小麦卷叶突变体RL1的生理特性及遗传研究
刘 叶 李 越 苑名杨 卫乃翠 关攀锋 赵佳佳 武棒棒 郑兴卫 郝宇琼 乔 玲,* 郑 军,*
研究简报
小麦卷叶突变体的生理特性及遗传研究
刘 叶1,2,**李 越1,**苑名杨1卫乃翠1关攀锋3赵佳佳1武棒棒1郑兴卫1郝宇琼1乔 玲1,*郑 军1,*
1山西农业大学小麦研究所 / 农业农村部有机旱作农业重点实验室(部省共建), 山西临汾 041000;2山西大学生命科学学院, 山西太原 030006;3郑州大学农学院, 河南郑州 450001
小麦叶片在逆境下会发生可逆的折叠或卷曲, 通过脱水回避的形态学变化降低非生物胁迫的损害。目前小麦叶片卷曲的生理和遗传调控机制尚不清楚。本文利用EMS诱变晋麦47获得了卷叶突变体(),在整个生育期叶片呈现卷曲, 初生叶片沿中轴脉向近轴面微卷, 随着叶片生长加速卷曲, 直至为筒状。与野生型相比,株高降低、穗长变短、旗叶变窄和千粒重降低。氯化三苯基四氮唑(TTC)染色结果表明种子活力低, 且种子发芽率降低了22%。抽穗10 d后,的叶绿素含量与野生型基本一致, 净光合速率、蒸腾速率、气孔导度、细胞间隙CO2浓度差异不显著, 但水分利用率降低。低温、高温和干旱促进的叶片卷曲; 石蜡切片观察表明,的大叶脉和小叶脉偏少, 在中脉区域远轴面厚壁细胞和近轴面薄壁细胞数目减少, 且维管束间泡状细胞的面积和数量均明显低于野生型;叶片不同部位泡状细胞缩小以及维管束减少导致整个叶片向近轴面极度卷曲。遗传分析表明该性状受1对不完全显性的核基因控制, 位于1D染色体短臂上, 精细定位将目标区间锁定在9.42 Mb范围内。
小麦; 卷叶突变体; 细胞学分析; 生理特性; 遗传分析
植物感受到外界不利环境后, 除生理和基因表达等内在响应外, 植株形态往往发生变化[1]。叶片卷曲是植物应对强光、干旱、盐和热等非生物胁迫的自我保护机制, 叶片折叠或卷曲从而减少受光面积、削弱强光辐射、减轻叶片损伤, 进而降低蒸腾作用、减少水分流失, 达到降低干旱影响的目的[2]。因此, 叶片卷曲是逆境条件下叶片免受光损伤的脱水回避形态学策略[3]。此外, 适当的叶片卷曲也有利于保持叶片直立, 可改善群体整体的受光条件。因此, 研究叶片卷曲的生理特性和调控机制, 有利于解析作物对逆境响应的适应机制[4]。
根据叶片近轴面和远轴面的极性发育可将叶片卷曲分为两类, 即内卷和外卷, 相关机制在拟南芥、水稻、玉米均有研究报道, 水稻中研究最为深入, 主要的卷曲机制主要有4种, 一是通过调节泡状细胞形态、大小、数量以及分布来改变叶片卷曲程度, 如半卷叶抑制叶片近轴面泡状细胞的生成, 导致叶片向近轴面卷曲[5];和也是通过影响泡状细胞的发育使得叶片卷曲[6-7]。二是调节薄壁和厚壁细胞的大小和数量影响叶片卷曲, 如通过影响远轴面厚壁组织细胞的发育导致叶片极度内卷[8]。三是改变维管束中细胞特性, 如由于叶脉维管束韧皮部中筛管细胞增多, 整个韧皮部的面积显著增大, 使叶脉远轴面皱缩导致叶片外卷[9]。四是角质层、表皮细胞和叶肉细胞的变化, 如通过与富亮氨酸拉链转录因子互作负向调节角质层的发育, 过表达引起叶片内卷[10]。在玉米中发现的叶片卷曲基因主要通过调节近轴面细胞大小,和改变近轴面细胞使得叶片卷曲[11-12]。
小麦是异源六倍体, 基因组庞大(17G左右)且多为重复序列, 叶片卷曲基因克隆和调控机制的研究远落后于水稻和玉米, 目前只报道了少数QTL/基因。Zhu等[13]通过全基因组关联分析在323份小麦材料中鉴定出调控叶片卷曲的候选基因。Aakriti等[14]通过构建作图群体及同源性比对, 选定可能为卷叶候选基因。利用集群分离分析(BSA)结合660K芯片在7A染色体发现可能为叶片卷曲的候选基因[15]。最近, Bian等[16]利用卷叶突变体检测到2个卷叶性状的主效QTL (和), 并将精细定位到6 Mb的范围内。可见, 开展小麦叶片卷曲相关基因的克隆及调控研究, 有助于深入了解小麦响应逆境的调控机制。本文利用甲基磺酸乙酯(ethylmethane sulfonate, EMS)诱变著名旱地品种“晋麦47”, 获得稳定遗传的卷叶突变体, 对农艺性状、叶片卷曲特征、细胞学及遗传特性进行了研究, 为后续基因克隆和基因功能研究奠定基础。
1 材料与方法
1.1 试验材料
晋麦47号, 旱地冬性品种, 1995年审定后, 一直是我国黄淮旱地生产上的主要品种, 也是国家黄淮旱地区试和山西省南部旱地区试的对照品种[17]。晋麦47和及相关材料种植于山西农业大学(山西省农业科学院)小麦研究所试验基地(山西省临汾市, 36°2'N, 111°18'E), 每年10月上旬播种, 翌年6月中旬收获。每个材料播种1行, 行长2 m, 每行40粒。于越冬期和拔节期灌溉, 灌溉量均为700 m3hm–2。所有生育期内未发生极端天气和严重自然灾害, 小麦生长情况良好。
春化后种植在温室中, 在光照14 h、22℃的条件下生长, 拔节期设置不同光照(400、800和1000 μmol m–2s–1)、不同平均温度(10℃、22℃和30℃)及不同土壤水分含量(27%~ 30%和干旱处理14%~17%), 研究环境因素对叶片卷曲的影响; 每个处理设置3次重复, 调查叶片卷曲指数。
1.2 农艺性状和叶片卷曲指数的测定
选择长势均匀的材料各10株, 抽穗20 d后测量株高、穗长、各节间长度、穗粒数、小穗数等主要农艺性状, 同时测量旗叶长和宽, 通过叶长×叶宽×0.77计算叶面积[18]。籽粒收获后, 测量千粒重。参考Shi等[19]的卷曲指数(LRI)测定方法进行计算, 公式为LRI (%)=(w–n)/w×100%, 其中w为测量叶片最宽处展开后的叶缘间距,n为叶片最宽处卷曲状态下的叶缘间距, 平展叶当LRI为0; LRI大于0, 叶片向近轴面对折为微卷, 叶片呈筒状为高度卷曲。
1.3 种子活力及苗期性状检测
在适宜的条件下, 分别取100粒野生型和结构完整的种子播种于育苗盘, 15 d后统计发芽数, 计算发芽率[20]; 采集10株长势一致、根系完整的幼苗统计根系性状。选取成熟的野生型和籽粒, 用温水浸泡4 h, 使种子充分吸胀, 沿种胚中央准确切开将切好的种子放在培养皿中, 加入现配的质量体积浓度为0.1%氯化三苯基四氮唑(TTC), 25℃染色40~60 min, 观察种胚着色情况。
1.4 叶绿素含量和光合参数的测定
抽穗10 d后利用叶绿素测定仪SPAD-502 (Konica- Minolta, 日本)测定旗叶叶绿素含量, 取旗叶底部、中部和顶部的均值作为测定结果, 每个材料测5株计算平均值。于晴天上午09:30—10:30, 利用便携式光合测定仪(TARGAS-1, PP Systems, Amesbury, 美国)测定旗叶中部的净光合速率(n)、蒸腾速率(r)、气孔导度(s)、细胞间隙CO2浓度(i)等气体交换参数, 每个材料测5株, 通过n/r计算叶片水分利用效率(WUE)[21]。
1.5 突变体RL1的组织学分析
选取抽穗10 d后的旗叶进行组织学分析。叶片在FAA固定液中浸泡24 h以上, 经脱水浸蜡后包埋于石蜡中, 蜡块冷却后使用组织摊片机(KD-P)切片, 厚4 μm左右。将切片依次放入环保型脱蜡透明液、无水乙醇和水中进行漂洗; 置于番红染色液2 h, 固绿染色6~20 s, 在正置光学显微镜(NIKON ECLIPSE E100, 日本)下观察。
1.6 突变体RL1的遗传分析和基因定位
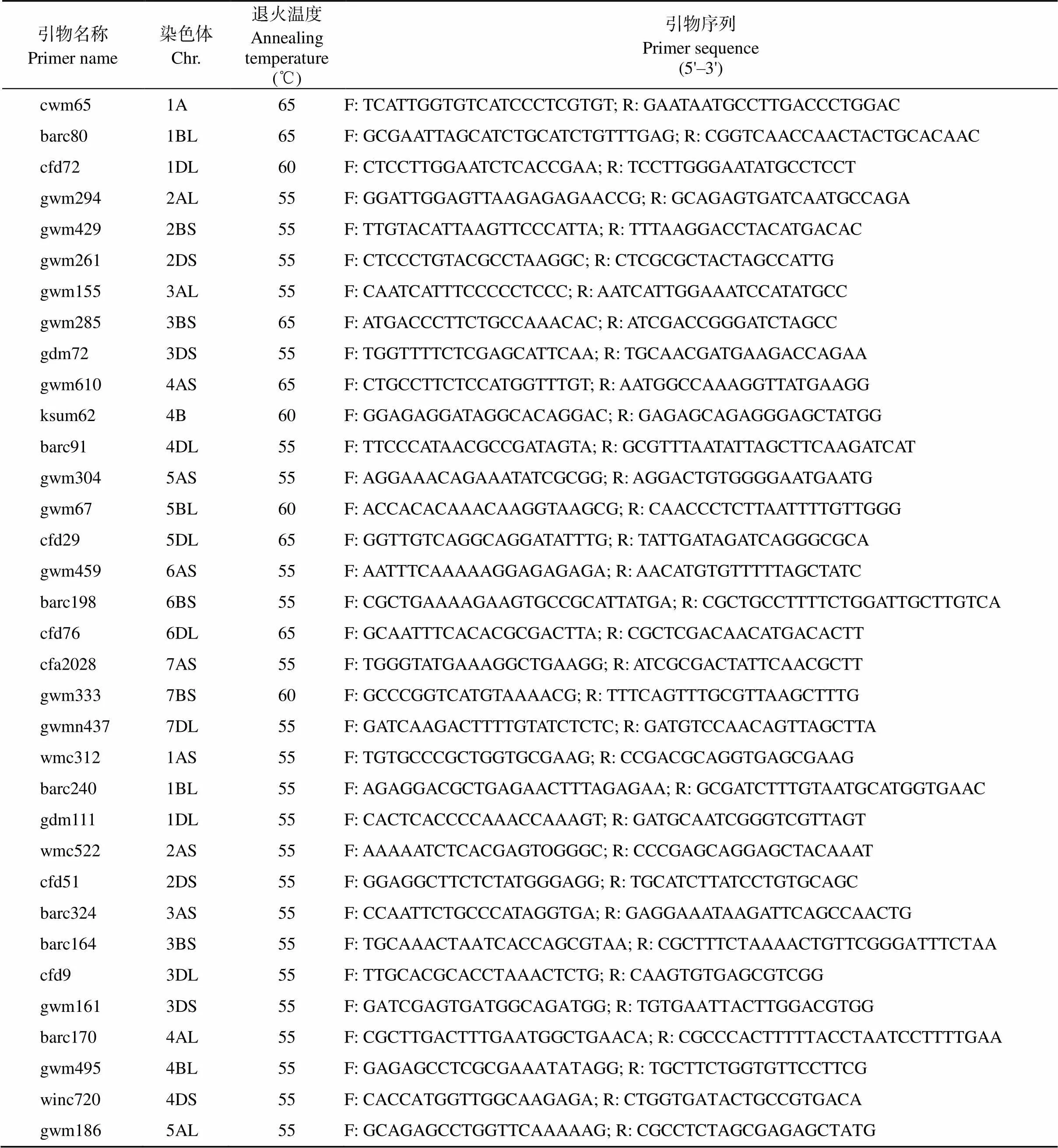
分别与晋麦47和临汾5064进行正反交, 以/晋麦47和/临汾5064构建F2定位群体, 大田观察F2表型并通过F3进行验证, 卡方检测分离比例。DNA提取采用改良的CTAB法, 从F2中选取平展叶与高度卷叶各20株构建极端混池用于基因初定位。混池及亲本由北京中玉金标记公司进行660K SNP芯片检测。筛选亲本间及后代间纯合有差异的SNP位点, 在小麦多组学数据库(http://wheatomics.sdau.edu.cn/)的中国春参考基因组序列中查找SNP位点上下游100 bp碱基序列, 设计KASP (kompetitive allele specific PCR)特异性引物进行基因精细定位(表1)。引物由北京博迈德生物技术有限公司合成。
2 结果与分析
2.1 突变体RL1的获得及表型观察
卷叶突变体由晋麦47经EMS诱变处理后, M2筛选到叶片卷曲的突变株, 连续3代套袋自交后, 与晋麦47回交3代, 自交获得到的一个稳定遗传的卷叶突变体株系。选取不同染色体上的共42对SSR引物(附表1), 对突变体和野生型进行遗传背景测定, 未发现多样性差异, 说明该卷叶植株是晋麦47的诱变株, 命名为。
叶片卷曲特性随生育进程而发生变化(图1-c), 新抽出的叶片基本上沿中轴脉纵向微卷, 伴随着生长叶片加速卷曲, 直到卷曲为筒状。与野生型相比,生长势较弱, 从苗期开始出现轻微卷曲现象, LRIs为0.11~ 0.25 (图1-b)。越冬前LRIs为0.31~0.60。返青期后, 新抽出的叶片仍然微卷, 随着叶片生长沿中轴逐渐向内卷曲, 直至叶片内卷成为近似圆筒状, LRIs为0.43~0.62, 且叶片的中下部约2/3卷曲、中上部正常。拔节期, LRIs为0.57~0.70。抽穗后, 旗叶、倒二叶卷曲程度高, 倒三、倒四叶依然为轻微卷曲, 叶鞘高度卷曲, 此时的旗叶LRIs为0.91~0.98 (图1-a)。而野生型植株的叶片始终接近为平展叶。
2.2 突变体RL1的农艺性状分析
对比和野生型株高相关性状, 发现穗长及节间缩短从而导致植株的矮化(图2)。通过比较和野生型的其余9个主要农艺性状, 发现的株高、旗叶宽、穗粒数和千粒重均极显著低于野生型, 而抽穗期、成熟期、旗叶面积、旗叶长和小穗数差异不显著(表2)。
2.3 种子活力及苗期性状检测
种子发芽率进行统计发现, 野生型的发芽率为98%, 而发芽率仅为76%。苗期地上部长势较弱, 地下部长势无显著差异, 其中根数目、主根长也无明显差异(图1-b和表2)。TTC染色结果表明, 野生型籽粒的胚着色较深, 而胚染色较浅, 说明种子活力较弱(图3)。

图1 野生型与突变体RL1的表型鉴定
a、b: 分别代表WT与的抽穗期、苗期表型; a中, 标尺为20 cm; b中, 标尺为10 cm。c: 野生型与不同时期卷曲程度的比较, 左为野生型, 右为, 标尺为1 cm。
a, b: plant phenotypes of WT andat heading stage and the seeding stage, respectively; Bar: 20 cm, in a; Bar: 10 cm, in b. c: the comparison of curl degree between wild type andat different stages, wild type on the left andon the right, Bar: 1 cm.
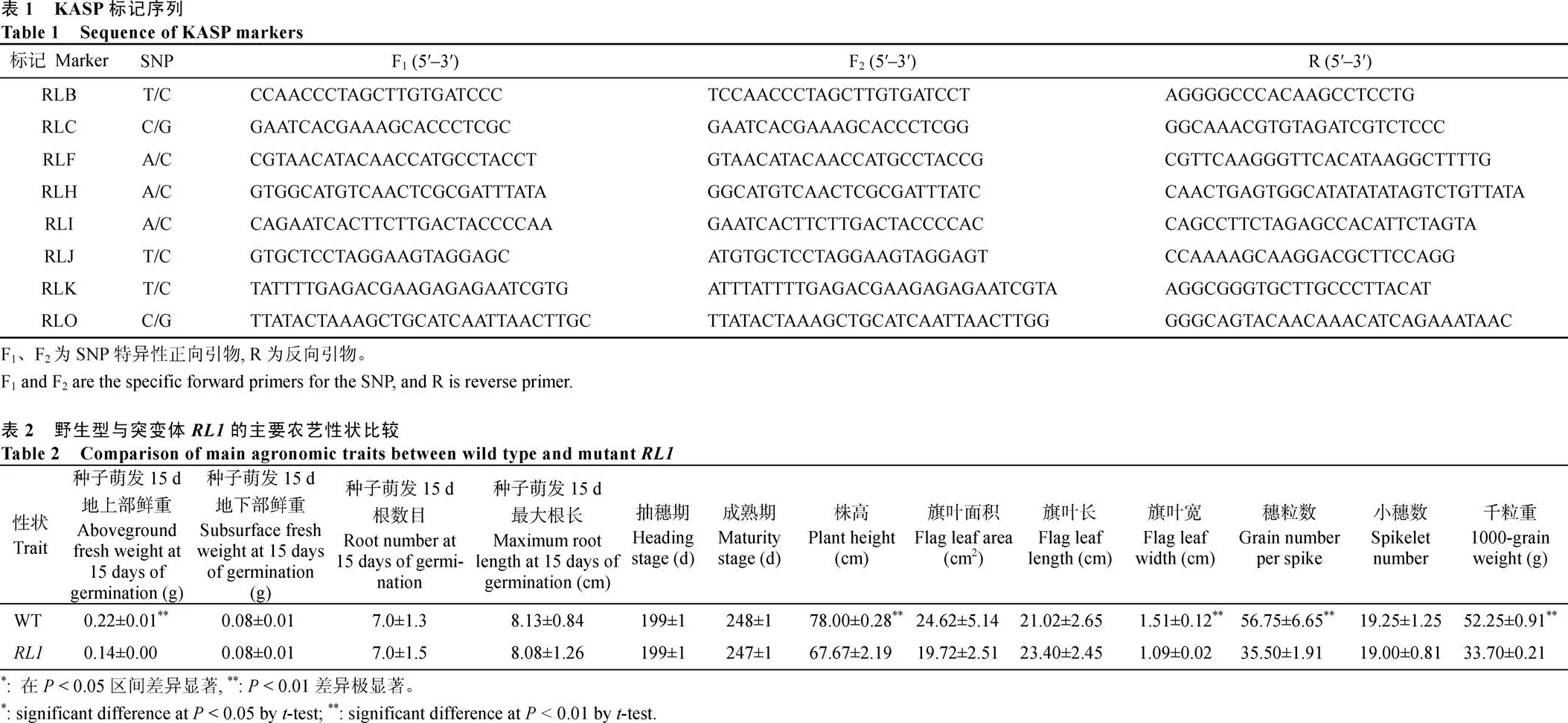
图2 野生型与突变体RL1的株型比较
Fig. 2 Comparing of plant type between RL1 mutant and its wild type counterpart
a: WT与的茎秆, 标尺为 20 cm; b、c: 成熟期WT与穗长及各节间长(I、II、III、IV), 标尺为10 cm;*: 在< 0.05区间差异显著,**: 在< 0.01差异极显著。
a: stem of WT and, Bar: 20 cm; b and c: the length of spike and internodes of WT andat mature stage, Bar: 10 cm; *: significant difference at< 0.05 by-test; **: significant difference at< 0.01 by-test.
2.4 旗叶叶绿素和光合参数的测定分析
抽穗10 d后, 对的叶绿素含量和光合参数进行测定。与野生型相比,的净光合速率、蒸腾速率、气孔导度、细胞间隙CO2浓度无显著差异, 叶绿素含量基本一致。单位面积内野生型和的叶绿素含量和光合能力差异不大, 但在自然生长过程中过度卷曲, 叶片整体受光面积明显小于野生型。此外, 叶片水分利用率数值表明,的叶片水分利用率极显著降低(图4)。
2.5 光照、温度和干旱处理对突变体叶片卷曲的影响
正常生长条件下的LRIs为0.10~0.31, 降低光照强度(400 μmol m–2s–1)和增加光照强度(1000 μmol m–2s–1)时,的LRIs分别为0.10~0.28和0.12~0.32, 表明光照强度基本不影响叶片卷曲。与22℃培养条件下的叶片卷曲度(LRIs为0.10~0.31)相比, 在平均温度10℃生长条件下,叶片卷曲度增加(LRIs为0.24~0.36); 平均温度30℃的培养条件也增加了叶片卷曲度(LRIs为0.32~ 0.38)。与正常浇水的叶片卷曲度(LRIs为0.10~0.31)相比, 干旱明显增加了叶片的卷曲程度(LRIs为0.46~0.61)。可见, 低温、高温和干旱促进的叶片卷曲。

图3 野生型与突变体RL1成熟籽粒的TTC染色
a: 种胚着色图, 标尺为2 mm; b、c: 种胚放大图, 标尺为200 μm。
a: seed embryo staining, Bar: 2 mm; b, c: magnified diagrams of seed embryos, Bar: 200 μm.

图4 野生型与突变体RL1的光合参数与SPAD的分析
a: 蒸腾速率; b: 气孔导度; c: 净光合速率; d: 细胞间隙CO2浓度; e: 叶绿素含量; f: 水分利用率。**:< 0.01差异极显著。
a: transpiration rate; b: stomata conductance; c: net photosynthetic rate; d: the intercellular CO2concentration; e: chlorophyll content; f: water use efficiency. **: significant difference at< 0.01 by-test.
2.6 突变体RL1的组织学分析
为了进一步了解叶片卷曲的机制, 对的叶片组织切片观察发现, 叶片泡状细胞、近远轴面的细胞层均发生了变化。与野生型相比,具有较少的大叶脉和小叶脉。野生型中脉一侧含有5~6个大叶脉和18~22个小叶脉, 而中仅有3~4个大叶脉和13~15个小叶脉(图5-a)。另外,的中脉区域, 远轴面厚壁细胞和近轴面薄壁细胞数目减少(图5-c, e)。维管束间泡状细胞的面积和数量都显著低于野生型, 发生卷曲部位的泡状细胞, 以及叶片边缘的泡状细胞都显著皱缩减少(图5-b, d, f)。叶片各部位泡状细胞缩小、维管束减少, 且叶肉细胞层数变薄, 导致整个叶片向近轴面极度卷曲。
2.7 突变体RL1的遗传分析
分别与晋麦47和临汾5064正反交得到的F1表型一致(附表2), 灌浆期的旗叶微卷, 介于野生型和突变体之间, 说明为细胞核遗传。在/晋麦47和/临汾5064的F2群体中出现明显的分离, 旗叶分别表现平展、微卷和高度卷曲, 经卡方检验, 平展叶单株∶微卷叶单株(中间型)∶高度卷叶单株符合1∶2∶1分离比(表3), 表明卷叶性状由1对不完全显性的单基因控制。
2.8 突变体RL1基因定位
小麦660K SNP芯片结果表明, 亲本及混池中有2170个多态性SNPs, 其中1115个分布在1DS上(51.38%), 表明决定卷叶形成有关基因可能在1D染色体上。通过计算每0.1 Mb的SNP数量, 初步将定位在62.82 Mb。利用8个高质量的KASP标记扫描320个F3分离家系, 根据目标区段内的重组家系, 将进一步定位在标记RLI和RLK间, 物理距离为9.42 Mb (图6), 其中包含了66个高可信度注释基因。
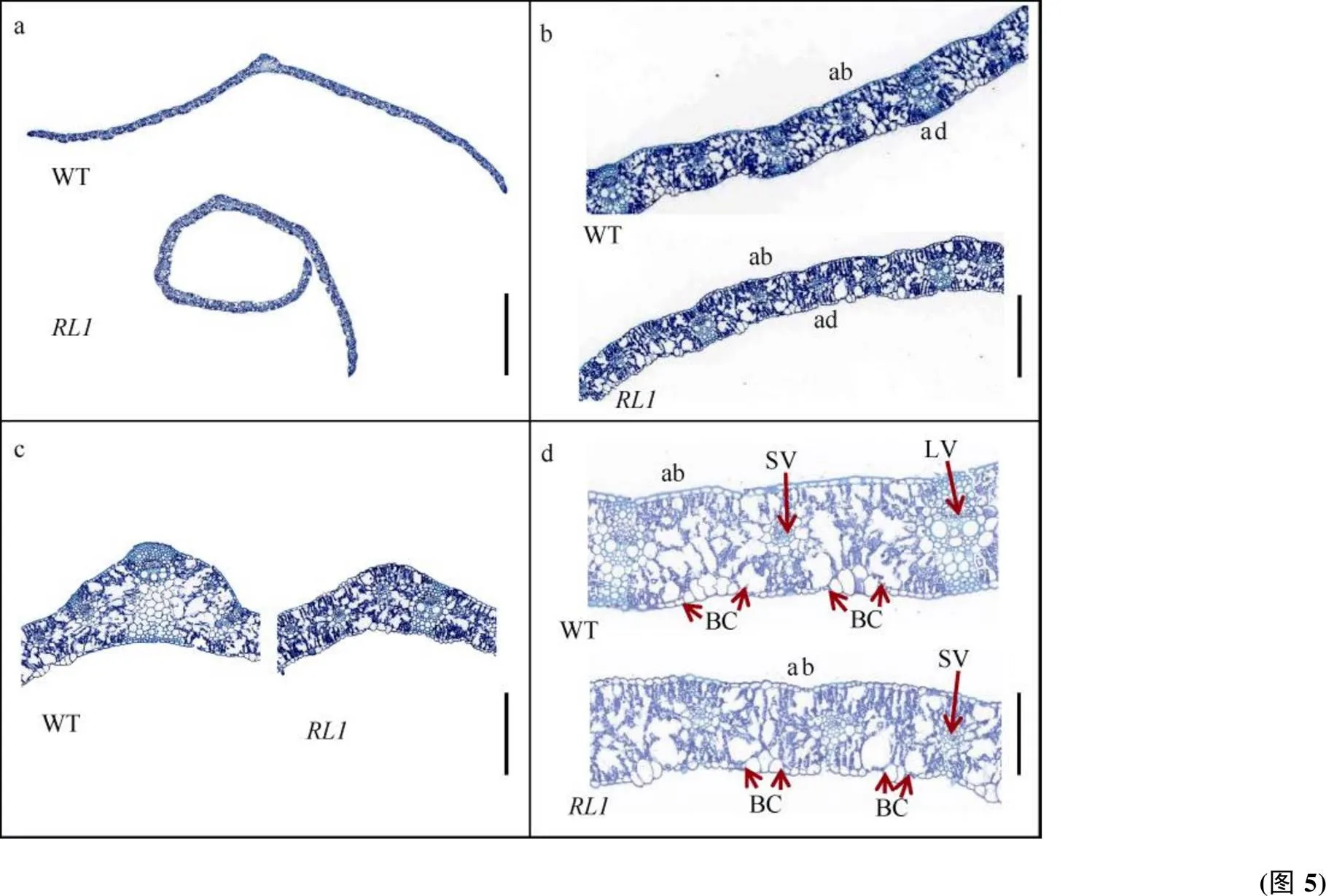
a: WT和的旗叶横切图, 标尺为1000 μm; b、c: 为a图的放大图, 标尺为 500 μm; d、e: 分别为b、c图的放大图, 标尺为200 μm; f: 两维管束之间的泡状细胞的数量和面积。SC: 厚壁细胞; PC: 薄壁细胞; BC: 泡状细胞; LV: 大叶脉; SV: 小叶脉; ad: 近轴面; ab:远轴面。**: 在0.01概率水平上差异显著。
a: cross cutting diagrams of flag leaves in WT and, Bar: 1000 μm; b, c: magnified diagrams of picture a, Bar: 500 μm; d, e: magnification of figure b and c, respectively, Bar: 200 μm; f: the number and area of bulliform cells between two vein. SC: sclerenchymatous cell; PC: parenchyma cell; BC: bulliform cell; LV: large vein; SV: small vein; ad: adaxial; ab: abaxial. **: significant difference at< 0.01 by-test.
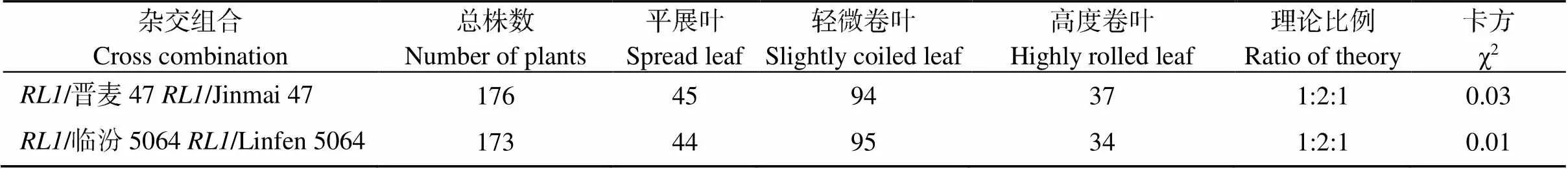
表3 F2代群体材料表型分离统计
χ2(0.05)(1)=3.84.
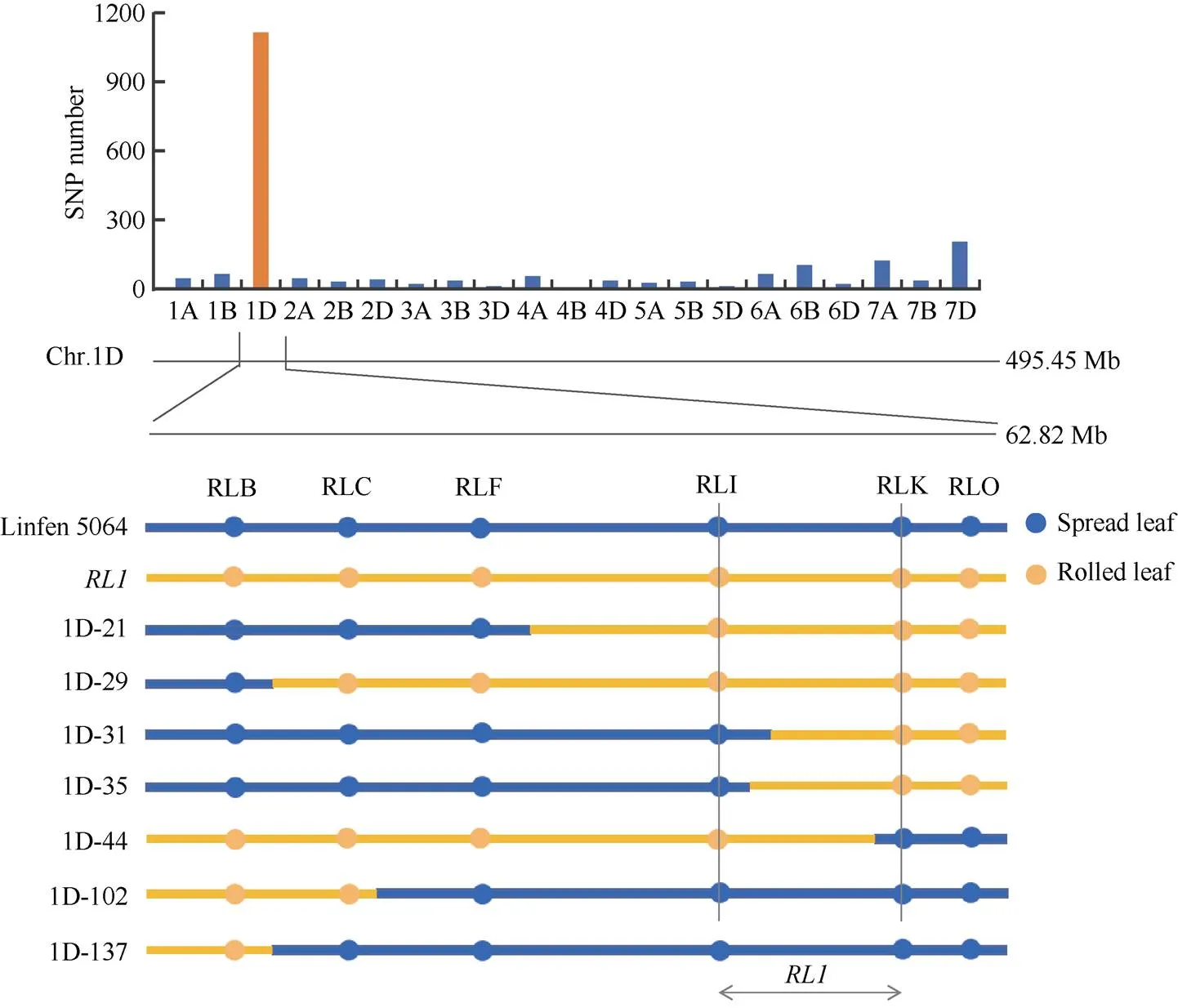
图6 RL1的精细定位
3 讨论
叶片卷曲作为一种植物抵抗非生物胁迫脱水回避的形态学策略, 可以减轻逆境带来的损伤, 因此研究叶片卷曲有利于了解作物对响应和抵御逆境胁迫的调控机制。根据植物叶片的近轴面和远轴面的极性发育, 可将卷叶性状分为正向和反向卷曲; 依照植株叶位表现, 可分为全株叶片卷曲和部分叶片卷曲; 从卷曲程度看, 有高度卷曲成筒状的, 也有中度卷曲和微卷[22]。本文获得的为全株正向卷曲, 新抽出的叶片微卷, 随着叶片生长沿着中轴向内卷曲, 直至叶片内卷成为近似圆筒状, 苗期卷曲程度轻,抽穗后的旗叶、倒二叶卷曲程度增高, 而倒三、倒四叶依然为轻微卷曲, 且旗叶叶鞘也出现卷曲。已发现的水稻卷叶突变体大多长势较弱, 育性差, 如的茎秆变短, 种子干瘪, 根细长, 根数量减少, 叶和穗变短小[23];株高变矮, 每穗粒数与结实率均下降, 且叶绿素含量和光合速率也显著降低[24]。的株高、旗叶宽、千粒重分别降低了13.25%、27.81%和35.50%, 这与、等类似[25-26]; 但苗期根系与野生型相比无明显差异, 单位面积内叶绿素含量和光合特性影响较小, 因为光合作用效率虽不受影响, 但其叶片卷曲造成受光面积减小, 因而造成长势较弱。虽长势和育性较差, 但其光合作用并未受到太大影响, 与水稻等[24]不同, 在研究作物应对外界胁迫响应和平衡基础代谢, 保证正常光合作用方面具有一定的研究利用价值。
禾本科叶片泡状细胞分布在两维管束之间, 位于叶片的近轴面, 一般只有4~5个, 中间大两边小, 类似扇形, 是调节水分的薄壁细胞。已有研究报道, 卷叶基因可通过调控泡状细胞的发育来控制叶片的卷曲, 借助大液泡内水分的得失来调控叶片的平展和卷曲度, 从而改变叶片的光合作用和蒸腾作用[27]。泡状细胞数量或体积的改变是调控叶片形态的关键因素, 增加泡状细胞的数量或大小会导致叶片外卷, 减少泡状细胞的数量或大小通常会导致叶片内卷[28]。的过表达导致泡状细胞增加,进而引起叶片反向卷曲[29]。过表达植株叶片表现为正向卷曲[30], 而过表达叶片则为反向卷曲[6]。此外, 增加的表达量会引起泡状细胞的缩小使得植株呈现出叶片正向卷曲的表型[31]。泡状细胞数量和面积均减小, 维管束及大小叶脉数量也减少, 两者共同作用引起叶片卷曲。
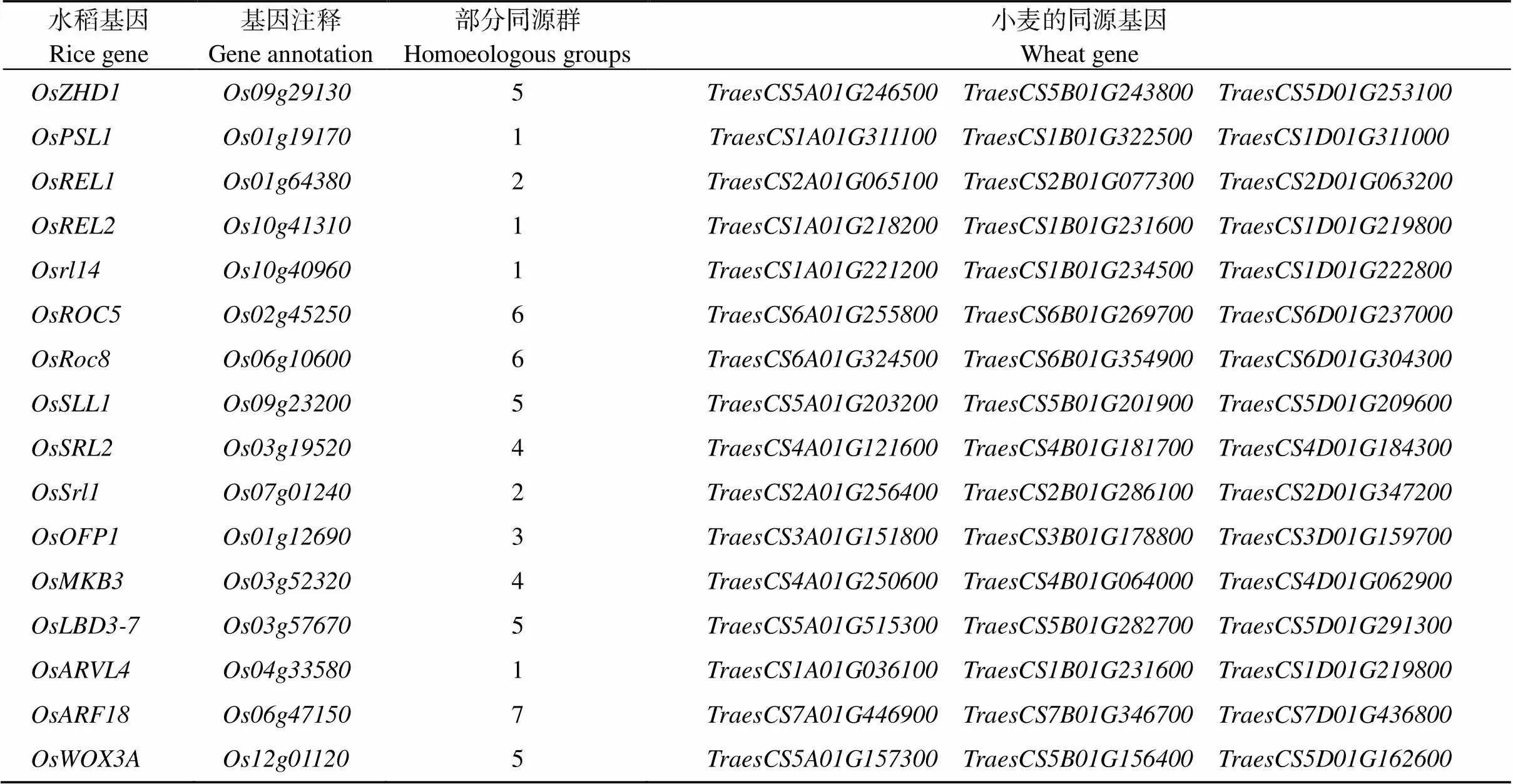
禾本科作物中卷叶基因克隆已有大量的研究报道。水稻叶片卷曲绝大多数为隐性性状, 受1对或多基因控制, 如、、、、、、、、、等都是隐性基因[32-33]。普通小麦含有3个亚基因组, 性状调控受基因冗余性影响表型分离不明显, 增加了QTL和图位克隆的难度。Zhu等[13]利用323份小麦材料通过全基因组关联分析鉴定了一个调控卷叶的候选基因。Aakriti等[14]通过QTL在5D染色体上定位到1个卷叶候选基因, 该基因是水稻外卷叶基因的同源基因。Yang等[15]通过BSA结合660K芯片在7A染色体717.82~720.18 Mb间定位到一个卷叶候选基因, 与拟南芥中的一个气孔发育调控因子同源。最近, EMS诱变卷叶突变体的1A和5A染色体上分别存在2个主效QTL, 其中被最终定位到6 Mb的范围内[16]。是连续自交得到的稳定遗传的卷叶突变体, 受环境影响小, 控制卷叶的主效基因定位在1DS染色体。水稻中克隆的卷叶基因有[6]、[28]、[10]、[31]、[34]、[19]、[35]、[36]、[24]、[37]、[29]、[38]、[39]、[40]、[41]、[7]、[5]、[8]、[25]、[42]、[43]、[26]、[30]、[44]、[45]、[46], 它们在小麦中的同源基因并未出现在功能区段中(表4), 因此可能是一个新的控制叶片卷曲的基因, 对其进一步精细定位和功能研究有助于深入了解小麦卷叶“脱水回避”发育机制, 解析小麦对于抵御逆境胁迫的应答调控。

表4 水稻叶片卷曲相关基因在小麦中的同源基因
(续表4)
附表1 42对SSR引物信息(国标: NY/T 2859–2015)

(续附表1)
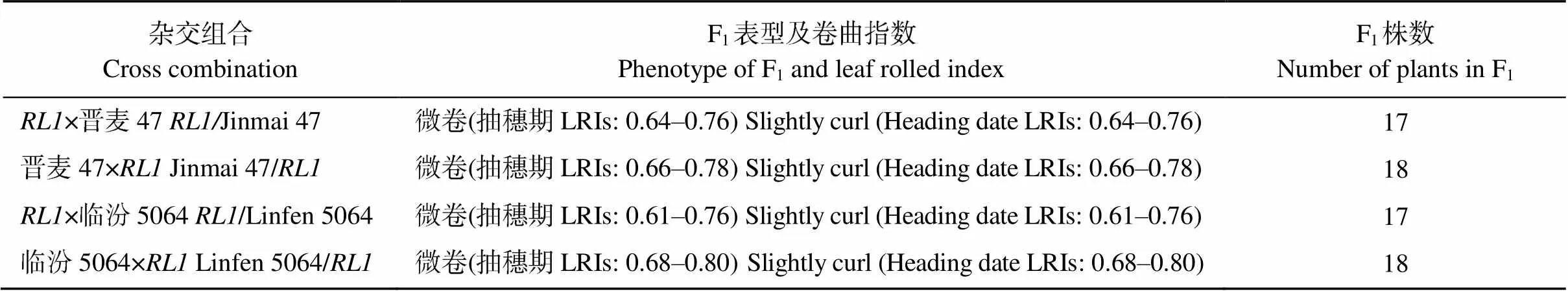
附表2 RL1与晋麦47/临汾5064正反交F1
[1] Bogard M, Hourcade D, Piquemal B, Gouache D, Deswartes J C, Throude M, Cohan J P. Marker-based crop model-assisted ideotype design to improve avoidance of abiotic stress in bread wheat., 2021, 72: 1085–1103.
[2] Merrium S, Ali Z, Tahir M H N, Habib-Ur-Rahman M, Hakeem S. Leaf rolling dynamics for atmospheric moisture harvesting in wheat plant as an adaptation to arid environments., 2022, 29: 48995–49006.
[3] Sirault X R R, Condon A G, Wood J T, Farquhar G D, Rebetzke G J. ‘Rolled-upness’: phenotyping leaf rolling in cereals using computer vision and functional data analysis approaches., 2015, 11: 52.
[4] Zhang X Y, Jia H Y, Li T, Wu J Z, Nagarajan R, Lei L, Powers C, Kan C C, Hua W, Liu Z Y, Chen C, Carver B F, Yan L L.modifies spike architecture and enhances grain yield in wheat., 2022, 376: 180–183.
[5] Sun J, Cui X A, Teng S Z, Zhao K N, Wang Y W, Chen Z H, Sun X H, Wu J X, Ai P F, Quick W P, Lu T G, Zhang Z G. HD-ZIP IV generegulates the size of bulliform cells and lignin content in rice., 2020, 18: 2559–2572.
[6] Li L, Shi Z Y, Li L, Shen G Z, Wang X Q, An L S, Zhang J L. Overexpression of() increased bulliform cells and induced abaxial curling of leaf blades in rice., 2010, 3: 807–817.
[7] Zou L P, Sun X H, Zhang Z G, Liu P, Wu J X, Tian C J, Qiu J L, Lu T G. Leaf rolling controlled by the homeodomain leucine zipper class IV genein rice., 2011, 156: 1589–1602.
[8] Zhang G H, Xu Q, Zhu X D, Qian Q, Xue H W. SHALLOT- LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development., 2009, 21: 719–735.
[9] 邹良平. 水稻卷叶突变体的细胞形成机制以及基因的克隆和功能研究. 中国农业科学院博士学位论文, 北京, 2012. Zou L P. Cytological Mechanism of Rolled-feaf Formation and Functional Analysis ofControlling Leaf Roll in Rice. PhD Dissertation of Chinese Academy of Agricultural Sciences, Beijing, China, 2012 (in Chinese with English abstract).
[10] Wu R H, Li S B, He S, Wassmann F, Yu C H, Qin G J, Schreiber L, Qu L J, Gu H Y. CFL1, a WW domain protein, regulates cuticle development by modulating the function of HDG1, a class IV homeodomain transcription factor, in rice and., 2011, 23: 3392–3411.
[11] Juarez M T, Twigg R W, Timmermans M C P. Specification of adaxial cell fate during maize leaf development., 2004, 131: 4533–4544.
[12] Canales C, Grigg S, Tsiantis M. The formation and patterning of leaves: recent advances., 2005, 221: 752–756.
[13] Zhu Z, Wang J Y, Li C N, Li L, Mao X G, Hu G, Wang J P, Chang J Z, Jing R L. A transcription factor TaMYB5 modulates leaf rolling in wheat., 2022, 13: 897623.
[14] Verma A, Niranjana M, Jha S K, Mallick N, Agarwal P, Vinod. QTL detection and putative candidate gene prediction for leaf rolling under moisture stress condition in wheat., 2020, 10: 18696.
[15] Yang X, Wang J Y, Mao X G, Li C N, Li L, Xue Y H, He L H, Jing R L. A locus controlling leaf rolling degree in wheat under drought stress identified by bulked segregant analysis., 2022, 11: 2076.
[16] Bian R L, Liu N, Xu Y Z, Su Z Q, Chai L L, Bernardo A, Amand P St, Fritz A, Zhang G R, Rupp J, Akhunov E, Jordan K W, Bai G H. Quantitative trait loci for rolled leaf in a wheat EMS mutant from., 2023, 136: 52.
[17] 赵佳佳, 乔玲, 武棒棒, 葛川, 乔麟轶, 张树伟, 闫素仙, 郑兴卫, 郑军. 山西省小麦苗期根系性状及抗旱特性分析. 作物学报, 2021, 47: 714–727. Zhao J J, Qiao L, Wu B B, Ge C, Qiao L Y, Zhang S W, Yan S X, Zheng X W, Zheng J. Seedling root characteristics and drought resistance of wheat in Shanxi province., 2021, 47: 714–727 (in Chinese with English abstract).
[18] 李浩然, 李慧玲, 王红光, 李东晓, 李瑞奇, 李雁鸣. 冬小麦叶面积测算方法的再探讨. 麦类作物学报, 2018, 38: 455–459. Li H R, Li H L, Wang H G, Li X D, Li R Q, Li Y M. Further study on the method of leaf area calculation in winter wheat., 2018, 38: 455–459 (in Chinese with English abstract).
[19] Shi Z Y, Wang J, Wan X S, Shen G Z, Wang X Q, Zhang J L. Over-expression of ricegene induces upward curling of the leaf blade that enhanced erect-leaf habit., 2007, 226: 99–108.
[20] Selim D A H, Zayed M, Ali M M E, Eldesouky H S, Bonfill M, El-Tahan A M, Ibrahim O M, El-Saadony M T, El-Tarabily K A, AbuQamar S F, Elokkiah S. Germination, physio-anatomical behavior, and productivity of wheat plants irrigated with magnetically treated seawater., 2022, 13: 923872.
[21] 张礼霞, 刘合芹, 于新, 王林友, 范宏环, 金庆生, 王建军. 水稻卷叶突变体的生理学分析及基因定位. 中国农业科学, 2014, 47: 2881–2888. Zhang L X, Liu H Q, Yu X, Wang L Y, Fan H H, Jin Q S, Wang J J. Molecular mapping and physiological characterization of a novel mutantin rice., 2014, 47: 2881–2888 (in Chinese with English abstract).
[22] 严长杰, 严松, 张正球, 梁国华, 陆驹飞, 顾铭洪. 一个新的水稻卷叶突变体的遗传分析和基因定位. 科学通报, 2005, 50: 2757–2762. Yan C J, Yan S, Zhang Z Q, Liang G H, Lu J F, Gu M H. Genetic analysis and gene fine mapping for a rice novel mutantwith rolling leaf character., 2005, 50: 2757–2762 (in Chinese with English abstract).
[23] Duan P G, Ni S, Wang J M, Zhang B L, Xu R, Wang Y X, Chen H Q, Zhu X D, Li Y H. Regulation ofby OsmiR396 controls grain size and yield in rice., 2015, 2: 15203.
[24] Li Y Y, Shen A, Xiong W, Sun Q L, Luo Q, Song T, Li Z L, Luan W J. Overexpression ofresults in pleiotropic effects on plant type architecture and leaf development in rice., 2016, 9: 46.
[25] Liu X F, Li M, Liu K, Tang D, Sun M F, Li Y F, Shen Y, Du G J, Cheng Z K.modulates rice leaf rolling by regulating abaxial side cell differentiation., 2016, 67: 2139–2150.
[26] Shimano S, Hibara K I, Furuya T, Arimura S I, Tsukaya H, Itoh J I. Conserved functional control, but distinct regulation, of cell proliferation in rice andleaves revealed by comparative analysis oforthologs., 2018, 145: 159624.
[27] Jane W N, Chiang S H T. Morphology and development of bulliform cells inHack., 1991, 36: 85–97.
[28] Hibara K L, Obara M, Hayashida E, Abe M, Ishimaru T, Satoh H, Itoh J L, Nagato Y. Thegene functions in leaf and embryonic pattern formation in rice., 2009, 334: 345–354.
[29] Xu Y, Wang Y H, Long Q Z, Huang J X, Wang Y L, Zhou K N, Zheng M, Sun J, Chen H, Chen S H, Jiang L, Wang C M, Wan J M. Overexpression of, a zinc finger homeodomain class homeobox transcription factor, induces abaxially curled and drooping leaf in rice., 2014, 239: 803–816.
[30] Li C, Zou X H, Zhang C Y, Shao Q H, Liu J, Liu B, Li H Y, Zhao T.overexpression induced adaxially rolled leaves in rice., 2016, 11: e0156413.
[31] Yang C H, Li D Y, Liu X, Ji C J, Hao L L, Zhao X F, Li X B, Chen C Y, Cheng Z K, Zhu L H. OsMYB103L, an R2R3-MYB transcription factor, influences leaf rolling and mechanical strength in rice (L.)., 2014, 14: 158.
[32] Kinoshita T. Gene analysis and linkage map. Tokyo: Japan Scientific Societies Press, 1984. pp 187–274.
[33] Khush G S, Kinoshita T. Rice karyotype, marker genes, and linkage groups. In: Khush G S, Toenniessen G H, eds. Rice Biology. Wallingford: CAB International and International Rice Research Institute, 1991. pp 83–108.
[34] Wang J, Hu J, Qian Q, Xue H W. LC2 and OsVIL2 promote rice flowering by photoperoid-induced epigenetic silencing of., 2013, 6: 514–527.
[35] Woo Y M, Park H J, Su'udi M, Yang J I, Park J J, Back K, Park Y M, An G. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio., 2007, 65: 125–136.
[36] Hu J, Zhu L, Zeng D L, Gao Z Y, Guo L B, Fang Y X, Zhang G Z, Dong G J, Yan M X, Liu J, Qian Q. Identification and characterization of, a novel gene regulating leaf morphology and plant architecture in rice., 2010, 73: 283–292.
[37] Dai M Q, Zhao Y, Ma Q, Hu Y F, Hedden P, Zhang Q F, Zhou D X. The ricegene is involved in the feedback regulation of gibberellin metabolism., 2007, 144: 121–133.
[38] Zhang G H, Hou X, Wang L, Xu J, Chen J, Fu X, Shen N W, Nian J Q, Jiang Z Z, Hu J, Zhu L, Rao Y C, Shi Y F, Ren D Y, Dong G J, Gao Z Y, Guo L B, Qian Q, Luan S.encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice., 2021, 229: 890–901.
[39] Chen Q L, Xie Q J, Gao J, Wang W Y, Sun B, Liu B H, Zhu H T, Peng H F, Zhao H B, Liu C H, Wang J, Zhang J L, Zhang G Q, Zhang Z M. Characterization ofin regulating leave morphology in rice., 2015, 66: 6047–6058.
[40] Yang S Q, Li W Q, Miao H, Gan P F, Qiao L, Chang Y L, Shi C H, Chen K M., a gene encoding an unknown function protein which contains DUF630 and DUF632 domains controls leaf rolling in rice., 2016, 9: 37.
[41] Fang L K, Zhao F M, Cong Y F, Sang X C, Du Q, Wang D Z, Li Y F, Ling Y H, Yang Z L, He G H.is a 2OG-Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves., 2012, 10: 524–532.
[42] Xiang J J, Zhang G H, Qian Q, Xue H W.encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells., 2012, 159: 1488–1500.
[43] Xiao Y H, Liu D P, Zhang G X, Tong H N, Chu C C. Brassinosteroids regulate OFP1, a DLT interacting protein, to modulate plant architecture and grain morphology in rice., 2017, 8: 1698.
[44] Wang L, Xu J, Nian J Q , Shen N W, Lai K K, Hu J, Zeng D L, Ge C W, Fang Y X, Zhu L, Qian Q, Zhang G G. Characterization and fine mapping of the rice generegulating leaf morphology and leaf vein development., 2016, 78: 345–356.
[45] Huang J, Li Z Y, Zhao D Z. Deregulation of thetarget genecauses growth and developmental defects with an alteration of auxin signaling in rice., 2016, 6: 29938.
[46] Cho S H, Yoo S C, Zhang H T, Pandeya D, Koh H J, Wang J Y, Kim G T, Paek N C. The riceandloci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development., 2013, 198: 1071–1084.
Physiological characteristics and genetic research of() in wheat (L.)
LIU Ye1,2,**, LI Yue1,**, YUAN Ming-Yang1, WEI Nai-Cui1, GUAN Pan-Feng3, ZHAO Jia-Jia1, WU Bang-Bang1, ZHENG Xing-Wei1, HAO Yu-Qiong1, QIAO Ling1,*, and ZHENG Jun1,*
1Institute of Wheat Research, Shanxi Agriculture University / Key Laboratory of Sustainable Dryland Agriculture (Co-construction by Ministry and Province), Ministry of Agriculture and Rural Affairs, Linfen 041000, Shanxi, China;2School of Life Science, Shanxi University, Taiyuan 030006, Shanxi, China;3School of Agricultural Sciences, Zhengzhou University, Zhengzhou 450001, Henan, China
Wheat leaves tend to fold or curl when exposure to stresses, the dehydration avoidance in morphology can reduce the damage of abiotic stress. At present, the physiological and genetic regulation mechanism associated with leaf curling is not clear in wheat. This study reported a novel() from the ethyl methyl sulphonate (EMS) mutagenesis cultivar Jinmai 47. The leaves of the mutantwere curled during the growth period, and the primary leaves were slightly curled along axial vein to paraxial plane. Leaf curling was accelerated with the growth until the leaf was tubular. Compared to wild type (WT), plant height, ear length, flag leaf narrowing, and 1000-grain weight were decreased in mutant. Triphenyltetrazolium Chloride (TTC) staining showed that seed vigor ofwas low, together with the decreased germination rate by 22%. Additionally, there was no significant differences in chlorophyll content, net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2concentration betweenand WT, while water utilization rate was decreased inafter heading for 10 days. Low temperature, high temperature, and drought led to the leaf rolling in.showed fewer leaf/lobular veins via paraffin section assay, and the number of abaxial sclerenchyma and adaxial parenchyma cells were reduced in midrib region of. Moreover, the area and counts of vesicular cells between the vascular bundles were significantly reduced in, together with the vesicular cells at the midvein region of leaves compared with WT. Vesicular cells and vascular bundles shrunk and decreased, respectively, resulting in the situation that the entire blade was extremely crimped to the adaxial plane. Genetic analysis demonstrated that the mutant trait was localized on the short arm of chromosome 1D, regulated by a pair of nuclear genes with incomplete dominance and fine mapping analysis further locked the target interval at 9.42 Mb.
wheat; rolled leaf mutant; cytological analysis; physiological characteristics; genetic analysis
2023-06-29;
2023-07-18
10.3724/SP.J.1006.2023.31004
通信作者(Corresponding author): 乔玲, E-mail: qiaolingsmile@163.com;郑军, E-mail: sxnkyzj@126.com
**同等贡献(Contributed equally to this work)
刘叶, E-mail: liuye12345202@126.com
2023-01-09;
本研究由山西农业大学省部共建有机旱作农业国家重点实验室自主研发项目(202002-1)和山西省科技重大专项计划“揭榜挂帅”项目(202201140601025-2-01)资助。
This study was supported by the Research Program Sponsored by State Key Laboratory of Integrative Sustainable Dryland Agriculture, Shanxi Agricultural University (202002-1) and the Science and Technology Major Special Plan Project “Reveal the Title” of Shanxi Province (202201140601025-2-01).
URL: https://kns.cnki.net/kcms2/detail/11.1809.S.20230717.1245.002.html
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).

