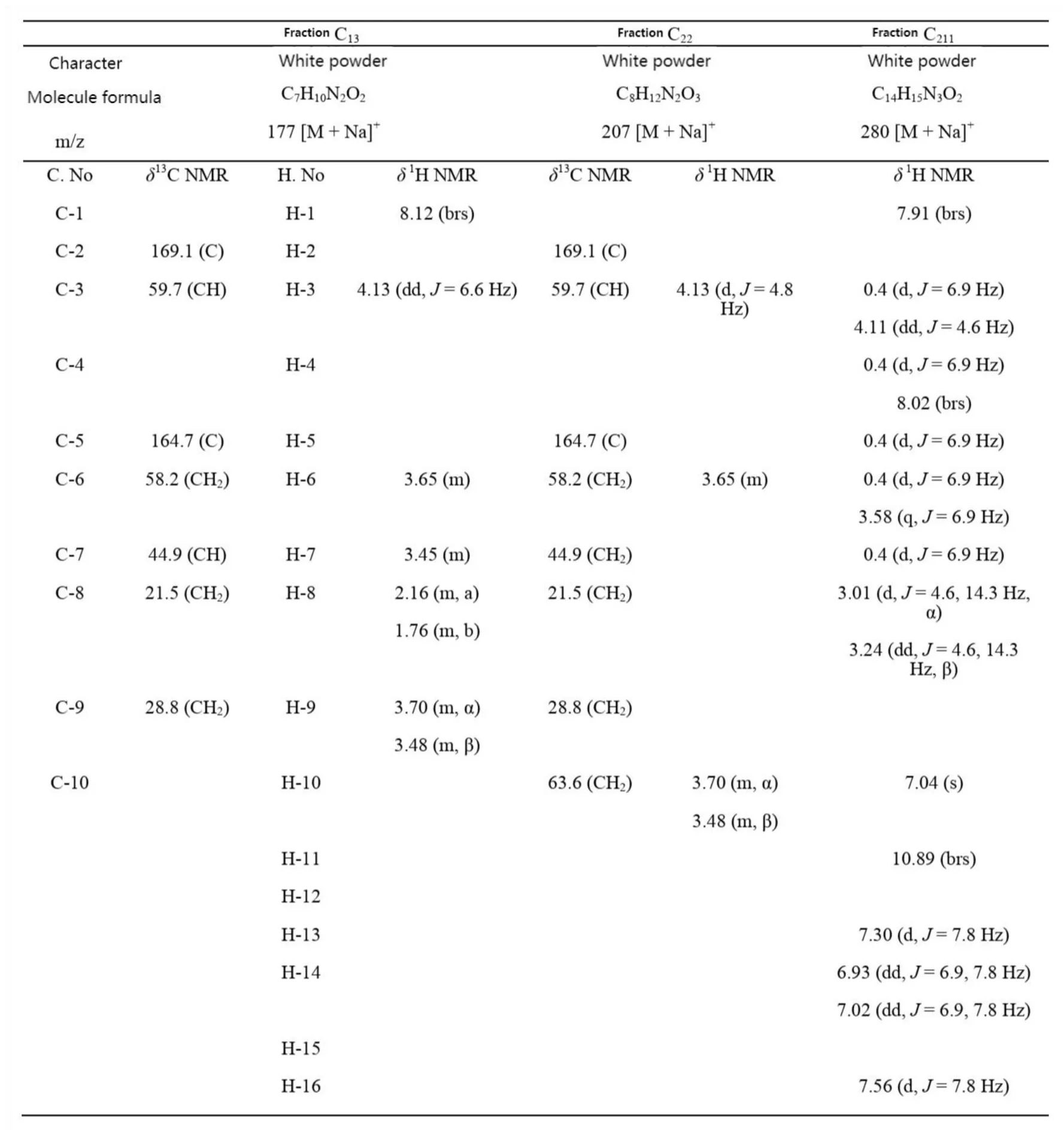
Tab.2 NMR spectroscopic data of 3 purified components from Gracilaria lemaneiformis in CDCl3
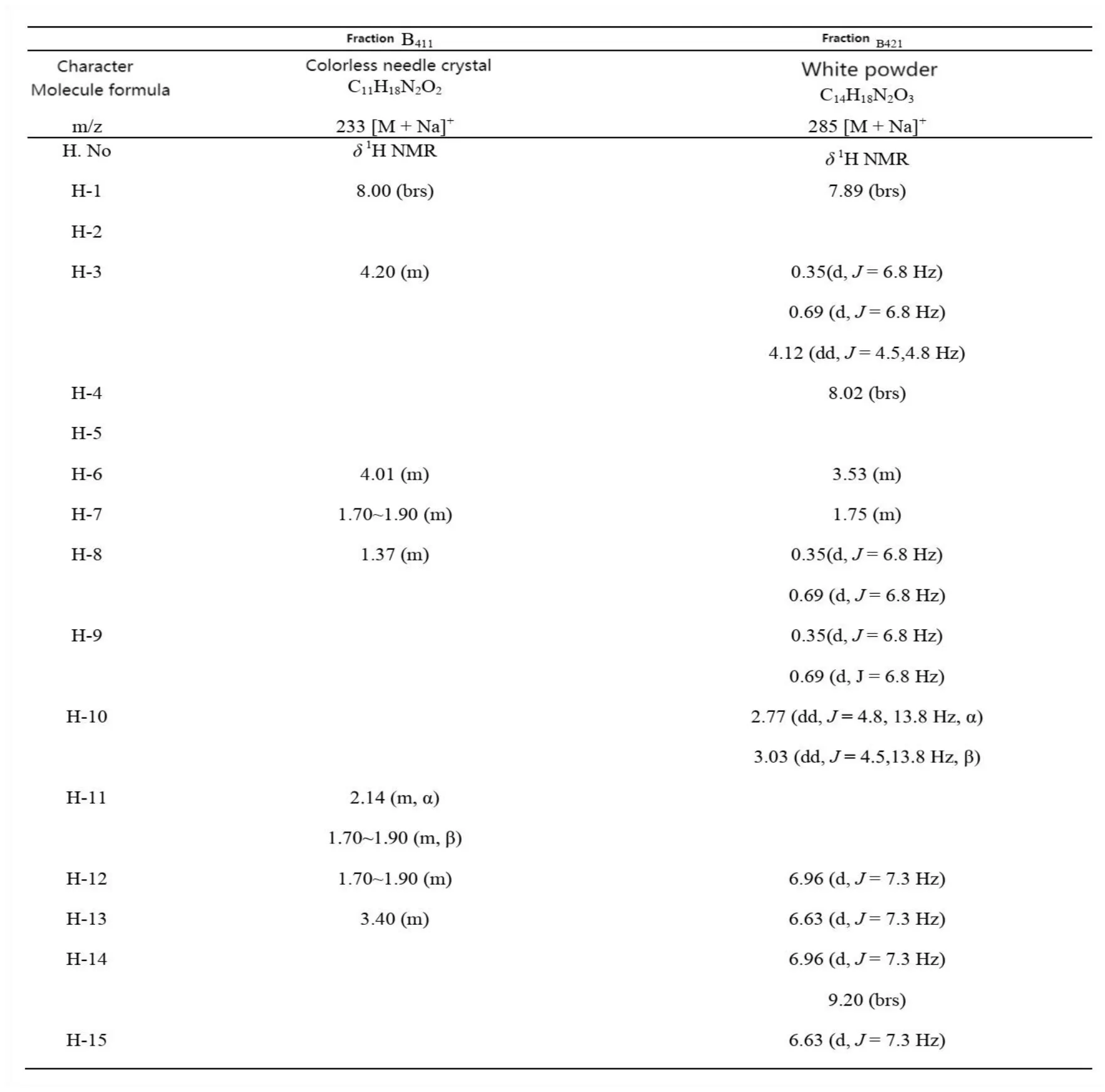
Tab.3 NMR spectroscopic data of 3 purified components from Neoporphyra yezoensis in CDCl3
Cyclic dipeptides[24,33]are biodegradable and are one of the ideal materials for developing into an environment-friendly algal inhibitor against red tide microalgae.The five cyclic dipeptides obtained in this paper are commercially available except for cyclo(Ala-Try). Among them, the inhibitory effect of cyclo(Ser-Pro) on the growth of red tide microalgae such asAmphidinium carteraehas been clarified in our previous study[10]. In view of this, we analyzed the inhibitory effects of cyclo(Pro-Gly), cyclo(Leu-Pro) and cyclo(Val-Tyr) on the growth ofAmphidinium carterae,Heterosigma akashiwo,Karenia mikimotoi,Phaeocystis globosaandSkeletonema costatum(Fig. 5). The three cyclic dipeptides exhibited wider inhibitory effects onAmphidinium carterae,Heterosigma akashiwo, andPhaeocystis globosa, strongly inhibiting their growth. However, they showed selective inhibition on the growth ofKarenia mikimotoiandSkeletonema costatum.Among them, only the cyclo(Pro-Gly)(or cyclo(Leu-Pro)) inhibitedKarenia mikimotoi(orSkeletonema costatum).The selective inhibitory effect of compounds on the growth of red tide microalgae has been found in other studies,which may be related to the mechanism of algal inhibition of the compounds and may also be related to the algal cell structure[2,4-8,10].At present, there are very few studies on the antialgal activity of cyclic dipeptides at home and abroad[10,22,25], let alone the research on the antialgal activity mechanism of cyclic dipeptides. In order to obtain the possible reasons for the differences in the inhibitory effects of cyclic dipeptides on the growth of tested red tide microalgae,this paper observed the effects of three kinds of cyclic dipeptides such as cyclo(Pro-Gly) on the morphology of algae cells. It was found that the cell morphology ofKarenia mikimotoi,Heterosigma akashiwoandPhaeocystis globosachanged significantly, for example, the cell membrane contour was blurred,the cell became hollow,elongated,and the contents came out(Fig.6).Based on these results, we believe that the three cyclic dipeptides such as cyclo(Pro-Gly)may have changed the permeability of the cell membrane, resulting in the release of contents,or the cyclic dipeptides may have caused electrolyte disturbance in the algal cells,and the cells swelled, hollowed or elongated, resulting in the contents to come out. The researchers pointed out that the inhibition process of the compound on microalgae may affect its function by binding to the biofilm[34-36]. In the study of cyclic peptides, the interaction between cyclic peptides and cell membranes is the main reason for their biological activity[11]. Researchers believe that the activity of cyclic peptides is the result of the changes in membrane permeability caused by the interaction of their structure, net charge and hydrophobicity with membrane[37,38]. It was found that cyclic dipeptide has antibacterial[39]and antioxidant effects[40]. Thus, we speculate that the antialgal activity mechanism of cyclic dipeptide may also be related to its antibacterial or antioxidant effect,which needs to be investigated in subsequent experiments.In addition,the effects of cyclic dipeptides on cell sub-microstructures, as well as important organelles such as chloroplasts and mitochondria need to be analyzed to determine the site of inhibitory effects of cyclic dipeptides.
Further, EC50-96hvalues of cyclo(Pro-Gly), cyclo(Leu-Pro) and cyclo(Val-Tyr) on the growth of test red tide microalgae could be obtained(Tab.4).According to the literature[41],cyclo(Pro-Gly)showed moderate to high toxicity to five red tide microalgae;cyclo(Leu-Pro)showed moderate to high toxicity toAmphidinium carterae,Heterosigma akashiwoandPhaeocystis globosa; cyclo(Val-Tyr) showed only moderate toxicity toHeterosigma akashiwo. Potassium dichromate is a common positive control used in algal toxicity experiments. Compared with EC50-96hvalues of potassium dichromate for these five tested red tide microalgae[10],EC50-96hvalues of cyclic(Pro-Gly)on the growth ofKarenia mikimotoiandPhaeocystis globosa, the EC50-96hvalues of cyclo(Leu-Pro) and cyclo(Pro-Gly) were significantly lower than that of potassium dichromate for corresponding red tide microalgae.It is noteworthy that EC50-96hvalue of cyclo(Pro-Gly) on the growth ofPhaeocystis globosais even lower than EC50-96hvalues of other antialgal compounds reported for this red tide microalgae[7,8,10]. This showed that the three cyclic dipeptides were more advantageous than potassium dichromate in the inhibition ofKarenia mikimotoi,Phaeocystis globosaandHeterosigma akashiwo.These three cyclic dipeptides are secondary metabolites produced byGracilaria lemaneiformisorNeoporphyra yezoensis, which are usually degradable and are expected to be developed as an environment-friendly algal inhibitor against red tide microalgae.
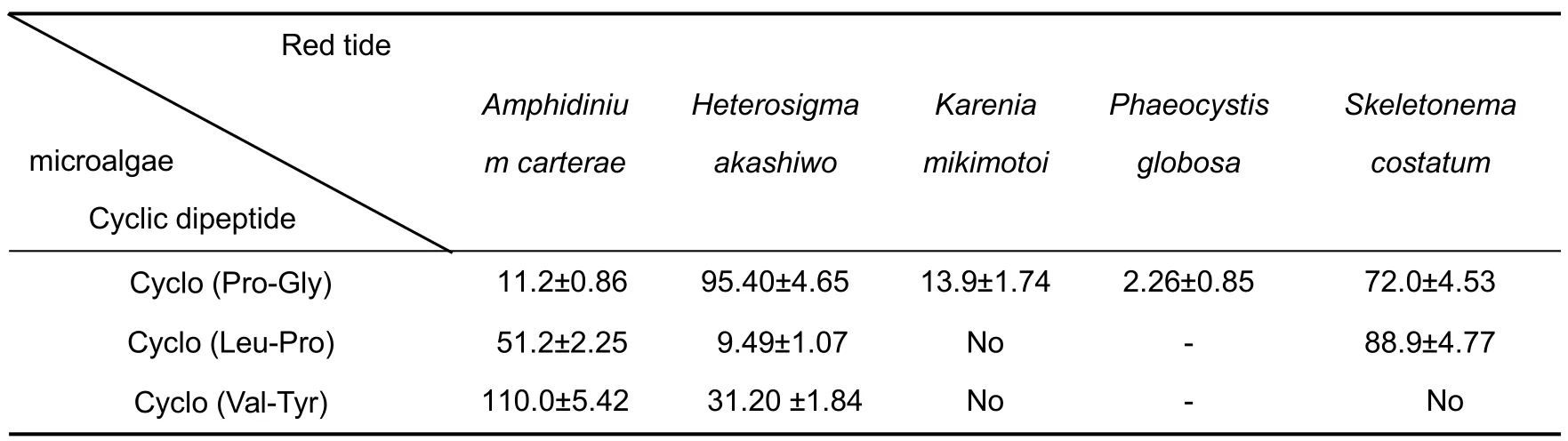
Tab.4 EC50-96h(µg/mL)of cyclic dipeptides against red tide microalgae
This paper fills the gap in the isolation and purification of cyclic dipeptides with antialgal activity inGracilaria lemaneiformisandNeoporphyra yezoensisat home and abroad.However,these results are not enough to use them for biological control of red tide microalgae. In the follow-up work, it is not only necessary to clarify the structure-activity relationship between cyclic dipeptide and its growth inhibitory effect on red tide microalgae,but also to deeply understand the antialgal activity mechanism of cyclic dipeptide.
 Marine Science Bulletin2023年1期
Marine Science Bulletin2023年1期
- Marine Science Bulletin的其它文章
- Characteristics of storm surge disasters along Fujian coast in recent 10 years
- Leveraging open source software and programming best practices for sustainable web applications in support of marine data sharing-BCO-DMO case
- Methods and empirical research on Chinese sea area resource assets value accounting
- Design and development of international buoy data operational processing system
- Data analysis of HF surface wave radar in Zhoushan sea area
- Priority conservation pattern and gaps of Guangxi sea area based on systematic conservation planning
