Epidemiology of pancreatic cancer: New version,new vision
Wenhao Luo,Jun Wang,Hao Chen,Liyuan Ye,Jiangdong Qiu,Yueze Liu,Ruobing Wang,Guihu Weng,Tao Liu,Dan Su,Jinxin Tao,Chen Ding,Lei You,Taiping Zhang
Department of General Surgery,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China
Abstract Pancreatic cancer (PC) is a devastating malignancy with an extremely high mortality rate and poses significant challenges to healthcare systems worldwide.The prevalence of PC risk factors spiked over the years,leading to a global increase in PC incidence rates.The contribution of different risk factors,however,varied from region to region due to genetic predisposition,environmental,social,and political factors underlying disease prevalence in addition to public health strategies.This comprehensive review aims to provide a thorough analysis of the epidemiology of PC,discussing its incidence,risk factors,screening strategies and socioeconomic burden.We compiled a wide range of seminal studies as well as epidemiological investigations to serve this review as a comprehensive guide for researchers,healthcare professionals,and policymakers keen for a more profound understanding of PC epidemiology.This review highlights the essentiality of persistent research efforts,interdisciplinary collaboration,and public health initiatives to address the expanding burden of this malignancy.
Keywords: Pancreatic cancer;novel data;epidemiology;risk factors;future direction
Introduction
Pancreatic cancer (PC) is a formidable adversary within the field of oncology,characterized by its aggressive nature,late-stage diagnosis,and dismal prognosis (1,2).Its impact on public health and the healthcare system is indeed profound.Not only does PC incur a significant direct cost from highly specialized treatment due to late-stage diagnosis,but also an even higher societal economic cost due to the loss of workforce (3).Therefore,it is imperative to explore extensively PC’s epidemiology features before implementing prevention measures as well as early screening strategies for high-risk populations.
PC,primarily represented by pancreatic ductal adenocarcinoma (PDAC),had a five-year survival rate of approximately 12%,having one of the worst prognoses among all cancer types (4,5).Despite its relatively low incidence rate,PC was responsible for a disproportionately large number of cancer-related deaths,with a mortality/incidence ratio of 94% (6).The epidemiology of PC encompassed a wide range of factors,the most fundamental including incidence,risk factors,and prognosis,which were significantly influenced by demographic as well as geographic disparities.Interestingly,there was a positive correlation between PC incidence rate and the Human Development Index according to GLOBOCAN 2020 (7),where higher incidence rates of PC were observed in North America,Europe,and parts of Asia (8).Within these regions,incidence rates were further influenced on a more individual spectrum including non-modifiable factors such as age,gender,and ethnicity,as well as modifiable factors such as smoking,diet and exercise.
Identifying predictive risk factors associated with PC is pivotal in the implementation of cost-effective prevention and early screening strategies in order to lower not only the physiological stress on individual PC patients but also the burden of public health revenues.Among non-modifiable factors,genetic predisposition played an important role,where individuals with certain hereditary inflammatory and neoplasm syndromes were placed under an elevated risk (9).A large amount of additional research as well as technical advancement needs to be accomplished for more elaborated genetic mechanisms underlying PC development among the pre-disposed population.Currently,screening and manipulating non-modifiable factors are not the most costeffective ways to significantly reduce PC incidence.Modifiable factors,however,should be placed a higher priority when devising public health initiatives.Smoking,for instance,had long been identified as a major modifiable risk factor accounting for a substantial proportion of PC cases,in addition to other cancer cases (10).Yet,comprehensive smoking cessation and outreach programs integrated into the primary care health system had shown satisfying results in both cessation rate and costeffectiveness (11).
A productive early screening system for PC is necessary for monitoring as well as reducing the incidence rate of PC and requires at least two components,technical advancements for the detection of predictive biomarkers through the least invasive measure and a comprehensive risk assessment model that integrates patient history,current symptoms,genetic susceptibility,etc.Currently,locating predictive biomarkers for PC early detection remained one of the foremost challenges (12),but an ardent quest for accessible and accurate biomarkers in addition to innovative diagnostic tools is ongoing.Advancements in lipid biopsy,for example,allowed the detection of cellular and sub-cellular components through non-invasive measures,including circulating tumor cells,circulating tumor DNA,exosomes,etc (13).This technology served as a pivotal role in the understanding of communicative information ongoing in the early stages of PC.While the field of molecular oncology continues its exploration for more biomarkers and reducing their detection cost,ongoing research also unveiled the significance of a series of emerging risk factors easily accessible through medical records,including chronic pancreatitis,type 2 diabetes,and dietary factors (10).Patterns in patients’ medical history require an extensive amount of analysis and are easily precepted by human eyes.Artificial intelligence analysis such as deep learning,is applied more frequently in the early diagnosis and evaluation of PC.Plasticity in artificial intelligence models could integrate not only information from various biomarkers but also imaging data and past medical records catering to different data collections (14).Collectively,the above-mentioned factors underscored the multifaceted nature of PC etiology and may contribute to more accurate risk prediction models for PC.
The latest release of epidemiological data (1) provided an incentive for an updated evaluation of the epidemiology and mortality trends of PC.This review,therefore,strives to serve as a valuable resource for researchers,healthcare professionals,and policymakers by highlighting the essentiality of persistent research efforts,interdisciplinary collaboration,and public health initiatives to address the growing burden of PC.
Global PC incidence
It is undebatable that the global incidence of PC is increasing.The age-standardized incidence rate increased from 5.0 per 100,000 person-years in 1990 to 5.7 per 100,000 person-years in 2017,and a 2.3 times increase in number of deaths for both sexes within the duration (15).Population aged ≥70 years and between 50-69 years contributed most significantly to arising new cases each year (16).Globocan 2018 reported 458,918 registered PC cases (17) and 495,773 cases in 2020,with a male-to-female ratio of 1.0:1.1.The highest incidence rate of 8.6/100,000 was reported in Western Europe while the lowest 1.2/100,000 in South-Central Asia.Interestingly,migration might exert an effect on cancer risk,which was observed among people with similar genetic backgrounds but who migrated to countries of various development stages(18,19).Residential environment,access to medical care,genetic susceptibility,life expectancy,and dietary habits could all be referenced to the resulted incidence disparity among age groups,genders,and regions.Therefore,an indepth analysis may help us assign appropriate weights to each contributor and tailor prevention and screening efforts toward specific populations.
Regional disparities
North America and Europe
These regions consistently reported some of the highest PC incidence rates (20).North American areas,including the United States,Canada,and parts of Western Europe have particularly elevated rates compared to the global average.According to the latest Globocan data,the global incidence rate of PC is 4.9/100,000 while Western Europe had an incidence rate of 8.6/100,000,Northern America 8.0/100,000,and the pan-European region well above the global average (7).These disparities may be attributed to a combination of lifestyle factors,genetic predisposition,and variations in healthcare infrastructure.
Asia
While overall incidence rates of PC were lower in Asia than in Western countries,specific areas within Asia,such as China,reported relatively higher rates. While PC incidences in Eastern Asia and Western Asia were slightly above the global average,incidences in South-Eastern Asia and South-Central Asia were far below.In China,the incidence and mortality of PC were comparatively high in East China compared with Central or Western China,with a positive correlation between the rate of incidence with urbanization stages (21).
Sub-Saharan Africa
Incidence rates in sub-Saharan Africa were generally lower compared to other regions. However,limited data availability and underreporting may have contributed to this observation,indicating an urgent need for improved cancer registries in this region (20).Additionally,the risk of developing PC is highly correlated with age increment.The below-average incidence rates seen among these regions could also be explained by less-than-average life expectancy (16).
Urban-rural disparities
Disparities in PC incidence also manifested among urban and rural residents.Higher PC incidence rate in urban areas could be explained by several factors: Increased prevalence of tobacco use and obesity (22) in addition to increased concentration of healthcare centers and specialists that led to higher rates of diagnosis.Conversely,lower incidence rates reported from rural regions could be due to limited healthcare access,lower population density,and different lifestyle patterns (20).China witnessed a measurable increase in PC incidence rate from 1990 to 2019,paralleled by China’s economic development surge.A rise in the prevalence of overweight,diabetes,and smoking,the three major contributors to PC development,was also recorded during the three-decade period (23).Collectively,less healthy social and natural environments in urban areas might explain the incidence disparity.
In summary,the geographic variations in PC incidence reflected a complex interplay of genetic,environmental,and lifestyle factors.Recognizing and addressing these variations is vital for tailoring effective prevention and early detection efforts.
Morbidity and mortality
Mortality of PC was 4.5/100,000,ranking the 9thhighest mortality and 7thhighest number of deaths among other cancers in 2020 (7).According to the latest data,there was a steady increase in the estimated incidence and death cases of PC in the past 8 years (Table 1).
The morality of PC was not evenly distributed worldwide,and counterintuitively,higher-income regions,such as North America and Europe,reported a greater mortality,whereas lower-income regions often exhibited lower rates (20).The age-standardized death rate of PC was the highest in the high-income super-region from 1990to 2017,in Greenland and Uruguay,and the lowest in Bangladesh (15).Despite the variation in mortality rates from 7.8/100,000 in Western Europe to 1.1/100,000 in South-Central Asia,the incidence-to-mortality ratio differed little across developed and developing countries(7),depicting a devastating image of this malignancy.A substantial proportion of individuals succumb to the disease within a brief period after the initial diagnosis of PC (22).PC was shown to have a mortality-incidence ratio close to 1 and was responsible for at least 331,000 deaths per year in 2015 and 505,500 in 2023,a increase of 53% (8).However,the gradual increase in the 5-year survival rate from 7% in 2015 to 12% in 2023 needed to be recognized according to Cancer Statistics.Many factors could account for this promising improvement in prognosis: advancement in imaging technology such as magnet resonance imaging(MRI) and positron emission tomography-computed tomography (PET-CT),effective public health education programs,the discovery of new treatment strategies,etc.

Table 1 Numbers of new cancer cases,deaths and survival in the United States
A better understanding of agonizing as well as antagonizing factors to PC’s prevalence would aid in reducing the cost of PC,which appears in two ways: direct cost and indirect cost.According to Ciporaet al.,direct costs of PC were composed of surgical treatment,chemotherapy,adjuvant &neoadjuvant treatment,and supportive &palliative care where indirect costs were estimated by the loss of workforce from patients and their caregivers (1).A Swedish study on the economic burden of PC showed that as much as 26 million EUR estimated direct cost of PC,an estimated indirect cost was 99 million EUR,constituting 79% of the total cost (24).
The globally increasing incidence rate of PC poses a remarkable challenge to the overall security of health and the economy.It is therefore of absolute urgency to identify risk factors for PC and continue monitoring population changes in morbidity,mortality,and survival of PC.
Risk factors
A complex interplay of risk factors contributes to PC etiology and understanding these factors is pivotal for implementing prevention measures,early detection,and risk assessment algorithms (Figure 1).
Modifiable factors
Smoking
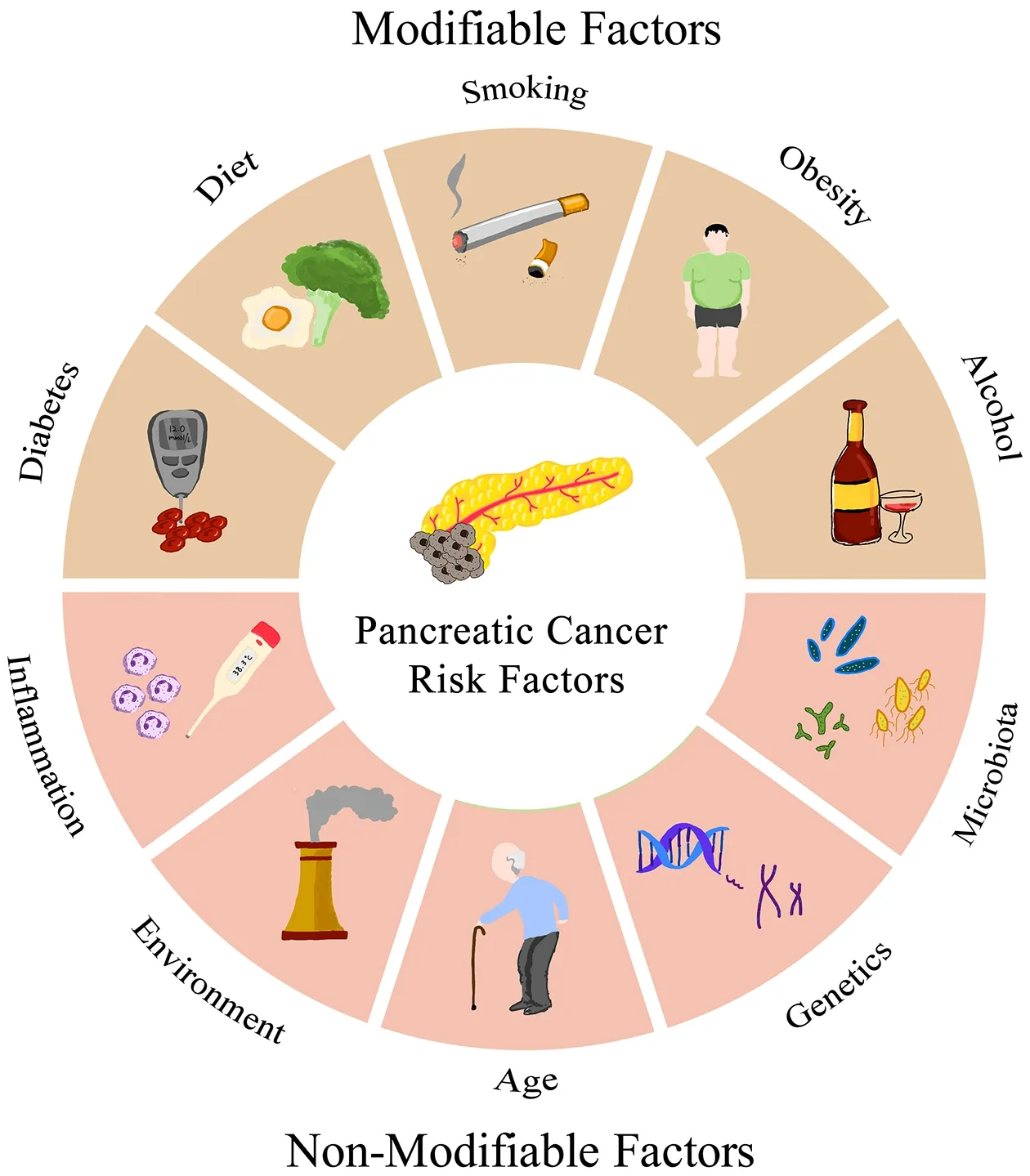
Figure 1 Risk factors of pancreatic cancer patients.
Cigarette smoking remained one of the most potent and modifiable risk factors for PC (25).And 25.9% of PC deaths in males and 16.1% in females can be accounted for by smoking (15).Interestingly,fluctuation in PC incidence rate could even reflect the smoking epidemic at various locations and times,where the incidence and mortality increased with the growth of cigarette consumption (26).Extensive research has reinforced the unnegligible association between cigarette smoking and increased PC risk.Cigarette smoke contains a cocktail of carcinogens,which,when inhaled and absorbed through lung capillaries,could travel through the blood and accumulate in the pancreas to induce malignancy.Recent studies have not only reasserted this association but unveiled the mechanisms underlying tobacco exposure and pancreatic carcinogenesis (27).Yuanet al.provided compelling evidence on the impact of cigarette smoking on PC survival,where the survival rate among PC patients who smoked was substantially reduced (25). Variants of carcinogen metabolism genes were independently associated with PC risk and may modify the risk posed by smoking (28).One possible explanation may be the inflammatory response caused by tobacco involved in carcinogenesis.
As one of the primary risk factors for PC,a reduction in tobacco consumption could obtain far-reaching effects on PC incidence,prevalence,and mortality.Luckily,costeffective smoking cessation programs could be implemented with acceptable costs.Mundtet al.(11)evaluated the cost-effectiveness of a tobacco cessation program integrating electronic health records and advocation of smoking cessation specialists.Collaborated efforts from outreach,medication,and counseling reached an 8.7% cessation rate while within the cost-effectiveness threshold (11).The rapid,effective,and cheap features of smoking cessation programs deserve more attention and funding from the public revenue to help people quit smoking.
Obesity and metabolic factors
Obesity and overweight,characterized by excessive adipose tissue accumulation,have emerged as a growing concern contributing to PC risk. Recent epidemiological investigations have highlighted the pivotal role of obesity and metabolic syndrome in the development of this malignancy. Elevated body mass index (BMI) and abdominal adiposity have been consistently associated with an elevated risk of PC,where a BMI≥25 kg/m2is considered overweight and a BMI≥30 kg/m2is considered obese.In 2020,Steeleet al.published a study in the Morbidity and Mortality Weekly Report and analyzed the association between cancer incidence and body weight in the United States,highlighting the substantial impact of obesity on PC incidence and the urgency of implementing strategies for obesity prevention and management (29).Increased weight or BMI has been shown to increase the risk of PC.After other risk factors such as age,smoking,diabetes,and so on,were calibrated,patients with a BMI>30 kg/m2showed a 1.72-fold increase in the relative risk of PC compared to individuals with a BMI<23 kg/m2(30).Weight gain after the age of 50 years was especially associated with an increased risk of PC (31).In a metaanalysis of 23 studies on BMI and PC,every 5 units of BMI increase corresponded to a 10% increase in PC risk (32).It can be safely inferred that maintaining a BMI of least less than 30 kg/m2among the general public and especially among the elderly population should reduce PC risk.
The expanding obesity epidemic is of great concern not only to public health but also to PC risk.According to WHO,in 2016 over 1.9 billion adults were overweight,among which 650 million were obese,consisting of 39%and 13% of the population respectively (33).The number of obesities is increasing but is preventable with an acceptable budget and should be of prioritized consideration in public health measures to diminish the incidence and mortality of PC.
Diabetes mellitus (DM)
Insulin resistance and type II DM (T2DM),often secondary to obesity,have been implicated as potential mediators to increase PC risk.It was demonstrated that diabetes,both type I and type II,was closely related to a 40% increased risk of PC (34).Researchers have identified the insulin-IGF-1 axis as a plausible mechanistic pathway stringing obesity to insulin resistance and then to pancreatic carcinogenesis (29).DM is indeed a risk factor for PC and can sometimes serve as an indicator for PC screening. Many patients with newly diagnosed PC reported a recent onset of DM or history of DM,suggesting that on one hand,DM could contribute to carcinogenesis of the pancreas,while on the other hand,pancreas malfunction due to its cancerous activity could present in the form of newly onset DM.Analysis of the Mayo Clinic patients’ data indicated that up to 1% of patients with newly diagnosed diabetes developed PC within 3 years of their initial DM diagnosis (35).A long history of T2DM also contributes to pancreatic carcinogenesis.It was shown that for a patient with a DM history exceeding 20 years,the risk of PC development could increase up to 30% compared to healthy individuals(36).A multiethnic cohort study conducted by Setiawanet al.confirmed the interacting relations between T2DM and PC development,where PC could present as newly onset DM and long-standing DM history could increase PC risk (37).Treatment of DM either through oral medication or insulin injections was shown to decrease PC risk,further testifying to the association of diabetes with PC (38).
Diabetes is a growing global health concern,especially among the Chinese population.Data collected in 2013 showed 35.7% of prediabetes prevalence and 10.9%diabetes prevalence in China,the largest epidemic in the world (39).The increasing prevalence of diabetes may be a contributor to the increasing age-adjusted incidence rates of PC.DM can be treated and well-managed with reasonable costs,and therefore its prevalence should be closely monitored and avidly controlled to further reduce its long-term effect leading to PC.
Dietary factors and lifestyle choices
Research interest in the impact of dietary and lifestyle factors on PC risk is rising since these factors could be easily modified in daily routine and could influence 30%-50% of PC development.The influential effect of dietary components on the risk of PC was observed (25).Red meat,especially when cooked quickly at high temperatures through grill,barbecue,and broil,was reported to increase the risk of PC (40) by about 48% (41).Ingestion of processed meat with a high content of nitroimines and fried food with high cholesterol also elevated PC risk (40). Apart from singular dietary component,dietary patterns,including the Mediterranean diet and consumption of fruits and vegetables,were investigated (25),where the Mediterranean dietary pattern was protective against PC (42),fruit,vegetables,and carbohydrate intake however,didn’t necessarily decrease the risk for PC (43,44).A meta-analysis conducted by Paluszkiewiczet al,however,suggested a 38% decreased risk in a diet enriched with vegetables and fruit intake (41).
Emerging evidence supported a dose-dependent association between increased PC risk and alcohol consumption.Nine or more alcoholic drinks per day led to a significantly increased risk [odds ratio (OR): 1.6;95%confidence interval (95% CI): 1.2-2.2] of PC compared to those consuming less than 1 drink per day and those who did not consume alcohol at all (45).An effective increase in PC risk could even be seen with more than three drinks/day (22).Another study showed a significant association between heavy alcohol use and PC but only apparent in males,not females (46).East Asians,such as the Chinese population,were especially vulnerable to the effect of alcohol on developing PC due to the prevalence of a reduced alcohol dehydrogenase activity and thus a decreased metabolism of alcohol (47).
Dietary factors presented a varying association with PC risk,but it could be safely suggested that a balanced meal with major contents of fruits and vegetables,minor contents of high-nitrite and high-fat food,and minimal intake of alcohol should maximumly reduce the risk of developing PC.
Non-modifiable factors
Age and gender
Individuals older than 65 years were at the highest risk of PC,and this group is expected to double in all regions due to population aging (48). Globally,age-standardized incidence rates of PC increased significantly from 1990 to 2017,and incidence rates for PC were higher among males than among females,particularly in the age group over the age of 75 years (15).About 49,000 people were diagnosed with PC in 2015 (49),and in 2020,the incidence rate of PCwas shown to be 11.7 per 100,000 persons (50),which could be due to a shift of population structure towards the elderly.
According to data released by Cancer Statistics in 2023,the estimated new cases of PC was 64,050,and the estimated death was 50,550 (1),a significant increase from 2015.A differential presentation between the two gender groups was also observed,where PC ranked 10thleading for males,8thfor females,and 4thfor new deaths in both males and females (1).In summary,old age is an unavoidable risk factor for PC and negatively affect males more than females.
Genetic predisposition and familial syndromes
Genetic factors play a non-negligible role in the risk of developing PC.A recent study explored the association between a family history of cancer and the risk of PC and found that a family history of PC in parents,siblings,and even children was associated with an increased risk of PC(51).A family history of PC was also clustered with the increased incidence of other cancers such as prostate cancer(51).A recent study indicated that individuals with a family history of PC among first-degree relatives had a nearly 7-fold increased risk of developing PC themselves (52).
Underlying genetic predisposition may be responsible.BRCA2andCDKN2Awere reported to account for the majority of mutations in familial PC (53).Moreover,research demonstrated an elevated risk of PC among individuals carrying pathogenicBRCA2compared to those without,andBRCA2mutation alone was significantly associated with a younger age at PC diagnosis (54).BRCA1,PALB2(encoding a binding partner ofBRCA2),the DNA repair geneATM,Lynch syndrome-related genes (MLH1,MSH2,MSH6,PMS2andEPCAM),Peutz-Jeghers syndrome-associated genes (STK11),and hereditary pancreatitis-related genes (PRSS1,CTFRandSPINK1) all shown a close association with an increased risk of PC (55).Moreover,mutations inKRAS,p53andSMAD4also account for the additional risk of PC.
Interestingly,blood types could also affect the risk of PC due to altered ABO glycosyltransferase activities (56).Blood group O showed a protective effect while about 15%-20% of all PC could be associated with non-O blood type (57,58).
As cheaper and more efficient sequencing technology continued to develop,a better mapping of the genetic predisposition in developing PC could be obtained to aid in screening procedures for susceptible individuals.
Chronic pancreatitis and inflammatory conditions
Chronic pancreatitis,characterized by persistent inflammation of the pancreas,stands as a recognized risk factor for PC,especially in the first few years.Timely diagnosis in addition to effective management of pancreatitis,however,could significantly mitigate the risk(59).The effect of chronic pancreatitis on developing PC seemed to dilute along with increased time from initial diagnosis.Pancreatitis patients within 1 year of diagnosis were associated with an OR of 21.35 (95% CI:12.03-37.86) to develop PC,and 2.71 (95% CI: 1.96-3.74)within 2 years (60).Despite the drop in OR from one year to two,these patients maintained a high relative hazard of 2.02 (95% CI: 1.57-2.61) when followed up beyond 5 years(61).It is worth noting that the significantly higher increased risk within the first year of diagnosis was not only due to inflammatory mediators but also the fact that PC patients could sometimes be misdiagnosed as chronic pancreatitis,resulting in a delay of up to 2 years of cancer diagnosis (59).Hence,patients newly diagnosed with chronic pancreatitis should be monitored more cautiously to avoid iatrogenic delay in the diagnosis and treatment of PC.
Apart from direct inflammation at the site of the pancreas,other inflammatory conditions induced by smoking,alcohol,diabetes,infection,microbiota,etc.could all potentially contribute to pancreatic carcinogenesis (62).
Microbiota
As an essential part of the digestive system,the pancreas’s status is tightly linked to the microbiota residing within the gasrtointestinal tracts,encompassing oral microbiota,gut microbiota and pancreatic microbiota.
A recent meta-analysis published in 2023 demonstrated a close association between PC and oral microbiota.Porphyromonas gingivalis (P.gingivalis) belonged to an oral bacteria strain found more commonly not only in PC patients compared to healthy volunteers (63) but also in pre-diagnostic blood samples of PC patients (64),suggesting P.gingivalis as a potential early diagnosis index for PC.Neisseria elongate and streptococcus mitis were two oral bacteria strains that presented a lower residence among PC patients and could be used collectively to distinguish health and PC subjects with high sensitivity as well as specificity (65).
A microbial profile analysis by Renet al.investigated gut microbes among Chinese PC patients and revealed a unique pattern in microbial diversity and composition among patients compared to controls.Despite a significant loss in diversity and probiotics,the gut environment in PC patients enriched pathogenic strains such as lipopolysaccharides-producing bacteria (66).In mouse strains engineered to develop PC,animals with intact gut microbes presented with poorer differentiated tumor cells and accelerated cancer progression compared to animals with depleted gut microbes (67).The regulating role of gut microbes in PC progression demonstrated by animal experiments could also be found in clinics where PC patients treated with antibiotics in combination with chemotherapy exhibited a better treatment outcome (68).
The pancreas itself is not a sterile organ but inhabited by numerous microbial strains.Lactobacillus,enterococcus faecalis,and escherichia coli were all found within pancreas tissues and presented distinctive patterns in healthy pancreas,pancreatitis and PC (62).
Oral,gut,and pancreas microbiota composition patterns all presented associations with PC,and pattern analysis of individual microbiomes might serve as a screening factor in the future.
Occupational and environmental exposures
Associations existed between occupational hazards,such as toxic chemical exposure,and the risk of developing PC(29). Compared to healthy individuals,PC patients reported more regular exposure to pesticides,asbestos,benzene and chlorinated hydrocarbons (69).A positive correlation was found between PC risk and the levels of lead,nickel,selenium,cadmium,and arsenic in participants’ toenail samples (70). Urbanization,air pollution,and environmental contaminants also exhibited intriguing associations with PC incidence that encourage further investigation (22).
In summary,smoking,alcohol,obesity,DM and diet continue to be prominent modifiable risk factors that could be altered rather rapidly with avid public health measures.Age,genetic predisposition,inflammatory conditions,microbiome and environmental exposures,on the other hand,also contribute to the intricate tapestry of risk.As we delve deeper into the complexities of PC epidemiology,these recent findings offer valuable insights for developing targeted prevention and early detection strategies,with the ultimate goal of maximumly alleviating the burden of this disease.
Early detection and screening for PC
Designing a cost-effective screening regimen for PC faces several formidable challenges,with the first being the asymptomatic nature of PC in the earlier stages,and the second,the lack of specific and accessible biomarkers for early detection.Currently,there is no blood test or imaging technique with sufficient sensitivity and specificity to reliably detect the disease at an early,curable stage in clinical practice.
Luckily,recent advancements in imaging modalities held promises for early detection and screening:
Endoscopic ultrasound has emerged as a valuable tool for detecting pancreatic tumors at an early stage.Its highresolution images allow for detailed visualization of the pancreas,enabling the identification of small lesions.Advanced MRI techniques,including diffusion-weighted imaging and magnetic resonance cholangiopancreatography,offer enhanced sensitivity in detecting pancreatic lesions (71).
Identifying novel biomarkers in laboratory settings that enable early diagnosis of PC with higher sensitivity and specificity received persistent interest from researchers(72).An investigation of red blood cells showed that PC patients had higher HbA1C levels prior to their PC diagnosis than cancer-free controls (73).Progresses were also made in biomarkers detection methods to catch PC early.Liquid biopsy was a recently developed technology that could be used to detect a wide range of cells as well as nucleic acids from various sample sources.Blood assays were mostly used in liquid biopsy but pancreatic juice,saliva,urine,and stool samples from patients could all be assayed for biomarkers.In addition to early detection of PC,liquid biopsy could be applied in treatment monitor and prognosis evaluation (13).With a liquid biopsy,Xuet al.developed a non-invasive circulating RNA-based biomarker panel that compared to CA19-9,presented an increased sensitivity in PC patient identification when applied singularly and enhanced the diagnostic performance of CA19-9 substantially when combined (74).Furthermore,the above-mentioned symbiotic microbiomes and their metabolic products could be indicative of early cancerous changes in the pancreas (75).
Considerable information from the health record,imaging data,genetic sequencing,and circulation components is essential in establishing comprehensive screening and risk assessment guidelines.With the deep learning and neural network features of artificial intelligence continuing to be exploited,algorithms could be the most accessible tool to evaluate PC risk,make diagnoses,evaluate prognosis and so much more (14).
Survival and prognosis
The global 5-year survival rate of PC was only 3% during 1975-1977,<5% in the 1990s (1),and rose to a mere 7%from 2000 to 2007 (76).In 2019,the 5-year survival rate of PC in China was only 7.2% (21),while the rate in the USA and Europe was around 9% in 2019 (77),11% in 2022 (78),and 12% in 2023 (1).The prognosis of PC remained pessimistic with only marginal improvements over the years.In a prospective study,Rahibet al.,projected cancer incidence and deaths by 2030,highlighting the alarming burden of PC in the United States and calling for urgent needs to enhance survival rates and improve disease outcomes (79).
Recent studies highlighted the significance of tumor biology and molecular profiling in estimating and enhancing prognosis.Wartenberget al.unraveled distinctive molecular subtypes among PC patients which were associated with improved survival outcomes in response to personalized treatment (80).Advancements in the treatment schemes brought hope to PC patients and influenced prognostic outcomes.Innovative therapies,such as immunotherapies and targeted therapies,showed promise in clinical trials.These treatments aimed to not only improve survival rates but also enhance the quality of life.The integration of multidisciplinary approaches,including surgery,chemotherapy,radiation therapy,and palliative care,contributed to better survival outcomes.Apart from clinical and tumor-related factors,patientrelated variables also play a crucial role in determining prognosis,which include age,overall health,comorbidities,and response to treatment.A holistic approach to patient care is integral to enhancing the long-term prognosis of PC patients.
Overcoming survival and prognosis bottlenecks in PC remains challenging,however,the combination of early diagnosis,treatment advancement,patient-related variables,and emerging biomarkers could add up and contribute to better PC prognosis from various aspects.
Prevention and public health strategies
Smoking cessation campaigns
As discussed above,a strong positive correlation existed between the number of cigarettes per day and PC risk among current smokers (81).Interestingly,the risk of PC in former smokers declined as the length of smoking cessation increased,and became comparable to that of never-smokers once the cessation duration reached 10-20 years (81,82),an encouraging message that it was never too late to quit smoking.
Smoking cessation is the most effective strategy to reduce the risk of PC.With smoking cessation,the risk of developing PC could gradually decrease,but at least a decade’s effort was required before smoking-related risk became negligible (83).
Obesity prevention and management
An increasing burden and challenge to public health,obesity is another significant,yet modifiable,risk factor for PC.A meta-analysis on obesity and PC risk found a higher risk of PC among the obese population,compared to the population with healthy weight (84).In healthcare facilities,weight management programs and clinics have been integrated and should be made more accessible to assist individuals in achieving as well as maintaining healthy body weights.Initiatives promoting healthy lifestyles,including balanced diets and regular physical activity,should be implemented to prevent excess weight gain then obesity.Social-economic disparity and food insecurity should also be considered in obesity management due to its prevalence among lower-income and urbanized regions (33).
Genetic counseling and testing
Acknowledging the role of genetic predisposition in PC,genetic counseling and testing became vital components of prevention strategies.Individuals with a family history of the disease or known genetic mutations associated with PC are encouraged to undergo genetic counseling and testing to assess their risk.Identifying high-risk individuals allows for tailored surveillance and preventive measures.
Lifestyle education
Promoting healthy lifestyle choices through education and community programs is the cornerstone of prevention efforts.These programs should provide individuals with information and tools to make informed decisions about their diet,physical activity,and overall health.Empowering individuals to adopt healthier lifestyles can significantly reduce the risk of PC.
Future directions
As we gain deeper insights into the epidemiology of PC,it is essential to chart a course for future research and initiatives.This section explores future directions in PC research and public health strategies.Current cancer treatment poses a tremendous economic burden on individuals and society with little patient benefit in return.Research on novel treatment plans should indeed be encouraged for the advancement of medicine.However,positive results from research work on PC early detection shall benefit the public even more.Thus,future studies should be oriented over two major areas: advancement in early screening and personalized medicine.
Advancement in early screening can be achieved through continuous exploration of promising biomarkers,including liquid biopsies and genetic signatures,to enhance early detection of PC.Understanding genetic risk factors is another crucial aspect of identifying individuals at higher risk of developing PC.Future research should delve deeper into genetic risk profiling,mapping out rare and common genetic variants associated with PC susceptibility.Moreover,the screening pipeline can be complemented more.Refinement of screening guidelines,evaluation of the cost-effectiveness of screening modalities,and implementation of public health measures should all be considered to shift the time of diagnosis toward an earlier stage when curative treatment options are more viable.Future studies should continue monitoring the varying impacts of lifestyle interventions on risk reduction,such as dietary modifications,physical activity promotion,and smoking cessation programs.
Simultaneously,as a better understanding of genetic profiling develops,personalized prevention strategies as well as treatment plans might be more accessible.Advancements in molecular profiling have already unveiled the heterogeneity of PC.Future studies could orientate on tailoring therapies to the unique genetic and molecular characteristics of individual tumors.Precision medicine approaches,including targeted therapies and immunotherapies,hold promise for improving treatment responses and overall survival.
Conclusions
The epidemiology of PC painted a sobering picture of a disease with a formidable impact on individuals and healthcare systems worldwide.Despite recent advancements in understanding risk factors,early detection,and treatment approaches,PC remains a challenge.Its aggressive nature and often late-stage diagnosis contributed significantly to its high mortality rates.However,ongoing research,innovative strategies,and a multidisciplinary approach have brought hope to improved outcomes and reduced the burden of this devastating cancer.The future lies in precision medicine,biomarker discovery,and robust prevention initiatives,all of which must be pursued with vigor to transform the prospect of PC care.
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities (No.3332023122);National Natural Science Foundation of China (No.82203158) and National High Level Hospital Clinical Research Funding (No.2022-PUMCH-D-001 and No.2022-PUMCH-B-004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2023年5期
Chinese Journal of Cancer Research2023年5期
- Chinese Journal of Cancer Research的其它文章
- MRD-directed and risk-adapted individualized stratified treatment of AML
- Inspired by novel radiopharmaceuticals: Rush hour of nuclear medicine
- Update of latest data for combined therapy for esophageal cancer using radiotherapy and immunotherapy: A focus on efficacy,safety,and biomarkers
- Genetic susceptibility loci of lung cancer are associated with malignant risk of pulmonary nodules and improve malignancy diagnosis based on CEA levels
- A novel multimodal prediction model based on DNA methylation biomarkers and low-dose computed tomography images for identifying early-stage lung cancer
- Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib
