二氧化硅层对氧化石墨烯表面生长碳纳米管的影响
朱静泊,邹杨君,唐小天,闫兵,岳建岭,刘愚*,黄小忠
二氧化硅层对氧化石墨烯表面生长碳纳米管的影响
朱静泊1,邹杨君1,唐小天2,闫兵1,岳建岭1,刘愚1*,黄小忠1
(1.中南大学 粉末冶金研究院,长沙 410083;2.陆军装备部航空军事代表局驻株洲地区航空军事代表室,湖南 株洲 412000)
通过在氧化石墨烯表面沉积二氧化硅过渡层,实现碳纳米管在其表面的可控生长。在氧化石墨烯(GO)的分散液中,滴入四乙氧基硅烷(TEOS),通过调节分散液的pH,使得TEOS水解,并在氧化石墨烯表面沉积二氧化硅层(SiO2),获得二氧化硅包覆的氧化石墨烯(GO@SiO2);然后,采用浮动催化剂化学气相沉积(CVD)方法,在GO@SiO2表面生长碳纳米管,通过调节沉积时间,获得二氧化硅包覆的氧化石墨烯–碳纳米管杂化材料(GO@SiO2-CNTs);将未沉积二氧化硅层的氧化石墨烯在同样条件下通过CVD得到氧化石墨烯–碳纳米管杂化材料(GO-CNTs);结合SEM、STEM、EDS分析,对比有无二氧化硅层的氧化石墨烯基底对生长碳纳米管的影响。在GO表面直接生长的CNTs不能实现全面均匀的包覆,相比之下,在SiO2包覆的GO表面生长的碳纳米管阵列均匀且致密,呈现典型的“刷”状结构,通过调节反应时间,可以控制碳纳米管的生长密度和长度。二氧化硅层可以有效地促进碳纳米管在氧化石墨烯基底的生长,实现碳纳米管形貌的可控调节。
CVD;氧化石墨烯;碳纳米管;二氧化硅层
利用碳基纳米材料的独特性质,将其作为添加剂与基体材料混合制备复合材料已成为材料设计与制备的一个重要发展趋势[1-2]。按照维度,可将碳基材料分为零维的炭黑、富勒烯,一维的碳纳米管、碳纤维,二维的石墨烯,三维的石墨等[3]。其中,典型代表为碳基纳米材料中2个最热门的材料——碳纳米管和石墨烯,它们不但具有低密度(全部由碳元素组成)和稳定的化学性能,而且具有优异的力学性能和导电导热性能等[4-5],将其作为填料在复合材料中已获得了重要应用。
对于纳米尺寸的石墨烯和碳纳米管,巨大的比表面积和表面能使其具有易堆叠、缠绕和团聚等特性,从而显著影响其在基体中的均匀分散,并严重制约它对复合材料性能的改善效果[6-8]。为此,将二维的石墨烯与一维的碳纳米管复合,优化两者的协同作用,有望在改善分散性的同时,实现复合材料性能的显著提升。如何制备这种包含碳纳米管和石墨烯协同作用的增强体,是获取高性能复合材料的关键。
机械共混法是获得石墨烯和碳纳米管复合填料常用的方法。研究人员采用机械共混方法制备的石墨烯和碳纳米管复合填料,相较于石墨烯或碳纳米管任一单一填料,均呈现出更好的力学增强效果,但性能的提升相对有限[9-11],这与石墨烯和碳纳米管在聚合物基体中相互隔离有关,因为未形成直接的协同物理作用。近年的研究表明[12-13],采用化学气相沉积方法可以在石墨烯表面直接生长碳纳米管。通过原位生成的一维碳纳米管,使得二维的衬底石墨烯彼此隔离,形成了一种新型的三维杂化材料,这是制备碳纳米管和石墨烯复合填料更有效的方法。然而,在未经改性处理的石墨烯表面直接生长碳纳米管时,其均匀性常常难以保障。相较而言,采用化学气相沉积方法在氧化铝、二氧化硅、玻璃纤维等衬底材料上生长碳纳米管已经有较多报道[12-15]。基于此,选择具有高活性的氧化石墨烯为前驱体,采用生长碳纳米管常用的二氧化硅衬底为改性层,在其表面生长碳纳米管的同时,将氧化石墨烯还原为石墨烯,这将是一种改善石墨烯表面碳纳米管生长均匀性的重要途径。
文中采用单层氧化石墨烯为模板,首先通过四乙氧基硅烷的水解在其表面沉积1层二氧化硅层,然后采用化学气相沉积方法,在氧化石墨烯表面生长碳纳米管,同时将氧化石墨烯还原,制备成碳纳米管包覆石墨烯的三维杂化材料,还研究了二氧化硅层对氧化石墨烯表面生长碳纳米管的影响。
1 实验
1.1 材料
实验用氧化石墨烯原料购自Standard Graphene公司,牌号为GO-V50。四乙氧基硅烷(TEOS)、二茂铁、二甲苯等试剂从国药集团化学试剂有限公司购入,等级为分析纯。化学气相沉积所需气体为高纯气体(体积分数为99.99%),从长沙高科气体有限公司购入。
1.2 实验过程
首先将450 mg氧化石墨烯加入400 mL无水乙醇与50 mL去离子水的混合试剂中,超声分散30 min。然后,在磁力搅拌下,向氧化石墨烯分散液中缓慢滴入氨水,将其pH调至9~10之间,再加入1 mL四乙氧基硅烷(TEOS),在室温下反应16 h。通过离心分离后获得沉淀物,分别采用乙醇清洗2次,去离子水清洗1次。将清洗干净的沉淀物重新加入75 mL的去离子水与2 mL乙醇的混合溶剂中,超声30 min,直至完全分散。最后,将分散液倒入石英皿中,并放入冷冻干燥机中处理48 h,获得GO@SiO2。
采用化学气相沉积方法,先将GO@SiO2放入管式炉中,通入240 mL/min氢气和700 mL/min氩气后开始加热,待温度升至650 ℃后,再通入0.05 mg/mL二茂铁/二甲苯催化剂溶液和60 mL/min乙炔,分别将反应时间控制在5 min和10 min,在样品表面进行碳纳米管生长,获得二氧化硅包覆的氧化石墨烯–碳纳米管杂化材料(GO@SiO2-CNTs)。采用同样的工艺条件,在未沉积二氧化硅层的氧化石墨烯表面生长碳纳米管,作为对照。
1.3 表征
实验所用扫描电子显微镜(SEM)的型号为TESCAN MIRA3 LMU,所用的透射电子显微镜(TEM)型号为FEI TITAN G2。EDS表征采用TEM仪器自带的设备。
2 结果及分析
在不同放大倍数下,GO的SEM图如图1a~b所示。文中所用的GO在使用前未经任何处理。从图1a可以看出,GO呈不规则的片状结构,直径在100 μm以下,在观测电压下,其片层结构为半透明状态,表明其厚度非常薄。从放大图(图1b)中可以看出,GO表面较光滑,无颗粒和其他杂质富集。
通常,GO由石墨或者石墨烯通过强氧化处理所得,其表面含有丰富的含氧官能团,在超声处理后能在水溶液中稳定分散。将溶液的pH调至9,使滴入GO分散液中的TEOS发生水解,利用GO表面的含氧官能团与水解产物中的硅氧键反应,在GO表面沉积二氧化硅。在不同放大倍数下,GO@SiO2的SEM图如图1c~d所示。从图1可以看出,沉积了二氧化硅层后,GO片层的形状并未发生明显变化,也呈现半透明状态,表明沉积的二氧化硅层非常薄。从图1d可以看出,GO@SiO2表面仍然非常光滑,并无颗粒富集。由图1可知,在沉积二氧化硅层前后,对应GO的SEM图并未发生明显变化。
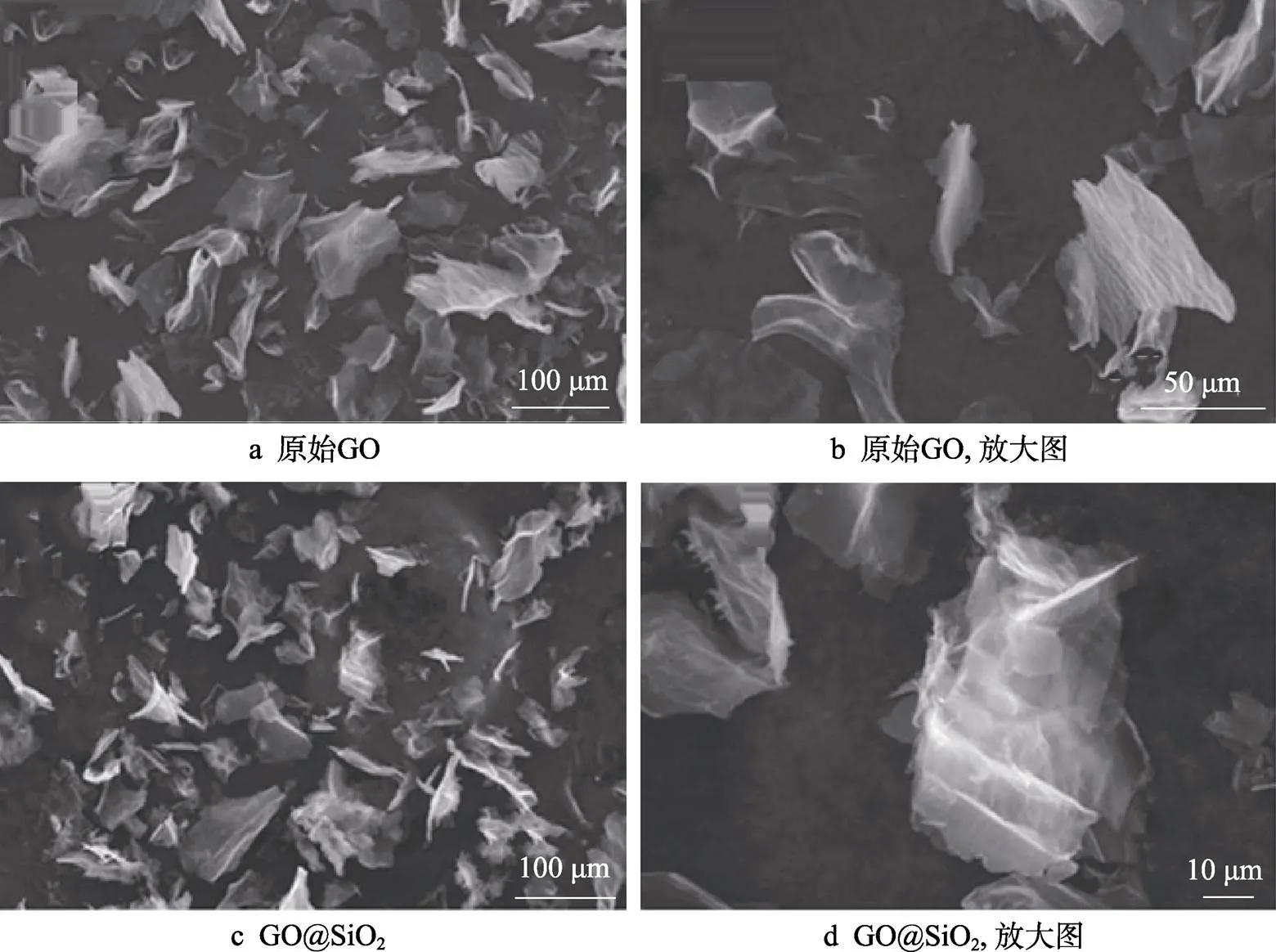
图1 GO与GO@SiO2的SEM图
通过STEM和EDS能够更清晰地观察沉积二氧化硅层前后GO的形貌和成分表征。GO的STEM图如图2a所示。STEM样品的制备:将稀释的GO分散液滴至含碳膜的铜网上。图2中所示的网状结构就是铜网中的碳膜,由于GO非常薄,因此在STEM图上表现为透明状态,中间覆盖有氧化石墨烯的地方比周围的碳膜稍亮些。对图2a中相应区域进行碳、氧、硅元素的EDS面扫描,结果分别如图2b~d所示。由图2可以看出,GO主要含有碳元素和氧元素,未含硅元素。图2 d中的少量亮点为噪点。
GO@SiO2的STEM图如图3a所示。由图3可见,GO@SiO2的STEM图与原始GO相比无变化。由对应的EDS元素分布图(图3b~d)可知,GO@SiO2除了含有碳元素和氧元素外,硅元素的含量也明显增加,而且硅元素的分布相对均匀。由此可见,通过TEOS的水解反应,可以在GO表面均匀地沉积二氧化硅层。由于沉积层的厚度非常薄,因此并不会明显改变氧化石墨烯的形貌。
制备的GO-CNTs杂化材料的SEM图如图4所示,反应时间为5 min。从图4a可以看出,少部分的GO表面覆盖有毛绒状碳纳米管,大部分的GO表面光滑,并未长出碳纳米管。从图4b~d可以看出,对于覆盖有碳纳米管的GO,其形貌也不尽相同,有的呈聚集状,也有的呈完全覆盖状,所生长的碳纳米管未表现为阵列状,而是较弯曲,且长度较短。
制备的GO@SiO2-CNTs杂化材料的SEM图如图5所示,反应时间为5 min。从图5a可以看出,在SEM观测区域内所有的GO片层都被碳纳米管覆盖,碳纳米管呈阵列状,整齐地垂直生长在GO表面。从图5b~d可以看出,在可观察到的杂化材料中,碳纳米管覆盖的形式均相同,呈阵列状,碳纳米管生长的长度在10 μm左右。所得的杂化材料非常规整,一维的碳纳米管和二维的石墨烯形成了一个三维的“刷”状结构。
为了研究不同CVD反应时间对碳纳米管在GO@SiO2表面生长的影响,将CVD的反应时间增至10 min,对应生成的杂化材料的SEM图如图6所示。从图6a可以看出,在SEM观测区域内GO片层均被碳纳米管覆盖,使得碳纳米管更密实,其长度也显著增至30 μm左右,仍然呈阵列状,整齐地垂直生长在氧化石墨烯表面。基于碳纳米管的包覆,难以清晰地观察氧化石墨烯边缘。从图6b~d可以看出,对于杂化材料,碳纳米管的覆盖形式都相同。
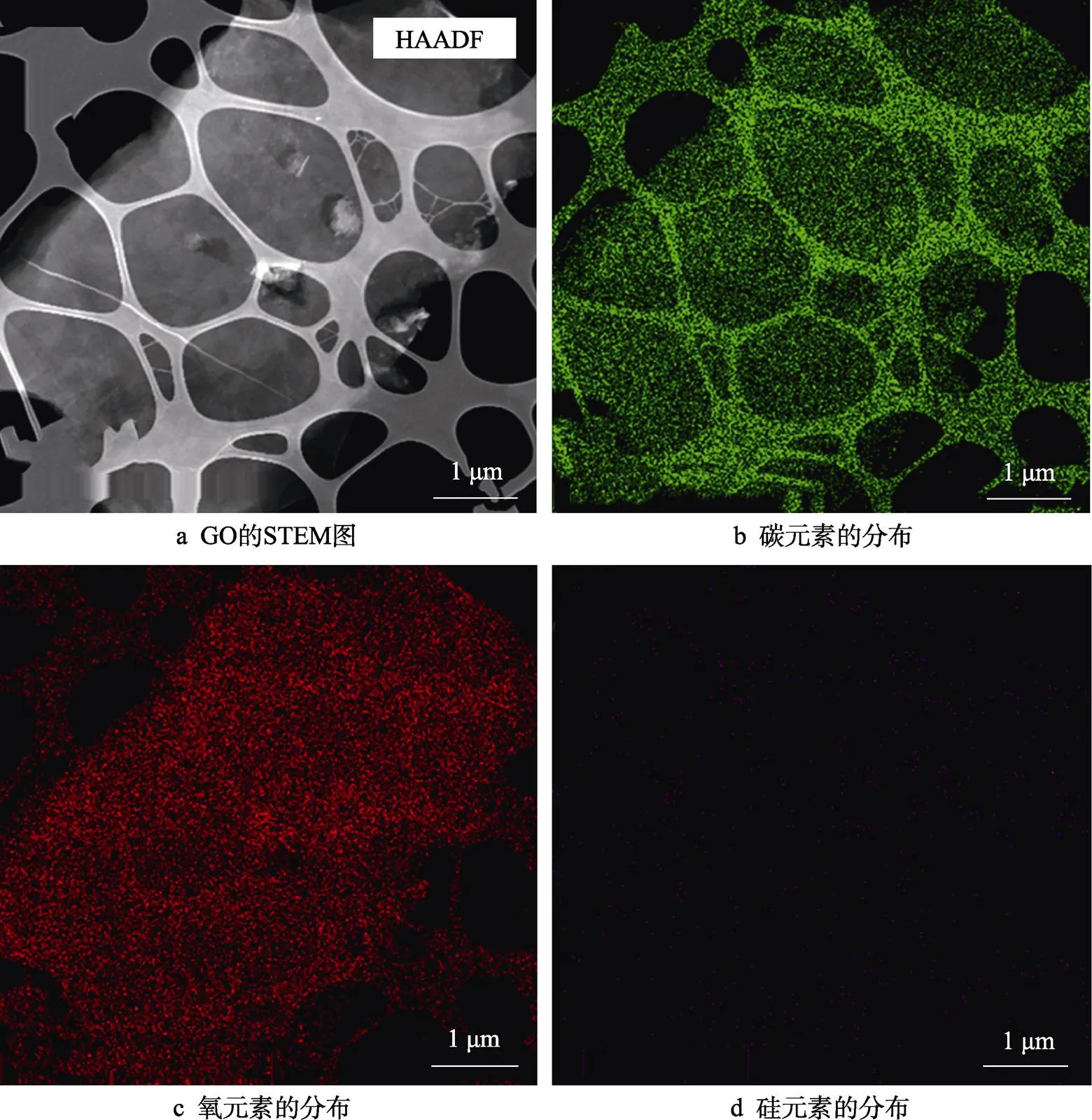
图2 GO的STEM图与EDS分析图
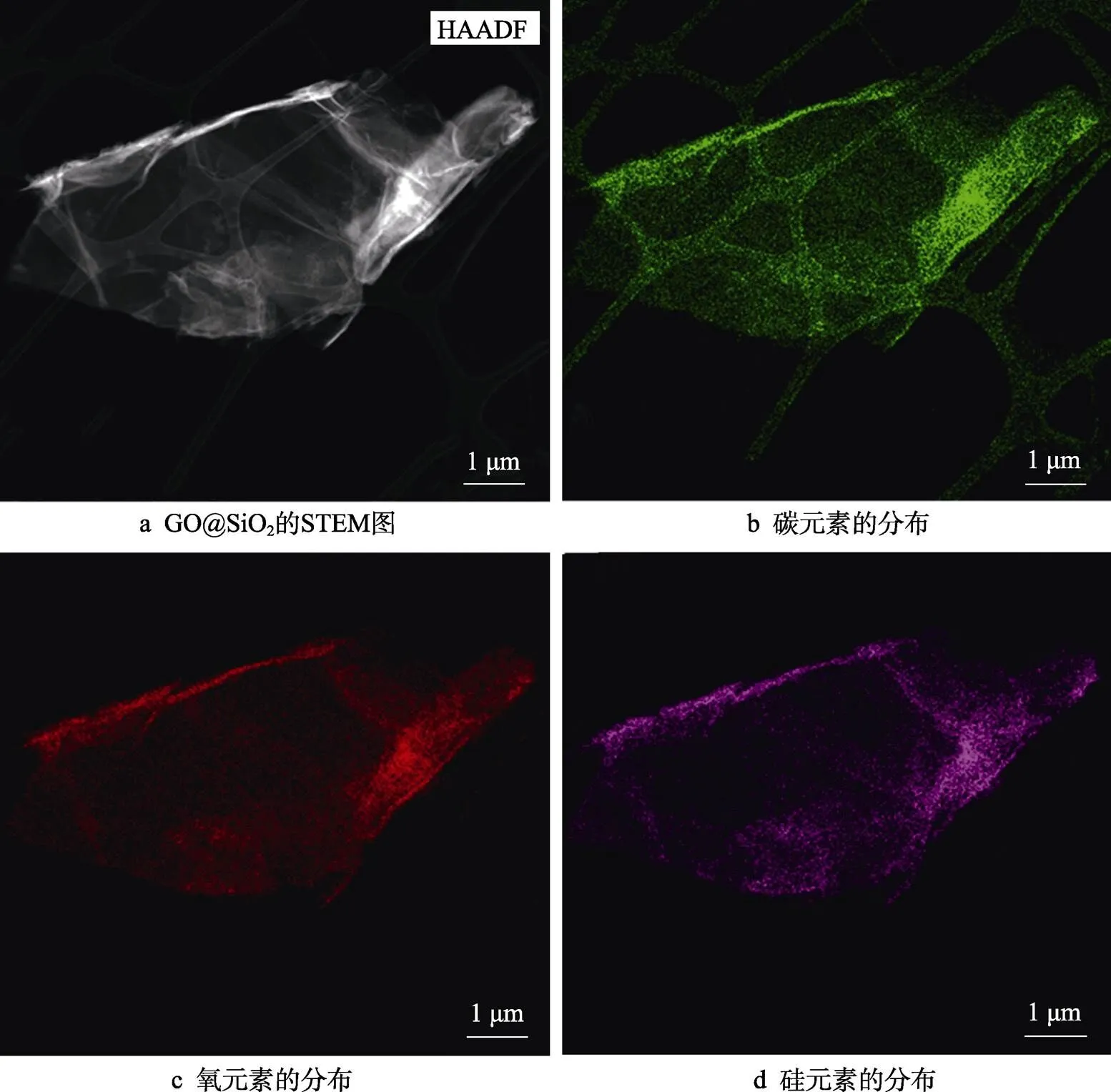
图3 GO@SiO2的STEM图和EDS分析
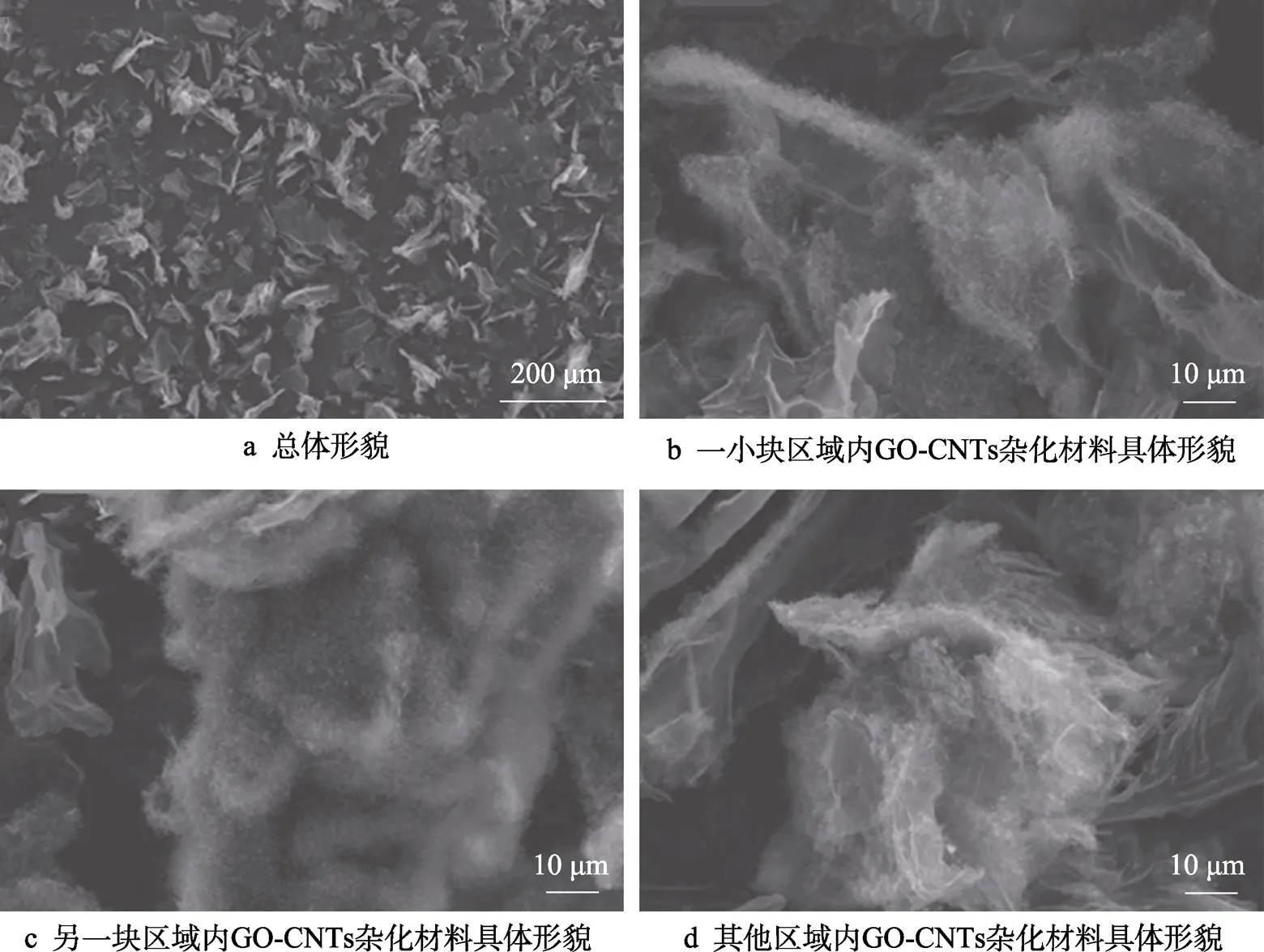
图4 GO-CNTs杂化材料SEM图

图5 CVD时间为5 min的GO@SiO2-CNTs杂化材料SEM图

图6 CVD时间为10 min的GO@SiO2-CNTs杂化材料SEM图
通过拉曼光谱分析,可以获得GO、GO@SiO2和杂化材料的更精确的结构和组成信息,结果如图7所示。原始GO和GO@SiO2在1 341、1 602 cm−1附近出现了2个特征峰,分别对应碳材料典型的D峰和G峰。D峰与G峰的强度比通常用于量化石墨化的排序。GO@SiO2与GO相比,D峰与G峰的强度比未发生明显变化。对于混合体,其D峰已转移至1 330 cm−1处,这是由于CNT在衬底表面生长。
为了明确采用CVD工艺制备的杂化材料的成分特征,对反应5 min后的杂化材料样品进行了TEM和STEM表征,结果如图8所示。从图8a~b可以看出,密实的碳纳米管将氧化石墨烯完全包覆,碳纳米管的形貌规整。在STEM模式下,对杂化材料进行了元素面扫描,如图8c~f所示。由图8可以看出,生成的杂化材料中分别含有碳、铁、硅和氧等4种元素,其中铁元素主要来自二茂铁催化剂,硅和氧元素来自二氧化硅包覆层。

图7 GO、GO@SiO2和GO@SiO2-CNTs的拉曼光谱
碳纳米管在衬底生长,究其原因是催化剂颗粒与衬底材料的相互作用。在碳纳米管的生长机理中,基于2个典型的理论——顶部生长原理、底部生长原理,它们都涉及催化剂颗粒与衬底材料的相互作用。早在二十多年前,斯坦福大学戴宏杰教授研究组就对氧化硅的表面进行了表征,结果表明,氧化硅的表面有一种羟基,使得氧化硅颗粒与金属发生相互作用[16]。氧化石墨烯在CVD过程中处于还有氢气的还原气氛中,使得其表面的含氧官能团减少,与催化剂颗粒的相互作用降低,这不利于碳纳米管在其表面的生长。根据以前发表的文献,对比硅衬底和氧化硅衬底,碳纳米管有选择性地在氧化硅衬底生长[15,17-18]。当选择氧化石墨烯作为衬底材料时,采用化学气相沉积在其表面生长的碳纳米管的形貌并不好,而经氧化硅过渡层修饰后,其生长状况得到明显改善。由此可见,相较于石墨烯衬底,碳纳米管有选择地在氧化硅表面生长,氧化硅衬底对于催化剂颗粒的吸附优于石墨烯。
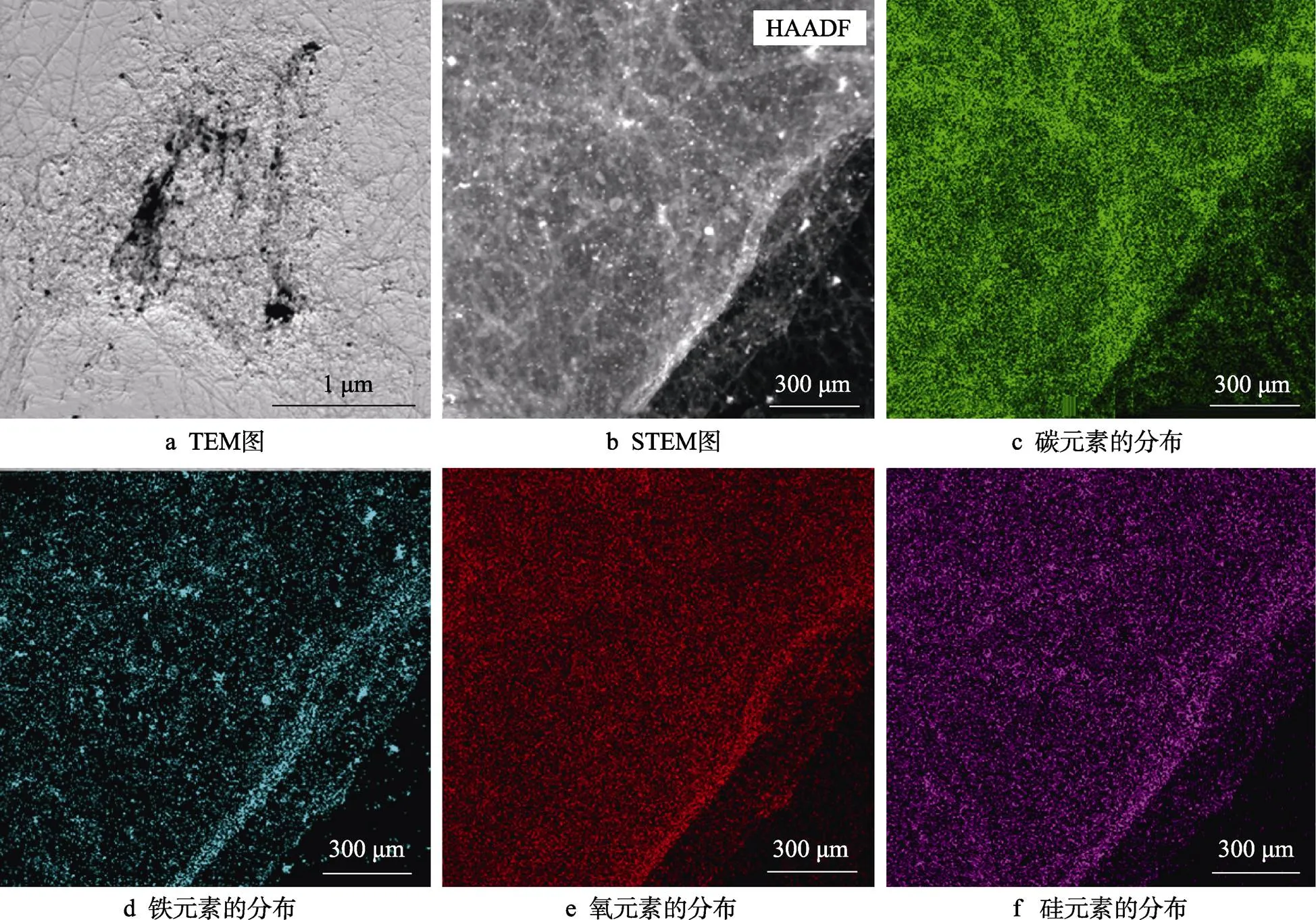
图8 GO@SiO2-CNTs杂化材料的TEM和STEM-EDS图
3 结论
采用四乙氧基硅烷在氧化石墨烯分散液中进行水解反应,在氧化石墨烯表面包覆一层二氧化硅,然后采用化学气相沉积方法在其表面生长碳纳米管,对比了二氧化硅层包覆前后氧化石墨烯表面的碳纳米管生长状态。结果表明,二氧化硅层的包覆可以有效促进碳纳米管在氧化石墨烯基底的生长,随着CVD反应时间的增加,碳纳米管的长度和生长密度都得到一定增长,所获得的杂化材料形貌规整,碳纳米管呈阵列状生长。
[1] KUILLA T, BHADRA S, YAO Da-hu, et al. Recent Advances in Graphene Based Polymer Composites[J]. Progress in Polymer Science, 2010, 35(11): 1350-1375.
[2] SENGUPTA R, BHATTACHARYA M, BANDYOPADHYAY S, et al. A Review on the Mechanical and Electrical Properties of Graphite and Modified Graphite Reinforced Polymer Composites[J]. Progress in Polymer Science, 2011, 36(5): 638-670.
[3] ROY N, SENGUPTA R, BHOWMICK A K. Modifications of Carbon for Polymer Composites and Nanocomposites[J]. Progress in Polymer Science, 2012, 37(6): 781-819.
[4] BAUGHMAN R H, ZAKHIDOV A A, DE HEER W A. Carbon Nanotubes—the Route Toward Applications[J]. Science, 2002, 297(5582): 787-792.
[5] KUMAR A, SHARMA K, DIXIT A R. Carbon Nanotube- and Graphene-Reinforced Multiphase Polymeric Composites: Review on Their Properties and Applications[J]. Journal of Materials Science, 2020, 55(7): 2682-2724.
[6] JANGAM S, RAJA S, REDDY K H. Effect of Multiwalled Carbon Nanotube Alignment on the Tensile Fatigue Behavior of Nanocomposites[J]. Journal of Composite Materials, 2018, 52(17): 2365-2374.
[7] YAO Hai-qing, HAWKINS S A, SUE H J. Preparation of Epoxy Nanocomposites Containing Well-Dispersed Graphene Nanosheets[J]. Composites Science and Technology, 2017, 146: 161-168.
[8] ARADHANA R, MOHANTY S, NAYAK S K. Comparison of Mechanical, Electrical and Thermal Properties in Graphene Oxide and Reduced Graphene Oxide Filled Epoxy Nanocomposite Adhesives[J]. Polymer, 2018, 141: 109-123.
[9] YAO Xu-ming, JIANG Jian-jun, XU Chu-meng, et al. Improved Interfacial Properties of Carbon Fiber/Epoxy Composites through Graphene Oxide-Assisted Deposition of Carbon Nanotubes on Carbon Fiber Surface[J]. Fibers and Polymers, 2017, 18(7): 1323-1329.
[10] LI Yun-yu, GUO Ling-jun, WANG Ya-wen, et al. A Novel Multiscale Reinforcement by In-Situ Growing Carbon Nanotubes on Graphene Oxide Grafted Carbon Fibers and Its Reinforced Carbon/Carbon Composites with Improved Tensile Properties[J]. Journal of Materials Science & Technology, 2016, 32(5): 419-424.
[11] MAKA H, SPYCHAJ T, PILAWKA R, et al. Influence of Hybrid Carbon Nanofillers on the Cure Behavior and Properties of Epoxy Materials[J]. Polimery, 2016, 61(3): 181-188.
[12] LI Wei-kang, HE De-long, DANG Zhi-min, et al. In Situ Damage Sensing in the Glass Fabric Reinforced Epoxy Composites Containing CNT–Al2O3Hybrids[J]. Composites Science and Technology, 2014, 99: 8-14.
[13] HE De-long, FAN Ben-hui, ZHAO Hang, et al. Design of Electrically Conductive Structural Composites by Modulating Aligned CVD-Grown Carbon Nanotube Length on Glass Fibers[J]. ACS Applied Materials & Interfaces, 2017, 9(3): 2948-2958.
[14] LI Wei-kang, DICHIARA A, ZHA Jun-wei, et al. On Improvement of Mechanical and Thermo-Mechanical Properties of Glass Fabric/Epoxy Composites by Incorporating CNT-Al2O3Hybrids[J]. Composites Science and Technology, 2014, 103: 36-43.
[15] CAO An-yuan, AJAYAN P M, RAMANATH G, et al. Publisher's Note: Silicon Oxide Thickness-Dependent Growth of Carbon Nanotubes[J]. Applied Physics Letters, 2004, 84(11): 2002.
[16] CASSELL A M, RAYMAKERS J A, KONG Jing, et al. Large Scale CVD Synthesis of Single-Walled Carbon Nanotubes[J]. The Journal of Physical Chemistry B, 1999, 103(31): 6484-6492.
[17] ZHANG Z J, WEI B Q, RAMANATH G, et al. Substrate- Site Selective Growth of Aligned Carbon Nanotubes[J]. Applied Physics Letters, 2000, 77(23): 3764-3766.
[18] ZHANG Z J, WEI B, WARD J W, et al. Select Pathways to Carbon Nanotube Film Growth[J]. Advanced Materials, 2001, 13(23): 1767-1770.
Effect of Silicon Dioxide Layer on the Growth of Carbon Nanotubes on the Surface of Graphene Oxide
1,1,2,1,1,1*,1
(1. Powder Metallurgy Research Institute, Central South University, Changsha 410083, China; 2. Air Force Military Delegate Bureau in Zhuzhou Military Representative Office, Army Armament Ministry, Hunan Zhuzhou 412000, China)
The work aims to achieve the controllable growth of carbon nanotubes (CNTs) on the surface of graphene oxide (GO) by depositing an intermediate layer of silicon dioxide (SiO2) on the surface of GO. A silicon dioxide-coated graphene oxide (GO@SiO2) was obtained through hydrolyzing drops of tetraethoxysilane (TEOS) in the dispersion of GO with adjusted pH. Then, a layer of SiO2was formed on the surface of GO. Subsequently, several SiO2-coated graphene oxide-carbon nanotubes hybrid materials (GO@SiO2-CNTs) were prepared by growing carbon nanotubes on the surface of GO@SiO2through the method of floating catalyst chemical vapor deposition (CVD) for different deposition time. For comparison, some graphene oxide-carbon nanotubes hybrid materials (GO-CNTs) were prepared under the same CVD condition with GO without the SiO2layer. The effect of the SiO2layer on the growth of carbon nanotubes was further studied by comparing the results of SEM, STEM and EDS of the GO@SiO2-CNTs and GO-CNTs. Also, the possible mechanism of the SiO2transition layer affecting the growth of CNTs was proposed. The result of EDS analyzation showed that a layer of silicon dioxide was uniformly deposited on the surface of graphene oxide after the hydrolyzing of TEOS at certain pH. SEM pictures indicated that the growth of CNTs on the GO was uneven with only few surfaces being covered by the CNTs, while the surface of GO@SiO2was uniformly and densely covered with carbon nanotube arrays, forming a typical "brush" structure. Moreover, the density and length of the CNTs on the surface of GO@SiO2can be modulated by altering the CVD time. The main reason for the growth of CNTs on the substrate is the interaction of the catalyst particles with the substrate material. There are two typical theories of the growth mechanism of CNTs: one is the top growth theory, and the other one is the bottom growth theory, which both involves the interaction of the catalyst particles with the substrate material. About 20 years ago, Professor Dai of Stanford University and his team characterized the surface of SiO2, indicating that there is a hydroxyl group like structure on the surface of SiO2that makes its particles have a certain interaction with the metal. While in the CVD process of GO, the oxygen-containing functional groups are reduced by the reducing hydrogen atmosphere, leading to the reduction of the interaction with catalyst particles, which finally restrains the growth of CNTs on the surface. According to previously published papers, CNTs prefer to grow on SiO2substrates rather than the silicon substrates. Thus, when GO is chosen as the substrate material, the morphology of CNTs grown on its surface by CVD is not good. Yet, after the modification with the SiO2transition layer, the growth condition is much improved. Therefore, compared with GO substrates, CNTs are selectively grown on the surface of SiO2, which further proves that the SiO2substrates are better than the GO substrates. In conclusion, the growth of CNTs on the GO substrate is greatly improved with the existence of SiO2layer, which also helps to control the growth of length and density of the CNTs.
CVD; graphene oxide; carbon nanotubes; SiO2layer
2022-10-10;
2022-10-16
TB321
A
1001-3660(2023)10-0287-08
10.16490/j.cnki.issn.1001-3660.2023.10.024
2022-10-10;
2022-10-16
国家自然科学基金青年项目(52002403);湖南省自然科学基金青年项目(2022JJ40607)
Youth Fund of the National Natural Science Foundation of China (52002403); Youth Fund of the National Natural Science Foundation of Hunan Province (2022JJ40607)
朱静泊, 邹杨君, 唐小天, 等. 二氧化硅层对氧化石墨烯表面生长碳纳米管的影响[J]. 表面技术, 2023, 52(10): 287-294.
ZHU Jing-bo, ZOU Yang-jun, TANG Xiao-tian, et al. Effect of Silicon Dioxide Layer on the Growth of Carbon Nanotubes on the Surface of Graphene Oxide[J]. Surface Technology, 2023, 52(10): 287-294.
通信作者(Corresponding author)
责任编辑:彭颋

