MRD-directed and risk-adapted individualized stratified treatment of AML
Yijing Zhao ,Hanfei Guo ,Yingjun Chang
1Peking University People’s Hospital,Peking University Institute of Hematology,Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation,Beijing 100044,China;2Stanford University Medical School,VA Palo Alto Health Care System,Palo Alto 94304,USA;3the First Hospital of Jilin University,Cancer Center,Changchun 133021,China
Abstract Measurable residual disease (MRD) has been widely recognized as a biomarker for deeply evaluating complete remission (CR),predicting relapse,guiding pre-emptive interventions,and serving as an endpoint surrogate for drug testing.However,despite the emergence of new technologies,there remains a lack of comprehensive understanding regarding the proper techniques,sample materials,and optimal time points for MRD assessment.In this review,we summarized the MRD methods,sample sources,and evaluation frequency according to the risk category of the European Leukemia Net (ELN) 2022.Additionally,we emphasize the importance of properly utilizing and combining these technologies.We have also refined the flowchart outlining each time point for preemptive interventions and intervention paths.The evaluation of MRD in acute myeloid leukemia (AML) is sophisticated,clinically applicable,and technology-dependent,and necessitates standardized approaches and further research.
Keywords: Measurable residual disease;acute myeloid leukemia;risk stratification
Introduction
Measurable residual disease (MRD),also known as minimal residual disease,refers to a small population of leukemic cells that persist in patients during or after treatment,even when the patient is in remission (1).MRD is recognized as a primary driver of leukemia relapse.In the fight against acute myeloid leukemia (AML),the timely and precise monitoring of MRD serves as an alert system,prompting physicians to take defensive actions and utilize available interventions to prevent AML relapse (2).In this sense,MRD is functioning as a component of the microscopiclevel package for understanding the progression of leukemia.
With the development of the detecting technology,MRD assessment utilizing sensitive molecular biology techniques can now detect levels as low as one cancer cell in a million normal cells (10-5-10-6).This remarkable sensitivity allows for the routine monitoring of submicroscopic leukemic cells in patients with acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML) throughout their disease journey,facilitating treatment guidance and adjustments (3).With increasing evidence on the significance of MRD status in determining AML treatment outcomes,the experts from the European Leukemia Net (ELN) MRD Working Party emphasized in a recently published document that MRD is an independent,post-remission prognostic factor in AML(1).This led to the definition of MRD-positive complete remission (CRMRD+) MRD-negative complete remission(CRMRD-),complete remission with MRD low level(CRMRD-LL),and MRD relapse as an outcome definition in the recently published ELN MRD guidelines (4) (Figure 1).
However,the assessment of MRD in AML currently remains sophisticated,incomplete,and inconsistent.MRD detection in subtypes of AML is still a relatively new and inadequately defined area,primarily owing to the distinctive biological characteristics of AML and the wide array of available of technologies (5).AML evolved dynamically over time,influenced by therapeutic interventions.Furthermore,the optimal time point for MRD detection has not been nailed down.It is an urgent need to transition from a generalized treatment approach(“one size fits all”) to an individualized and stratified treatment model based on the MRD assessment (6).There is a lack of consensus regarding MRD-based individualstratified chemotherapy treatment and the pre-treatment considerations for bone marrow (BM) transplantation in AML.
In this review,we aim to provide an overview of the current status of MRD-based clinical decision-making and the prognostic value of MRD in AML.Additionally,we will discuss the selection of optimal time points for MRD detection,the assessment technology,and sample resources according to the ELN 2022 risk category.We intend that this review will contribute to the establishment of best practices and facilitate further advancements in the utilization of MRD for the benefit of AML patients in the future.
MRD technologies
Currently,the two most extensively evaluated methodologies are multiparameter flow cytometry-based MRD (MFC-MRD) and molecular MRD (Mol-MRD)assessed by quantitative polymerase chain reaction (qPCR),droplet digital PCR (dPCR),and next-generation sequencing (NGS) (4,7).
MFC
MFC is a widely utilized method for MRD detection (8),and it is applicable in 90% of AML.Evaluation of residual leukemic stem cells (LSC) by MFC-MRD has been associated with a higher sensitivity and lower false negativity (9,10).LSC can be immunophenotypically defined as CD34+/CD38lowcells combined with an aberrant marker not present on normal HSCs (11).In addition,if AML belongs to monocytic,the biomarker should be detected as CD64,CD11b,and CD4 (1).
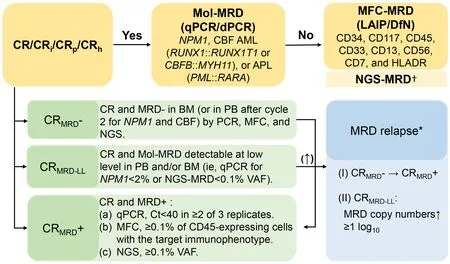
Figure 1 MRD response categories and detection methodology.MRD,measurable residual disease;CR,complete remission;qPCR,quantitative polymerase chain reaction;dPCR,droplet digital polymerase chain reaction;MFC-MRD,multiparameter flow cytometrybased MRD;LAIP,leukemia-associated immunophenotype;DfN,different from normal;NGS,next-generation sequencing;CRMRD+,MRD-positive complete remission;CRMRD-,MRD-negative complete remission;CRMRD-LL,complete remission with MRD low level.†,NGS-MRD is insufficient to stand alone as a MRD-technique,which is use to refine prognosis in addition to MFC (4);*,Results of (I) or(II) should be rapidly confirmed in a second consecutive sample,preferably from the bone marrow.
Currently,two major approaches,namely leukemiaassociated immunophenotype (LAIP) (12) and different from normal (DfN) (13),are commonly employed for routine MRD evaluation.Aberrant expression of immature blast markers includes cross-lineage expression of nonmyeloid CD markers on myeloid blasts (e.g.,CD7,CD56),asynchronous expression of mature CD markers on immature cells (e.g.,CD11b),lack or overexpression of CD markers (CD13,CD33,CD34).These leukemic blasts are known as the LAIP (12).The LAIP approach involves defining individual-specific surface marker patterns at the time of diagnosis and subsequently tracking these markers in subsequent assessments (1).However,the LAIPs that arise due to clonal evolution will be missed as the LAIPbased methods only measure dominant LAIPs detected at diagnosis (14).On the other hand,the DfN approach focuses on identifying aberrant surface marker profiles at follow-up time points.This approach can be applied even in cases where the diagnostic material is unavailable.By comparing the surface marker profiles of leukemic cells to those of normal cells,the DfN approach can detect immunophenotypic shifts indicative of MRD (8).Both LAIP and DfN approaches are valuable tools in MRD evaluation using MFC,providing important insights into the presence and dynamics of residual leukemic cells in AML patients.
qPCR
Around 60% of patients with AML are expected to possess a molecular marker suitable for validated qPCR-based MRD analysis.The count of molecular markers continues to rise as new molecular targets are identified (15).Around 15%-35% of patients with AML carry a gene fusion that can be monitored using qPCR,examples of which includeRUNX1::RUNX1T1,CBFB::MYH11,andPML::RARA(16).Fewer specific markers such asNPM1,WT1,andEVI1could be used as MRD markers by leukemia-specific PCR assays.The assays have been standardized and rely on assessing the relative expression of the mutation target in leukemic blasts compared to a reference housekeeping gene,commonlyABL1.The ELN consensus suggests that hematologists should use qPCR to monitorAMLgene mutations at the time of diagnosis,after completing two cycles of induction/consolidation therapy,and then follow-up at the end of treatment (24 months,every 3 months) (1).The response to MRD monitoring and the kinetics of relapse vary significantly depending on the specific target,which will be further explored in the subsequent part of this review.
dPCR
dPCR is a potentially powerful technique for monitoring MRD (17).The number of positive molecules for dPCR is determined by counting the number of successfully amplified fluorescent partitions with a sensitivity comparable to that of qPCR,but does not require quantification by reaction kinetics and plasmid standards with is required in qPCR (18).The dPCR has shown promising results in the detection of single nucleotide variants (SNVs),with a greater ability to distinguish mutant from normal alleles,and in the MRD in hematological malignancies using RNA-and DNA-based approaches (19).Leukemia-related abnormalities suitable for qPCR monitoring include gene mutation (e.g.,NPM1),gene fusions (e.g.,CBFB::MYH11,RUNX1::RUNX1T1,KMT2A::MLLT3,DEK::NUP214,andBCR::ABL1),andWT1expression (4).Validation is most robust forNPM1-mutated,as well asCBFB::MYH11andRUNX1::RUNX1T1gene fusion AML (20).
NGS
NGS (21) demonstrates the potential benefits of being highly sensitive,allowing for the simultaneous detection of multiple mutations at once,and has been used as a promising tool for sensitive MRD monitoring (16,22-24).DNA-based focused target enrichment strategies (gene panels) represent an appealing solution for NGS-MRD due to their broader applicability to a larger population of patients compared to single-gene molecular testing (25).For NGS-MRD,the 2021 ELN MRD Working Party recommend considering all detected mutations as potential MRD markers,and the germline mutations [variant allele frequency (VAF) of~50 in genesANKRD26,CEBPA,DDX41,ETV6,GATA2,RUNX1,andTP53] should be excluded as they are non-informative for MRD (4).
The strength of NGS lies in its capacity to deduce the population architecture of all disease clones and subclones,enabling the tracking of their evolving mutational dynamics through sequential measurements (26).By leveraging NGS,it becomes possible to ascertain the MRD burden for each disease clone and subclone,a capability beyond the limited assessment of specific clones achievable with current methods like qPCR or MFC (24).Despite the potential advantages,several obstacles must be addressed before the widespread implementation of NGS for MRD detection in AML and MDS,including confounding factors due to preleukemic mutations,time-consuming procedures,limited availability of the technology,high error rates,the complexity,and the current lack of standardization and uniform bioinformatics pipeline/platform for NGS-MRD variant calling (4).Therefore,for the time being,the NGS MRD is not considered a stand-alone technique for MRD assessment and is recommended to be used in combination with qPCR or MFC.
Frequency,source,and score system
Currently,the optimal time point for MRD assessment in AML lacks standardization.Different time points may provide different information.Early assessment (following induction and/or consolidation) can help determine remission status and evaluate disease response kinetics (27).Conversely,later assessments can be valuable for detecting potential relapse (16).
Frequency
The ELN consensus suggests that MRD monitor at the time after completing two cycles of induction/consolidation therapy,and then follow-up at the end of treatment (24 months,every 3 months).Making clinical decisions based on d 14 BM results may result in some patients receiving an unnecessary second induction treatment,with a higher risk of treatment-related mortality and increased utilization of hospital resources,including greater use of intravenous antimicrobials,longer hospital stays,and a greater need for blood products (28).Evaluations of BM on d 14 after “7+3”induction therapy cannot reliably predict the likelihood of achieving complete remission (CR).Some patients may achieve CR without requiring additional chemotherapy.Therefore,the decision to re-induce can be prudently delayed in many cases,particularly for those with favorable risk cytogenetics (29).In the context of transplantation,having persistent MRD-positive before the procedure,rather than MRD-positive at a single time point,has been shown to predict a poorer outcome in both human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT) and haploidentical hematopoietic stem cell transplantation (haplo-HSCT) settings (30).A 6-month landmark analysis indicated that achieving pretransplant MRD negative is clinically beneficial in terms of relapse,overall survival (OS),and leukaemia-free survival(LFS) at this time point (31).Patients with a positive MRD status before transplantation may have a higher risk of relapse and worse survival (32).
BM or peripheral blood (PB)
Mol-MRD is capable of monitoring lower levels of leukemia in PB and BM,with the feasibility of frequent sampling (33,34).Several studies have shown that MRD response in PB is a more accurate discriminator of prognosis in AML patients with specific recurrent fusion genes [such asRUNX1::RUNX1T1(34),CBFB::MYH11(35)or mutantNPM1(36)].MFC-MRD is mainly monitored by BM samples,and BM aspiration is an invasive procedure.In a cohort of children with CBF orKMT2A-rearranged AML,sustained MRD-positive and shifting from negative to positive MRD in PB were significantly associated with disease relapse.In contrast,MRD level in BM was not significantly associated with relapse risk.This study proposes an MRD increment of more than 5×10-4in both PB and BM as the definition of molecular relapse,as it always leads to hematologic relapse.And recommends PB collection every 4 weeks during follow-up as a source of qPCR MRD monitoring (37).In another study on early AML recurrence prediction,MFC data analysis of 205 paired BM and PB samples from 114 AML patients showed a significant correlation between PB and BM MRD(r=0.67,P<0.001),while the median PB MRD percentage was 4-5 times lower than BM MRD,accompanied by a significantly lower frequency of blasts(CD34+/CD117+/CD133+) in PB,suggesting that PB MRD detection is more specific than BM.The CR rate was 29% in PB MRD negative patients and 89% in PB MRDpositive patients (P<0.001) at 1 year after induction therapy(38).These results suggest that PB MRD may play an important role in the future assessment of MRD in AML.
MRD-based score system
In addition to MRD,other factors affect the prognosis of AML patients such as cytogenetic and molecular abnormalities,disease type,and transplantation status (39).A multifactorial correlation scoring system based on MRD will be the best tool for assessing risk before transplantation(40).One of the score systems is the AML-specific disease risk group (AML-DRG) developed to predict outcomes for AML patients post allogeneic hematopoietic cell transplantation (41). Hematopoietic cell transplantcomposite risk (HCT-CR) model by incorporating molecular data and MRD status of patients with AML.Both the AML-DRG and AML-HCT-CR demonstrated significantly superior discriminative capacity when compared to the disease risk index,ELN genetic risk model,and cytogenetic risk model.Prognostic models incorporating molecular data and MRD status allow better stratification and improved survival estimates of AML patients post-transplant (41).Another developed score system based on MRD status is a six-gene LSC score of prognostic value in pediatric AML,which predicted the outcome of transplant patients,suggesting it is a useful criterion for transplant referrals (42).
Intervention and treatment of MRD-positive patients
In AML,genetic risk defined according to ELN criteria and World Health Organization (WHO) criteria remains the most crucial factor in prognostication and decisionmaking processes (1,43). Despite the molecular heterogeneity of AML,treatment decisions still rely on a limited number of molecular genetic markers and morphology-based assessment of remission.Implementing a comprehensive risk category strategy,combining genetic features and MRD assessments,along with targeted chemotherapy and HSCT,has shown the potential to enhance outcomes in patients with AML (44).We summarized the MRD methods,sample sources,and evaluation frequency according to the risk category of ELN 2022 (Table 1).
Favorable risk
The presence of AML MRD has been associated with higher relapse rates.Identifying residual variants in the blood of adults with AML indicates an increased risk of relapse and poorer OS compared to those without these DNA variants (58).Sensitive detection of MRD could improve prognostication by identifying submicroscopic disease during remission (23).The ELN MRD Working Party has issued guidelines for the implementation of MRD in clinical practice (1).Numerous studies have demonstrated that monitoring MRD persistence can strongly predict the outcomes of AML patients and guide pre-emptive interventions (59-61),however,persistent lowlevel MRD may be associated with very low relapse risk in some favorable-risk patients,e.g.,NPM1mutations when measured at the end of consolidation chemotherapy (4).
RUNX1::RUNX1T1
The United Kingdom Medical Research Council AML-15 protocol study revealed that,following the first course of induction chemotherapy,a >3 log reduction inRUNX1::RUNX1T1transcripts,measured by quantitative RT-qPCR in BM of t(8;21) remission patients,was strongly associated with a remarkably low cumulative incidence of relapse (CIR) of only 4% (35).Another investigation indicated thatRUNX1::RUNX1T1transcripts with a <3 log reduction from diagnosis within 12 months and/or a <4 log reduction at ≥12 months after allogeneic hematopoietic stem cell transplantation (allo-HSCT) can effectively predict relapse in patients with AML (62).In contrast,the study from Peking University Institute of Hematology yielded different findings,indicating that MRD status after the second consolidation therapy might be the optimal timing for making treatment decisions.Furthermore,this MRD status served as an independent prognostic factor for relapse,disease-free survival (DFS),and OS (63).This distinct result compared with the AML-15 protocol may come from our different chemotherapy regimens (e.g.,low intense of induction daunorubicin dose,45 mg/m2).In terms of the monitoring interval,one of the studies highlights that conducting monitoring of PB every 3 months enables the prediction of hematological relapse and the identification of patients who may benefit from intervention therapy (64).A study conducted at the University of Texas MD Anderson Cancer Center suggests that HSCT may help overcome the unfavorable prognosis associated with CBF MRD-positive cases.The study found that MRD monitoring after transplantation allowed the identification of a subgroup of patients with a higher CIR.This assessment was carried out using qPCR on day +100 after HSCT (65).
CBFB::MYH11
AML carrying inv(16)/t(16;16),resulting in fusion transcriptCBFB::MYH11,belongs to the favorable-risk category according to 2022 ELN risk stratification (1).However,even though most patients achieve morphological CR after induction,about 30% of cases eventually relapse,and the 5-year OS rate is about 50%-60% (66),suggesting the need to detect more prognostic markers agents to optimize prognostic stratification-oriented therapy of these patients.After achieving remission,following the first course of induction chemotherapy,having a >10CBFB::MYH11copy number in PB among inv(16) patients emerged as the most valuable prognostic variable for relapse risk,as indicated by multivariate analysis (35).In another study involving BM(n=331) and/or PB (n=353) samples from 53 AML patients expressing theCBFB::MYH11fusion transcript,the results of qPCR MRD detection showed that BM samples were more sensitive during treatment,and the PB samples were equally informative with BM samples for follow-up.This study identified three-time points for clinical examination ofCBFB::MYH11fusion transcript as MRD to predict recurrence: 1) PCR negative in at least one BM sample during consolidation therapy;2) PCR negative in at least two BM or PB samples during consolidation therapy and early follow-up;and 3) conversion from PCR negative to positive with copy ratios >10 after consolidation therapy(67).The presence of MRD was associated with a poorer RFS in patients withCBFB::MYH1fusion transcript [hazard ratio (HR),4.55;95% confidence interval (95% CI),1.20-17.2;P=0.03] (68).MRD is important for assessing the prognosis of AML patients withCBFB::MYH11fusion transcript,and for patients identified at high risk of recurrence,other treatment strategies should be considered,including allo-HSCT during first remission,or adding gemtuzumab or oxazolicin to chemotherapy (69).
Nucleophosmin (NPM1) mutation
NPM1is the most commonly mutated gene in adult AML,found in approximately 25%-35% of patients (70).NPM1mutation was specifically observed at the time of relapse and proved to be a more reliable marker of disease status.Following the second chemotherapy cycle,a persistent presence ofNPM1-mutated transcripts in the blood was observed in 15% of the patients,and this was associated with a higher risk of relapse after a 3-year follow-up period,as well as a lower rate of survival compared to those without such transcripts.Notably,the presence ofNPM1-mutated MRD emerged as the sole independent prognostic factor for death according to the results of multivariate analysis (36).The presence ofNPM1-mutated MRD offered robust prognostic information that remained independent of other risk factors (36).The earlyNPM1-mutated PB-MRD demonstrates significant prognostic value,independent of the cytogenetic and molecular context.Furthermore,NPM1-mutated PB-MRD may serve as a predictive factor for determining the indication of allo-HSCT (71).A marked and highly significant contrast in OS after allo-HSCT was observed between patients who tested pre-transplant MRD-positive and MRD negative(with estimated 5-year OS rates of 40%vs.89%,respectively;P=0.007).Multivariable analyses focusing on time to relapse and OS indicated that pre-transplantNPM1MRD levels greater than 1% served as an independent prognostic factor for poorer survival after allo-HSCT.However,FLT3-ITD had no significant impact on the outcomes studied (72).Notably,the outcome of patients with pre-transplantNPM1MRD-positive >1% was as poor as that of patients transplanted with RD.Detecting positive mutatedNPM1MRD status using dPCR before allo-HSCT is associated with a poorer prognosis,regardless of other established prognostic markers.This holds even for patients undergoing non-myeloablative conditioning before transplantation (73).The NGS for Residual Variant Detection was utilized in a study involving 3,020 patients in their first CR from AML associated withFLT3,NPM1,IDH1,IDH2,and/orKITvariants who received their first allo-HSCT.The study revealed that the persistence ofFLT3internal tandem duplication orNPM1variants in the blood at an allele fraction of 0.01% or higher was associated with increased relapse rates and poorer survival when compared to those without these variants (23).
Through sequential monitoring of MRD,relapse was effectively predicted by an increase in the level ofNPM1-mutated transcripts,which emerged as the sole independent prognostic factor for death in multivariate analysis.Utilizing MFC-MRD can enhance outcome stratification by expanding the definition of partial response after the first induction,and it may also aid in identifyingNPM1-wt standard-risk patients with an unfavorable prognosis who could benefit from transplantation in the first CR (74).
Intermediate risk
Intermediate-risk cytogenetics represents a grey zone that requires other biomarkers for risk stratification.Multivariate analysis revealed age,MRD,and cytogenetics as independent variables (75).AML MRD importantly guided post-remission strategies in AML and should be incorporated into routine treatment (76).
FLT3-ITD
On multivariate analysis,immunophenotypic MRD at the end of induction andFLT3-ITD emerged as independent prognostic factors predictive of outcomes (76).Another study showed intermediate-risk cytogenetics and negativeFLT3-ITD patients with MRD levels ≥0.1% exhibited significantly lower 3-year event-free survival (EFS) and 3-year OS than those with MRD levels <0.1% (33.3%vs.83.3%,P=0.02 and 20.0%vs.76.9%,P=0.005,respectively)(77).This study suggested that MRD shows prognostic relevance in pediatric patients with intermediate-risk cytogenetics and negativeFLT3-ITD AML.A study from Peking University Institute of Hematology identified variables associated with CIR and survival in patients and selected adverse-risk variables that benefitted from a transplant.The study focuses on the cohort having normal cytogenetics and noNPM1mutation orFLT3-ITD,which takes about 25% of newly diagnosed AML.We evaluated 158 newly diagnosed adults with this genotype who achieved histological CR within two cycles of induction therapy and were assigned to two post-remission strategies with and without an allo-HSCT.We found out that patients with ≥1 of adverse-risk variables benefitted from a transplant,whereas the others did not.The adverse-risk variables including wild-type or mono-allelic mutatedCEBPA,mutatedNRAS,mutatedCSF3R,and MRDpositive after the second consolidation cycle were independently correlated with higher CIR (27).Another randomized,placebo-controlled,phase 3 QUAZAR AML-001 trial evaluated the survival outcomes in subgroups of patients defined byNPM1andFLT3mutation status at AML diagnosis,the results showed that oral azacitidine significantly improved survival independent ofNPM1orFLT3mutation status,cytogenetic risk,or post-intensive chemotherapy MRD status (78).
MLLT3::KMT2A
In mixed-lineage leukemia (MLL) gene-related gene fusion,such asKMT2A::MLLT3,researchers found that a rapid reduction of MRD level to ≤10-4appears to be a prerequisite for better OS and EFS during the treatment of AML.The MRD levels were basically in line with the clinical outcomes and may be of great importance in guiding early allo-HSCT treatment (79).In another study conducted on MLL-rearranged AML patients,transplant age (>45 years),allo-HSCT in the non-CR state,and MRD-positive before transplantation were negative prognostic factors in allo-HSCT (80).
Adverse risk
However,not all MRD detection in adverse-risk AML is associated with relapse.One of the famous studies from The New England Medicine found the detection of persistent DTA mutations (i.e.,mutations inDNMT3A,TET2andASXL1),which are often present in persons with age-related clonal hematopoiesis,was not correlated with an increased relapse rate (81).Patients who had active leukemia or MRD before transplantation had a worse outcome.Delayed hematologic recovery after induction or consolidation chemotherapy,adverse-risk AML genetics,donor-recipient HLA-DRβ3/4/5-DP mismatches,and history of cardiovascular diseases were also correlated with survival in multivariate analyses.The 57 MRD-negative patients with few other adverse prognostic factors had an excellent outcome,whereas the 58 patients with detectable leukemia and more than 1 other additional factor fared poorly (82).In patients with AML undergoing HSCT,the pre-transplant persistence of NGS-defined MRD imparts a significant,sensitive,strong,and independent increased risk for subsequent leukemic relapse and death.Given that NGS can simultaneously detect multiple leukemiaassociated mutations,it can be used in the majority of AML patients to monitor disease burdens and inform treatment decisions (83).
They had only one child, a son, who was their pride and joy, and for his sake they were ready to work hard all day long, and never felt tired or discontented with their lot
Other genes (Wilms tumor 1)
The role of cytogenetic karyotype-based risk systems has been challenged and complemented by MRD assessment.
Numerous studies have identifiedWT1overexpression as a prognostic factor that is independent of the wellestablished risk system.These findings suggest thatWT1overexpression could serve as an additional MRD tool for risk stratification in patients currently classified in CR (84).Evidence from recent studies shows serial monitoringWT1in PB predicted hematological relapse in allo-HSCT (85).TheWT1expression cutoff threshold in PB was >500 copies/µg RNA.The monitor time point before and at d 60 after allo-HSCT correlated with worse OS,additionally,WT1≥50 copies/µg RNA at d 30 were correlated with CIR(85).The pre-allo-HSCT MRD-WT1stratification in AML is a valuable tool to identify patients at high risk of post-HSCT relapse and can influence conditioning regimen intensification and/or post-HSCT pre-emptive strategies(86).In patients after allo-HSCT for AML,PB MRD(WT1) monitoring 3 months identified very adverse-risk patients,who could benefit from early pre-emptive treatment,and those who do not need such an intervention(87).Whereas,some studies showed the contrary results.Chemotherapy intervention though induced a decrease in theWT1expression level,no difference was seen between the survivals ofWT1-positive subgroups that expressed moderate or high levels ofWT1mRNA.A 1-log decrease inWT1expression without becoming negative did not affect prognosis,either (88).
The usage of theWT1expression level as an additional marker for more precise risk stratifications of AML patients could lead to more adapted,personalized treatment protocols (89).Some studies use FCM or specific cytogenetic types as co-predictors of the outcome.For instance,theWT1MRD stratification inFLT3-positive AML is a valuable tool with which to identify patients who are at adverse risk of relapse and that could be considered from post-allo-HSCT prophylaxis withFLT3inhibitors or other strategies (donor lymphocyte infusion,tapering of immunosuppression,azacitidine) (90).CombiningWT1gene expression status withNPM1andFLT3-ITD mutational status,the tumor behavior of intermediate patients (FLT3-ITD(-)/NPM1(-) double negative) withWT1(high) status is almost the same as the tumor behavior of the adverse risk group.Larger-scale randomized studies are warranted to define a cut-off value and monitor time point forWT1-positivity,rather than just using logarithmic figures of changes in gene expression,which might have prognostic use in post-induction AML or allo-HSCT patients (88,91).
MRD-driven individualized transplantation
The achievement in the HSCT provides choices for AML patients under distinct or similar disease statuses.MRD is a useful tool in the HSCT strategy selection as MRD status before transplantation was an independent predictor for both OS and RFS (12).And the clearance of MRD before HSCT could improve the prognosis after transplantation(92).The flowchart of MRD-driven individualized therapy is summarized inFigure 2.
Post-consolidation transplantation
Haplo-HSCT has been a choice for patients who lack HLA-matched donors (93).Autologous hematopoietic stem cell transplant (auto-HSCT) benefits from lower nonrelapse mortality,the absence of graft-versus-host disease(GVHD),and a better quality of life for long-term survivors (94). Several studies showed that MRD distinguished the intermediate risk subgroups benefit from auto-HSCT or allo-HSCT (95).The result of the GIMEMA AML1310 trial,patients of intermediate-risk category according to National Comprehensive Cancer Network (NCCN) criteria (NCCN-IR) can avoid allo-HSCT if MRD is not measurable;if MRD is positive,allo-HSCT can prolong OS and raise the DFS duration to the level of favorable-risk (NCCN-FR) patients or poor-risk(NCCN-PR) (96).Similar results were found in another research that investigated 172 AML patients with favorable or intermediate risk after one course of consolidation (95).Given the above research,in the AML patients with NCCN-IR and MRD-positive state,allo-HSCT is a better choice as it prolongs OS and DFS to equal those of the favorable-risk category. Notably,among patients of favorable-risk or intermediate-risk group who received chemotherapy only,those individual-MRD negative status presented favorable survival,which was comparable with that of the patients who accepted HSCT (12).This is to say not all patients need a choice between auto-HSCT and allo-HSCT,for some intermediate-risk MRD-negative patients receiving chemotherapy only may not be a bad path.MRD status can identify patients with significantly different outcomes in those with favorable-risk or intermediate-risk profiles.
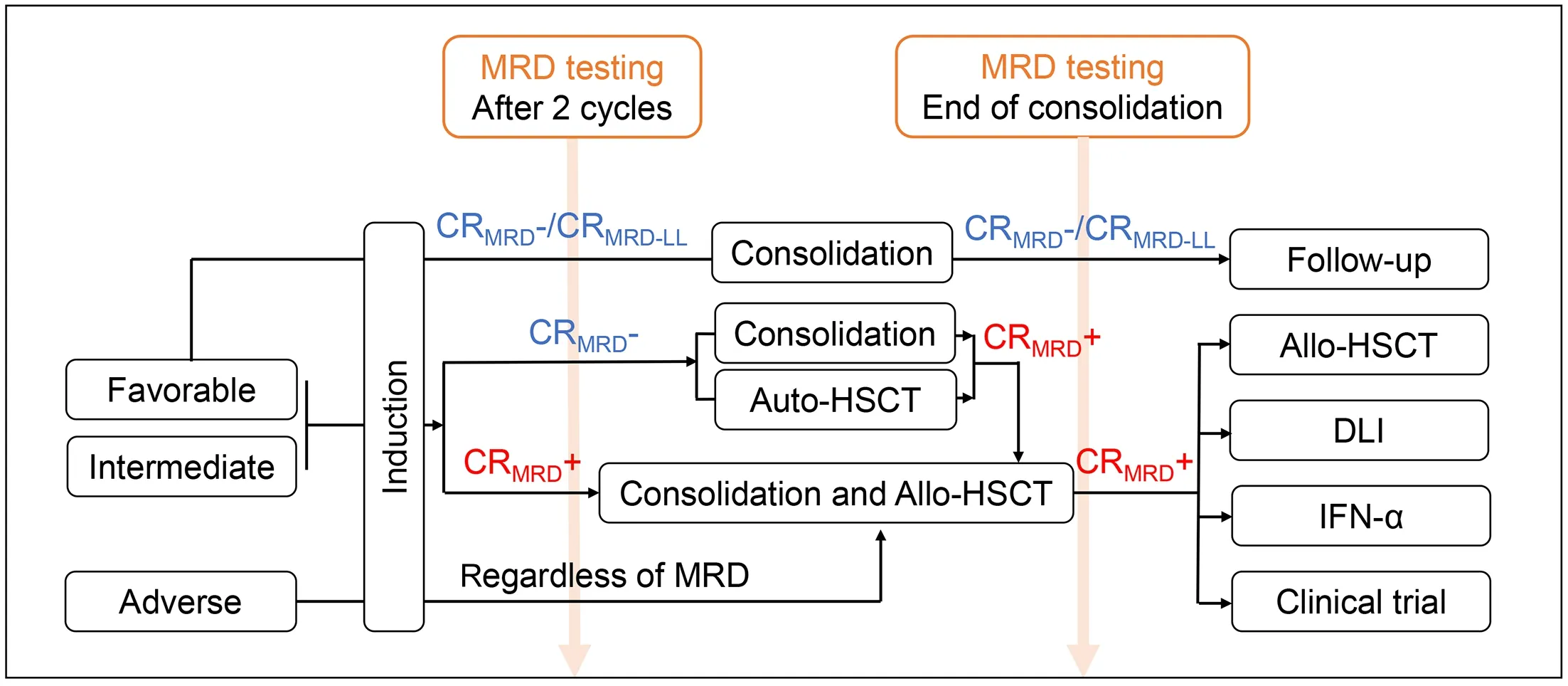
Figure 2 Flowchart of time point for pre-emptive interventions and intervention paths.MRD,measurable residual disease;CR,complete remission;allo-HSCT,allogeneic hematopoietic stem cell transplantation;auto-HSCT,autologous hematopoietic stem cell transplantation;IFN-α,interferon-α;CRMRD+,MRD-positive complete remission;CRMRD-,MRD-negative complete remission;CRMRD-LL,complete remission with MRD low level.
In terms of allo-HSCT,pre-transplant MRD also drove the transplantation subtype between haplo-HSCT and MSDT.In asserting superiority of HSCT,studies from our institution in either retrospective group or prospective group verified that for pre-transplantation MRD-positive cases,haplo-HSCT was associated with a low CIR and with better LFS and OS,suggesting a stronger anti-leukemia effect,compared to MSDT.Those who underwent MSDT had a higher CIR than those receiving haplo-HSCT (97).Another study focusing onFLT3-ITD pre-transplantation MRD-positive also concluded haplo-HSCT might overcome the negative impact of pre-MRD on patient outcomes compared to MSDT inconsistency with the above (98).For the subgroup of AML to hold a favorable cytogenetic subgroup both in NCCN guideline or ELN classification,disease relapse remains one of the most important causes leading to death,occurring in around one-third of the patients,allo-HSCT is needed as salvage treatment to improve OS (99,100).Studies from our institution provide the insight that patients who hold favorable-risk cytogenetics inv(16) with pre-transplantCBFB::MYH11MRD-positive were more suitable for haplo-HSCT compared to MSDT (101),which might overcome the negative impact of pre-MRD on patient outcomes (101).
Another study presented the dilemma of choosing between MUDT and CBT.This retrospective analysis of 582 consecutive patients who received initial myeloablative hematopoietic cell transplant found that in patients with pre-transplantation MRD,the probability of OS was at least as favorable with CBT as with MUDT and significantly higher than with HLA-mismatched unrelated donor.Furthermore,the probability of relapse was lower in the CBT group than in either MUDT or HLAmismatched unrelated donors (102).
The combination of cytogenetic classification and MRD monitoring correlated with the outcome of transplantation might help the choice among the different types of HSCT for patients with primary AML.
Pre-transplant consolidation
Multivariate analysis indicated that pre-transplant consolidation and the consolidation courses (<2vs.≥2 courses) did not have an impact on allo-HSCT outcomes.Allo-HSCT for candidate patients without further consolidation when first CR and negative MRD was attained was feasible (103).In AML patients undergoing allo-HSCT in MRD negative with first CR,a history of prior consolidation was associated with favorable outcomes.If the path to pre-HSCT MRD negativity includes consolidation,it may identify patients with improved prognosis following HSCT in MRD negative state.These results warrant validation in larger cohorts (104).
Pretreatment conditioning
Accumulative evidence provides support that MRD predicts the clinical outcomes before transplantation (105).MRD state before the transplantation consistently identifies patients at a higher risk of relapse and shorter survival relative to negative MRD (106).Therefore,pretreatment conditioning should be considered a strategy to improve the outcome of MRD-positive patients (107).Large retrospective analyses have verified that reduced transplant-related mortality (TRM) in reduced-intensity conditioning (RIC) is counterbalanced by increased relapse rates (108).Still,a few of the clinical trials failed to establish the preference for myeloablative conditioning(MAC) or RIC in MRD-positive patients (106,109-111).
Emerging data proved the benefit of MAC over RIC for those who tested MRD-positive by genomic sequencing.This study provides evidence that RIC was significantly associated with increased relapse,decreased relapse-free survival,and decreased OS compared with MAC before allo-HCT (112).The consistent results could be seen in another retrospective study,which suggested AML patients with first CR and positive MRD should preferentially be offered MAC allo-HCT (113).If fit enough,such patients should be considered candidates for a MAC regimen or an early taper of post-transplant immunosuppression (112).Given that MAC is not a viable conditioning option for many patients with AML because of age or comorbidity,the MRD-based new treatment approaches after transplantation should be well-established.
Researchers have shown that anti-thymocyte globulin(ATG) can significantly reduce the risk of both acute and chronic GVHD in the setting of MAC as well as RIC conditioning (114,115).In MRD-positive patients,ATG was associated with a lower incidence of chronic GVHD(total,HR=0.56,P=0.03;extensive,HR=0.40,P=0.01),without an impact on other allo-HCT outcome parameters,including relapse index (HR=1.02,P=0.92).The use of ATG was associated with reduced risk for GVHD.ATG did not increase the relapse index,even in high-risk AML patients who were MRD-positive before allo-HCT (116).
MRD-triggered post-HSCT maintenance therapy
Maintenance therapy provides a method to prevent relapse after HSCT,especially for patients with NCCN-PR.However,before initiating maintenance therapy,new diagnostics are needed to identify patients destined to experience relapse.Personalized treatment options are required for AML with different molecular characteristics,along with determining the optimal time to start post-HCT.In this clinical context,MRD assessment,based on continuous tracking of molecular mutations,plays a crucial role as a significant identifier,molecular characterizer,and time point selector that triggers pre-emptive strategies to avoid worse outcomes after relapse (117).
Based on the MRD-triggered maintenance therapy,the ideal maintenance agent is well tolerated,received only by those patients who would otherwise relapse,and can modify the course of the disease to achieve sustained remission.In recent decades,plenty of newly discovered AML maintenance therapies have been used in daily clinical practice (118),such as epigenetic modifiers(azacitidine) (119,120),tyrosine kinase inhibitors(sorafenib) (121),cellular therapies (122,123).These novel approaches improved in solving the shortage of maintenance therapies,for instance,off-target toxicity of effective agents,cytopenia,and additional immunosuppression.
Epigenetic modifiers
Relapse is the leading cause of treatment failure for myeloid malignancies treated with allo-HSCT.Treatment options are very limited and the use of azacitidine is one of the available options (124).Applying azacitidine as therapy for ongoing relapse after allo-HSCT may lead to stable disease (49) and allow for better performance of the second allo-HSCT.For CBF MRD-positive patients who would not have transplantation,maintenance therapy with hypomethylating agents (HMA),including decitabine(DAC) and azacitidine (AZA) after induction/consolidation,would be recommended for MRD elimination and relapsefree survival prolong within the first two cycles of HMA therapy (125).
Target therapy
Around one-third of AML patients carry mutations in theFLT3gene.The incorporation ofFLT3inhibitors,such as sorafenib,and the implementation of MRD monitoring have become important components of treatment and evaluation approaches for AML patients,leading to improved prognosis for these individuals (126,127).
Donor lymphocyte infusion
A significant number of studies have shown that preemptive administration of donor lymphocyte infusion(DLI) based on MRD and chimerism monitoring,as well as prophylactic DLI in AML patients at high risk of relapse is effective in preventing relapse (128-130).In addition,genetically engineered T cells,both chimeric antigen receptor (CAR) and T cell receptor (TCR) genetically modified T cells have the potential to enhance antitumor immunity,augment vaccine efficacy,and limit graft-versushost disease after allo-HSCT (122).Not only limited to T cells,researchers also found the allogeneic NK cell infusion combined with the consolidation of chemotherapy contributed to the further remission of AML patients and the reduction of long-term recurrence (131).
IFN-α
Salvage IFN-α treatment following DLI showed significantly better results compared to persistent MRD without IFN-α treatment. Additionally,the clinical outcomes between salvage DLI and IFN-α treatment groups were similar.Therefore,salvage IFN-α treatment could potentially enhance the outcomes of patients with unsatisfactory responses to MRD-directed DLI and may serve as a viable salvage treatment option after allo-HSCT(132).MRD-directed IFN-α treatment is effective for patients with t(8;21) AML who were MRD-positive after allo-HSCT (133). The ITI regimen (IFN-α-1b,interleukin-2 and thalidomide) can reduce the MRD level of patients with AML who are in hematologic remission but MRD-positive. The therapeutic effect could be improved by a higher dose administration of the ITI regimen,and the therapeutic effect may be negatively correlated with MRD level before treatment (134).
MRD-directed IFN-α treatment (i.e.,pre-emptive IFNα treatment) can eliminate MRD,effectively prevent relapse,and significantly improve long-term survival in patients with AML after allo-HSCT (135).MRD-positive status detected by quantitative polymerase chain reaction and MFC before IFN-α treatment,high-risk disease risk index before allo-HSCT,and receiving an identical sibling donor HSCT were all associated with an increased risk of relapse and reduced leukemia-free survival (136).
Conclusions
MRD serves as a valuable prognostic indicator in AML,playing a crucial role in evaluation of CR,early detection of relapse,and enabling pre-emptive intervention.In this review,we have compiled ample MRD-related information for each risk category-related biomarker.Additionally,we have presented a flowchart summarizing the definition of MRD and the changes in its status between each definition.Our review aims to empower clinical physicians in their selection of MRD technology,time point determination,and reservation of sample resources.By doing so,it will expedite the integration of MRD assessment as a powerful prognosticator and a significant tool in the management of AML.
Acknowledgements
This study was supported by Beijing Nova Program of Science and Technology (No. Z211100002121058);National Natural Science Foundation of China (No.82100168);Peking University People’s Hospital Research and Development Funds (No.RS2020-03,RDY2020-29);Peking University Medicine Fund of Fostering Young Scholars’ Scientific &Technological Innovation and “the Fundamental Research Funds for the Central Universities”(No.BMU2021PYB005).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2023年5期
Chinese Journal of Cancer Research2023年5期
- Chinese Journal of Cancer Research的其它文章
- Epidemiology of pancreatic cancer: New version,new vision
- Inspired by novel radiopharmaceuticals: Rush hour of nuclear medicine
- Update of latest data for combined therapy for esophageal cancer using radiotherapy and immunotherapy: A focus on efficacy,safety,and biomarkers
- Genetic susceptibility loci of lung cancer are associated with malignant risk of pulmonary nodules and improve malignancy diagnosis based on CEA levels
- A novel multimodal prediction model based on DNA methylation biomarkers and low-dose computed tomography images for identifying early-stage lung cancer
- Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib
