腰椎后路椎间融合术对椎旁肌损伤的影响研究
张晓 商亮 阮智

摘要:目的 评估腰椎后路椎间融合术(PLIF)后椎旁肌肉损伤状况。方法 前瞻性纳入接受PLIF治疗的侧隐窝型腰椎管狭窄症患者56例。记录术前、术后3个月疼痛视觉模拟评分(VAS)、Oswestry功能障碍指数(ODI);使用表面肌电图(sEMG)测定在抬物动作试验三阶段椎旁肌均方根(RMS)和中位频率(MF)变化;检测术前及术后第1、3、7天血清肌酸激酶(CK)和C-反应蛋白(CRP)水平。结果 患者术后3个月的疼痛VAS评分和ODI评分均较术前降低(P<0.05)。在测试阶段一、二中,患者术后3个月椎旁肌RMS水平低于术前,在测试阶段三中患者术后椎旁肌的MF值高于术前(P<0.05)。血清CK术后第1、3天较术前水平升高,且在术后第1天达到峰值(P<0.05),术后第7天血清CK水平基本恢复至术前水平。CRP水平术后各时点均高于术前,于术后第3天达到峰值,术后第7天较前下降(P<0.05)。结论 sEMG显示PLIF对椎旁肌組织造成了一定程度的损伤,这可能是引起患者术后椎旁肌功能下降和术后腰痛仍未完全缓解的主要原因。
关键词:椎管狭窄;腰椎;脊柱融合术;肌酸激酶;肌电描记术;椎旁肌
中图分类号:R681.5文献标志码:ADOI:10.11958/20221658
The influence of posterior lumbar interbody fusion on paraspinal muscle injury
ZHANG Xiao, SHANG Liang, RUAN Zhi
Department of Spine Surgery, the First Affiliated Hospital of Shihezi University Medical College, Shihezi 832000, China
Corresponding Author E-mail: 1725660475@qq.com
Abstract: Objective To evaluate paraspinal muscle injury after posterior midline lumbar interbody fusion. Methods Fifty-six patients with lateral recess lumbar spinal stenosis who received PLIF were prospectively included. Visual analog scale (VAS) and Oswestry Disability Index (ODI) were recorded before surgery and 3 months after surgery. Surface electromyography (sEMG) was used to measure the root mean square (RMS) and median frequency (MF) of paravertebral muscle in the three stages of lift test. Serum levels of creatine kinase (CK) and C-reactive protein (CRP) were detected before surgery and at 1, 3 and 7 days after surgery. Results The VAS score and ODI score were significantly lower in patients 3 months after surgery than those before surgery (P<0.05). In test stage 1 and 2, the level of paraspinal muscle RMS was significantly lower 3 months after surgery than that before surgery, and in test stage 3, the postoperative paraspinal muscle MF value was significantly higher than that before surgery (P<0.05). Serum CK level was significantly higher on the 1st and 3rd day after surgery than that before surgery, and which reached the peak on the 1st day after surgery (P<0.05). Serum CK level basically recovered to the preoperative level on the 7th day after surgery, and CRP level was higher at all time points after surgery than that before surgery, and reached the peak on the 3rd day after surgery, and decreased on the 7th day after surgery (P<0.05). Conclusion sEMG reveals that posterior lumbar interbody fusion causes some paraspinal muscle tissue injury, which may be the primary cause of postoperative paraspinal muscle function decline and postoperative low back pain.
Key words: spinal stenosis; lumbar vertebrae; spinal fusion; creatine kinase; electromyography; paraspinal muscles
腰椎管狭窄症(lumbar spinal stenosis,LSS)是脊柱退行性病变导致椎管或神经根管内径变窄的疾病[1]。腰椎后路椎间融合术(posterior lumbar interbody fusion,PLIF)是治疗LSS的经典手术[2-3]。然而,在手术过程中对椎旁肌肉产生的创伤可引起患者术后腰背部疼痛、肌无力及萎缩等并发症[4]。研究表明,腰椎术后出现腰部不适持续不缓解与椎旁肌的损伤密切相关[5]。因此,探究LSS患者术后椎旁肌肉功能变化尤为重要。表面肌电图(surface electromyography,sEMG)有助于客观评价肌肉功能变化,与针极肌电图相比,sEMG技术具有简便性和无创性,且可重复性强,可在受试者运动过程中持续观察肌肉活动的变化[6]。此外,研究表明血清肌酸激酶(creatine kinase,CK)和C-反应蛋白(C-reactive protein,CRP)可以量化手术对肌肉组织的损伤程度[7-8]。基于此,本研究通过评估PLIF对椎旁肌损伤的影响,以期助力指导术后康复方案的制定,促进术后腰椎旁肌功能恢复。
1 对象与方法
1.1 研究对象 前瞻性纳入2021年1月—2022年9月于石河子大学医学院第一附属医院脊柱外科接受PLIF治疗的侧隐窝型腰椎管狭窄症患者,所有患者单侧神经根症状和体征与影像学检查相符。纳入标准:(1)经保守治疗无效。(2)合并有腰椎不稳或滑脱。(3)单节段腰椎管狭窄。排除标准:(1)合并脊髓相关疾病如脊髓肿瘤、脊髓炎、脊髓梗死者。(2)有病理性脊柱疾病、脊柱感染性疾病如先天性脊柱畸形致脊髓受损、脊柱肿瘤、化脓性感染、脊柱结核和伴有椎体骨折者。(3)既往有脊柱手术史者。(4)术后发生感染者。(5)失访。最终纳入56例,男26例,女30例;年龄32~86岁,平均(63.17±9.34)岁,平均体质量指数(25.79±2.23)kg/m2。手术节段:L3/4 4例,L4/5 35例,L5/S1 17例。依据Bartynski等[9]提出的侧隐窝型腰椎管狭窄程度分级:B级19例,C级31例,D级6例。本研究经医院伦理委员会批准(批准号:KJX-2021-011-02),所有患者及家属均知情同意并签署书面知情同意书。
1.2 治疗方法 所有患者均由同组医师完成手术,均取后正中切口,充分剥离椎体两侧椎旁肌肉,充分显露责任节段椎间隙,在高倍显微镜辅助下摘除椎板、钙化增厚的黄韧带及切除椎间盘,使用咬骨钳咬除部分骨质以扩大侧隐窝及神经根管,随后将切下的骨质制成骨粒紧密填充至椎间融合器内,植入椎间隙恢复椎间盘高度,并使用椎弓根螺钉和刚性钛合金棒固定(型号:SINO6.0,山东威高医疗有限公司)。术后患者均接受相同治疗,使用腰部护具辅助3个月,能够进行日常活动,但不能体育训练及负重劳动。
1.3 临床疗效评估 所有患者于术前及术后3个月采用疼痛视觉模拟评分(visual analogue scale,VAS)评估腰痛(0分:无疼痛;10分:极度疼痛);使用Oswestry功能障碍指数(ODI)评分问卷评估日常活动改善情况。
1.4 椎旁肌sEMG检测 sEMG检查室的室温维持在25~28 ℃,使用75%乙醇棉球对检查部位局部皮肤进行脱脂处理以降低皮肤和电极片之间的电阻。采用一次性吸附表面电极片(Via Buccari,21-16153 Genova-Italy)沿所测肌肉纤维的长轴方向,将3块电极片粘贴在肌腹最饱满的部位。其中测试电极片间距2 cm,参考电极粘贴在距离测试电极外侧3 cm的位置。采用NIHON-KOHDEN型肌电图仪(QP-946BK,NIHON-KOHDEN,日本)分别检测术前及术后3个月患者在抬物动作试验[10]3个阶段的椎旁肌肉肌电信号变化情况并分析。测试阶段一:受试者保持站立位,双下肢直立,双足与双肩等宽,检测1 min,选取中间50 s波段的均方根值(root mean aquare of the spectrum,RMS)。阶段二:受试者躯干前屈90°,双手握起置于受试者前方质量约2 kg的四脚凳,然后受试者躯干伸直并后伸5 s左右,取躯干伸直时中间3 s的RMS值。阶段三:受试者双手臂均保持水平位置,当其无法处于水平位置时(以双手臂下垂>10°为标准)应立即停止,从30 s后开始连续检测1 min的中位频率(median frequency,MF)取平均值。休息5 min后重復以上动作。上述操作均由同一神经内科医师完成。
1.5 血清CK和CRP水平测定 术前及术后第1、3、7天晨起固定时间采血送检,均由我院检验科完成检测。使用免疫速率散射比浊法在PA-990pro特定蛋白分析仪上测定CRP值。采用比色法在AU-5821分析仪上检测血清CK。正常参考范围按照制造商、国际临床化学和检验医学联合会(IFCC)推荐标准,分别是:CRP<10 mg/L和CK 50~310 U/L。
1.6 统计学方法 采用SPSS 26.0进行数据分析,计量资料以x±s表示,组内治疗前后比较采用配对t检验;组内多时间点比较采用单因素重复测量方差分析。检验水准α=0.05。
2 结果
2.1 受试者术前和术后3个月疼痛VAS评分和ODI评分比较 患者术后3个月的疼痛VAS评分较术前降低(3.52±0.83 vs. 6.75±0.92,t=29.106,P<0.05),术后3个月的ODI评分较术前降低(22.88±2.85 vs. 40.30±2.87,t=46.989,P<0.05)。
2.2 sEMG对椎旁肌功能评估 在测试阶段一、二中,患者术后3个月椎旁肌RMS水平低于术前,在测试阶段三中患者术后椎旁肌的MF值高于术前(P<0.05),见表1。
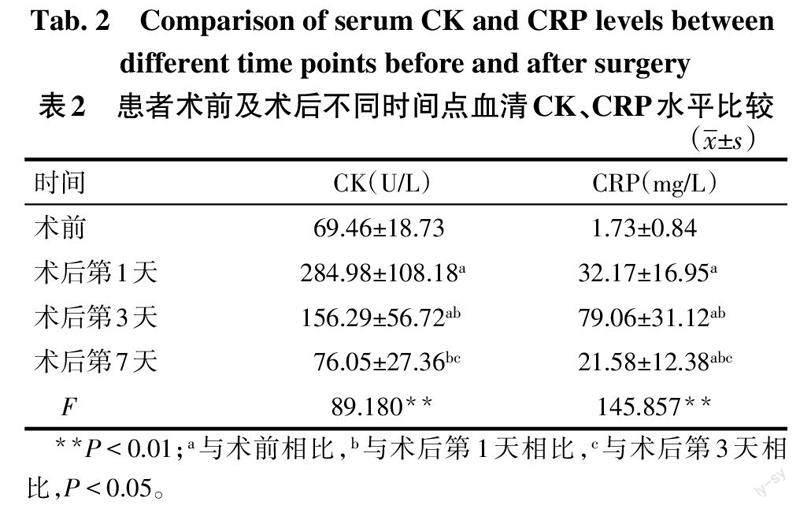
2.3 血清CK、CRP水平比较 血清CK水平术后第1、3天比术前升高,且在术后第1天达到峰值(P<0.05),术后第7天血清CK水平基本恢复至术前水平。CRP水平术后各时点均高于术前,于术后第3天达到峰值,术后第7天较前下降(P<0.05),见表2。
3 讨论
PLIF手术因广泛剥离易造成椎旁肌肉组织损伤,此外撑开器的持续使用引起椎旁肌的压力显著增加,毛细血管内灌注减少,导致肌肉缺血性损伤[11]。本研究患者术后VAS和ODI评分较术前均有下降,表明患者的腰痛得以改善且日常生活能力有所提升;但患者术后的VAS和ODI评分仍高于基础无疼痛及无功能障碍水平,这可能是由于手术对椎旁肌创伤所引起。
目前关于LSS患者通过PLIF手术治疗后,采用sEMG方式评估椎旁肌肉功能变化的研究鲜有报道。本研究利用sEMG技术对椎旁肌肉功能变化进行检测,sEMG具有对肌肉疲劳性和收缩力变化检测较为灵敏的优势,在临床诊断、评估腰背痛患者肌肉功能、神经肌肉疾病及康复运动治疗方面应用广泛[12-14]。RMS是评估肌肉收缩活动最常见的参考指标,其随着肌肉收缩力的增强而增加[15]。MF反映了骨骼肌收缩运动中肌纤维释放频率的变化,随着椎旁肌疲劳程度的增加,MF值减小,MF对肌肉疲劳程度的评估具有重要意义[16-17]。本研究显示,在静止站立状态和躯干抬物伸展时,术后椎旁肌电活动的RMS水平比术前下降,这可能是由于椎旁肌肉的损伤所造成肌肉收缩力下降。当术后腰椎旁肌功能下降时,有必要进行相应腰椎旁肌群的抗阻训练、力量训练以及中频电刺激治疗[18],这将会对PLIF术后患者神经肌肉功能的修复、避免和逆转肌肉萎缩起到重要作用。在抬物维持过程中,术前椎旁肌的MF值低于术后,表明术前患者的椎旁肌处于疲劳状态,这可能是由于LSS患者长期的腰部疼痛使椎旁肌产生保护性收缩,而肌肉长期处于激活状态使椎旁肌疲劳性增加所致。PLIF加强了脊柱的稳定性,从而缓解了患者部分腰部疼痛,一定程度提高了术后椎旁肌抗疲劳能力,但其与同龄健康人的差异仍需进一步验证。
血清CK常作为术后早期检测肌肉损伤程度的有效标志物之一[19],手术过程中对肌肉的创伤会使血清CK的浓度升高[20]。本研究中受试患者的血清CK浓度在术后第1天达到峰值,在术后第3天呈下降趋势,均明显高于术前,表明手术对椎旁肌肉损伤较明显,在术后第7天基本恢复至基线。这与Kumbhare等[21]在研究腰椎手术对肌肉的损伤程度时,受试者术后6、7 d内血清CK恢复至基线的结果基本一致。Hoeller等[22]認为CRP可作为评价术中组织损伤程度的指标,术后第3天CRP达到峰值是手术创伤所致。本研究测定的CRP浓度在术后第1、3、7天较术前明显升高,其峰值出现在术后第3天,术后第7天的CRP浓度值较前下降,证实了PLIF术过程中对机体造成了创伤。
综上所述,经PLIF治疗的LSS患者的椎旁肌组织受到了一定程度的损伤,这可能是引起患者术后椎旁肌功能下降和术后腰痛的主要原因。本研究不仅为探索PLIF对椎旁肌损伤提供了新的依据,而且对于指导经PLIF术治疗的LSS患者制定合理的椎旁肌康复训练方案提供了理论基础。但由于本研究样本量不足,未使用影像学对椎旁肌肉进行评价,且随访时间较短,可能对结果有一定影响。
参考文献
[1] JENSEN R K,LAURIDSEN H H,ANDRESEN A,et al. Diagnostic screening for lumbar spinal stenosis[J]. Clin Epidemiol,2020,12:891-905. doi:10.2147/CLEP.S263646.
[2] CHO S M,KIM S H,HA S K,et al. Paraspinal muscle changes after single-level posterior lumbar fusion:volumetric analyses and literature review[J]. BMC Musculoskelet Disord,2020,21(1):73. doi:10.1186/s12891-020-3104-0.
[3] FENTON-WHITE H A. Trailblazing:the historical development of the posterior lumbar interbody fusion(PLIF)[J]. Spine J,2021,21(9):1528-1541. doi:10.1016/j.spinee.2021.03.016.
[4] HE W,HE D,SUN Y,et al. Quantitative analysis of paraspinal muscle atrophy after oblique lateral interbody fusion alone vs. combined with percutaneous pedicle screw fixation in patients with spondylolisthesis[J]. BMC Musculoskelet Disord,2020,21(1):30. doi:10.1186/s12891-020-3051-9.
[5] PALPAN FLORES A,GARCíA FEIJOO P,ISLA GUERRERO A. Paraspinal muscle atrophy after posterior lumbar surgery with and without pedicle screw fixation with the classic technique[J]. Neurocirugia(Astur:Engl Ed),2019,30(2):69-76. doi:10.1016/j.neucir.2018.11.006.
[6] MORTKA K,WIERTEL-KRAWCZUK A,LISIńSKI P. Muscle activity detectors-surface electromyography in the evaluation of abductor hallucis muscle[J]. Sensors(Basel),2020,20(8):2162. doi:10.3390/s20082162.
[7] MAEZAWA K,NOZAWA M,GOMI M,et al. Changes in serum creatine kinase and C-reactive protein after posterior and direct anterior approaches in total hip arthroplasty[J]. Hip Int,2022,32(5):591-595. doi:10.1177/1120700020978643.
[8] MARGRAF A,LUDWIG N,ZARBOCK A,et al. Systemic inflammatory response syndrome after surgery:Mechanisms and protection[J]. Anesth Analg,2020,131(6):1693-1707. doi:10.1213/ANE.0000000000005175.
[9] BARTYNSKI W S,LIN L. Lumbar root compression in the lateral recess:MR imaging,conventional myelography,and CT myelography comparison with surgical confirmation[J]. AJNR Am J Neuroradiol,2003,24(3):348-360.
[10] 李永忠,朱一霄,陳文君,等. 基于表面肌电信号分析的退行性腰椎后凸患者手术前、后椎旁肌肉功能变化研究[J]. 中华物理医学与康复杂志,2018,40(4):290-295. LI Y Z,ZHU Y X,CHEN W J,et al. The functional status of the paraspinal muscles among patients with degenerative lumbar kyphosis before and after surgery[J]. Chinese Journal of Physical Medicine and Rehabilitation,2018,40(4):290-295. doi:10.3760/cma.j.issn.0254-1424.2018.04.012.
[11] TANDON R,KIYAWAT V,KUMAR N. Clinical correlation between muscle damage and oswestry disability index score after open lumbar surgery:Does open surgery reduces functional ability?[J]. Asian Spine J,2018,12(3):518-523. doi:10.4184/asj.2018.12.3.518.
[12] CHMIELEWSKA D,STANIA M,KUCAB-KLICH K,et al. Electromyographic characteristics of pelvic floor muscles in women with stress urinary incontinence following sEMG-assisted biofeedback training and Pilates exercises[J]. PLoS One,2019,14(12):e0225647. doi:10.1371/journal.pone.0225647.
[13] MERLETTI R,CERONE G L. Tutorial. Surface EMG detection,conditioning and pre-processing:Best practices[J]. J Electromyogr Kinesiol,2020,54:102440. doi:10.1016/j.jelekin.2020.102440.
[14] JERO S E,BHARATHI K D, RAMAKRISHNAN S. A method to differentiate fatiguing conditions in surface electromyography signals using instantaneous spectral centroid[J]. Annu Int Conf IEEE Eng Med Biol Soc,2020,2020:690-693. doi:10.1109/EMBC44109.2020.9176599.
[15] KRAJEWSKA-W?GLEWICZ L,BANACH M,FILIPIAK E,et al. The feasibility of surface electromyography in monitoring orbicularis oculi recovery after anterior approach levator aponeurosis advancement[J]. J Clin Med,2022,11(3):731. doi:10.3390/jcm11030731.
[16] KOUMANTAKIS G A,OLDHAM J A. Paraspinal strength and electromyographic fatigue in patients with sub-acute back pain and controls:Reliability,clinical applicability and between-group differences[J]. World J Orthop,2021,12(11):816-832. doi:10.5312/wjo.v12.i11.816.
[17] LI W,LIU Y C,ZHENG C F,et al. Diagnosis of compressed nerve root in lumbar disc herniation patients by surface electromyography[J]. Orthop Surg,2018,10(1):47-55. doi:10.1111/os.12362.
[18] JUNG G S,CHANG M C,SEO S W,et al. Transcutaneous neuromuscular electrical stimulation applied to optimal points on the lower abdomen and lumbar paraspinal region changes gait parameters in patients with lumbar degenerative kyphosis[J]. J Back Musculoskelet Rehabil,2018,31(2):267-274. doi:10.3233/BMR-169638.
[19] MATěJKA T,ZEMAN J,BELATKA J,et al. Creatine kinase and myoglobin levels as indicators of perioperative muscle damage during open- and mini-invasive stabilization of thoracic and lumbar spine fracture - a prospective randomized study[J]. Acta Chir Orthop Traumatol Cech,2020,87(1):9-16.
[20] AO S,ZHENG W,WU J,et al. Comparison of preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: Is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study[J]. Int J Surg,2020,76:136-143. doi:10.1016/j.ijsu.2020.02.043.
[21] KUMBHARE D,PARKINSON W,DUNLOP B,et al. Biochemical measurement of muscle injury created by lumbar surgery[J]. Clin Invest Med,2007,30(1):12-20. doi:10.25011/cim.v30i1.444.
[22] HOELLER S,ROCH P J,WEISER L,et al. C-reactive protein in spinal surgery: more predictive than prehistoric[J]. Eur Spine J,2021,30(5):1261-1269. doi:10.1007/s00586-021-06782-8.
(2022-10-17收稿 2022-11-24修回)
(本文編辑 李志芸)

