Reversible surface modification of PAN-based carbon fibers by a ferrocene-based surfactant
ZHANG Xiao-fang, YAO Ting-ting, LIU Yu-ting, WU Gang-ping,*
(1. CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China;3. National Engineering Laboratory for Carbon Fiber Technology, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China)
Abstract: The surface of carbon fibers (CFs) was modified by a surfactant (ferrocenemethyl)dodecyldimethylammonium bromide (FDDA) to enhance the interfacial ashesion between the CFs and surrounding matrix. Results showed that it could be electrochemically desorbed by a potentiostatic electro-oxidation method. The FDDA adsorption isotherm was attributed to the formation of multi-molecular layers mainly by non-electrostatic interactions. The adsorption and desorption of FDDA on the CFs have little effect on their tensile strength. The effects of FDDA modification on the interfacial properties of CF/epoxy composites were evaluated by a single-filament fragmentation test. Compared with the un-modified CFs, the FDDA-modified ones had significantly improved interfacial adhesion properties in the composites. This method provides a potential approach for preparing recyclable CF/resin composites.
Key words: Carbon fibers;FDDA;Reversible modification;Interfacial properties
1 Introduction
Carbon fibers (CFs) have been widely used in the aerospace, sports and automotive industries due to their excellent properties, such as high strength, high modulus, high temperature resistance, along with low density and low thermal expansion coefficient[1-3].However, CFs without surface treatment are chemically inert, which results in weak interfacial adhesion between the CFs and their surrounding matrix[4-5]. To solve this problem, various methods have been developed to improve their hydrophilicity by introducing chemically-active functionalities to CFs[6].
Conventional methods for CF surface modification are sizing[7-8], gas- or liquid-phase oxidation[9-10],electrochemical oxidation[11], plasma treatment[12],chemical grafting[13]and so on. Jiang et al.[14]carried out electrochemical oxidation treatment of CF by electrophoretic deposition technology, and its surface roughness and wettability increased. Altay et al.[15]treated the recycled carbon fiber with atmospheric pressure plasma at different powers (100, 200 and 300 W) to improve the mechanical properties of the recycled carbon fiber reinforced polypropylene composite. Li et al.[13]have established a direct and efficient method to fabricate graphene /CF graded reinforcement materials based on chemical bonding. The graphene/CF reinforced material was prepared by rapid insertion and addition reaction of diaryl carbene.However, these methods are mainly chemically in nature. They could readily cause damages and defects to CF surfaces, leading to degradation in mechanical properties of final CF-reinforced composites. More importantly, the chemical bonding between CFs and matrix makes it difficult to extract CFs in recycling composites.
It is desirable to find a surface modifier that can both modify the CF surface and be readily removed if necessary. Switchable surfactants have been reported to possess amphiphilic structures, and they can respond to changes in external conditions, such as light,temperature, redox conditions and so on[16-20]. By controlling external stimulus, the surfactants can be switched into active or inactive status. Peng et al.[16]have found that reversible trans cis photoisomerization of the azobenzene group occurred efficiently in dilute solutions for azo-surfactants under alternative UV and visible light irradiation. Tsuchiya et al.[17]have achieved control of the viscoelasticity of a mixing system of cationic redox switchable ferrocene and sodium salicylate by electrochemical redox reactions.
In this study, a cationic reversible surfactant,(ferrocenemethyl)dodecyldimethylammonium bromide (FDDA), was used for surface modification of CFs. The FDDA adsorption isotherms on the CFs and the factors affecting the adsorption capacity were investigated, and the transition between active and inactive state of the surfactant was adjusted by using electrochemically-stimulated redox reactions. Furthermore, the interfacial properties between CFs and epoxy matrix were investigated through a single-filament fragmentation test.
2 Experimental
2.1 Materials
The FDDA was purchased from Tokyo Chemical Industry Co., Ltd. (Japan), and its structure is shown in Fig. 1. The CFs were purchased from Toray Industry Co., Ltd. (T700, 12K, Japan), and they were further desized for adsorption of FDDA at 700 °C in nitrogen for 30 min. The epoxy resin was supplied by Shenzhen NO.1 Chemical Co., Ltd. (produced from bisphenol A and epichlorohydrin, China).
2.2 Adsorption and desorption experiments
2.2.1 Adsorption experiments
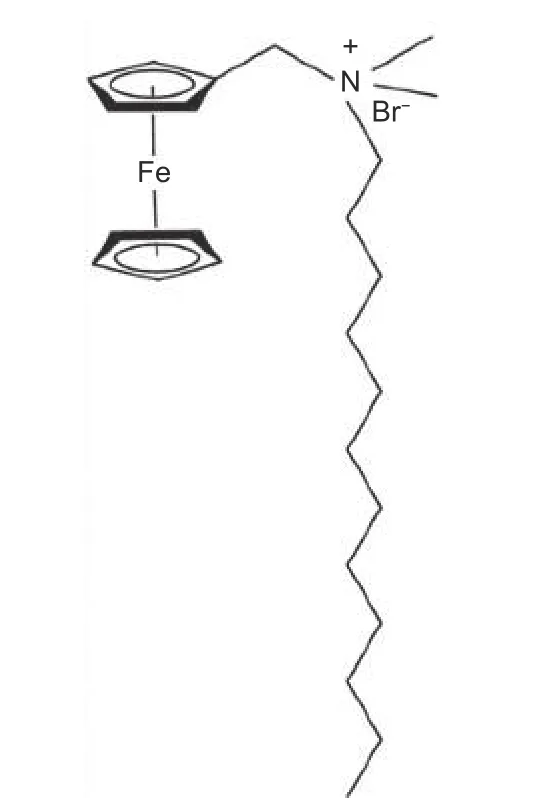
Fig. 1 Structure of FDDA
A certain amount of CFs were added to FDDA solutions with concentrations ranging from 0 to 2 mmol/L in 20 mL vials. The vials were then shaken in an Oscillator (HSHZ-B) at 20 r/min for 12 h at constant temperatures, and left it stand still for 12 h to ensure adsorption equilibrium. Finally, the samples were centrifuged at 3 000 r/min for 30 min, and the free FDDA in the supernatants were quantified by an ultraviolet spectrophotometer. The adsorbed amounts were determined from the difference in the FDDA concentrations before and after adsorption, as shown in Equation 1[21]:
whereΓis the equilibrium adsorption amount of FDDA,C0is the initial concentration of the FDDA solution,C1is the free FDDA concentration after the adsorption equilibrium,Vis the solution volume, andmis mass of CFs (adsorbents).
The effects of pH value and temperature on the adsorption capacity of FDDA on CFs were investigated. The adsorption experiments at various pH values adjusted by HCl or NaOH were performed in 0.6 or 1.4 mmol/L FDDA solutions at 20 ± 1 °C. Analogously, the adsorption experiments at various temperatures were performed in 0.6 or 1.4 mmol/L FDDA solutions at neutral pH. The experimental conditions were otherwise identical to the above-mentioned adsorptions.
2.2.2 Desorption experiments
The FDDA has good electrochemical reversibility verified by using cyclic voltammetry[22]. In this work, the FDDA adsorbed on CFs was removed by a potentiostatic electro-oxidation method (electrolysis at a constant voltage of 0.8 V for 24 h) under N2atmosphere. The electrolysis was conducted in a 3-electrode system, in which a bunch of FDDA-adsorbed CFs, a platinum plate and a saturated calomel electrode were used as the working, the counter and reference electrodes, respectively. The spacing between the CFs and platinum plate is about 4 cm. A 0.2 mol/L Li2SO4aqueous solution was used as the supporting electrolyte.
2.3 Tensile strength of the CFs
The tensile strength of CFs was tested by using a single fiber stretching machine. The stretching rate was set as 2 mm/min and gauge length 20 mm. The statistical distribution of CF tensile strength data is defined by the 2-parameter Weibull distribution described by Equation 2[23]:
Equation 2 can then be transform to Equation 3:
whereF(σ) is the cumulative probability distribution,σis the fiber tensile strength,σ0andmare the Weibull scale and shape parameters, respectively. The tensile strength data were linearly fitted by using the leastsquares method, and theσ0andmwere determined according to Equation 3.
2.4 Single-filament fragmentation tests
The CF/matrix interfacial adhesion properties were evaluated by a single-filament fragmentation test. During the unidirectional CF/matrix fabrication process, a dog-bone shaped mould was used, in which a single fiber was straightened along the longitudinal direction and fixed on both sides. Then the single fiber was cured by epoxy resin with a curing agent (polyamide) at 60 °C for 12 h, so as to obtain the specimen for the single-filament fragmentation test. The specimen was tensile-stretched up to 5% by a mini-tensile machine (Jinan Shidai Shijin Testing Machine Group Co. Ltd, WDW-T2, China) with a crosshead speed of 0.2 mm/min and a gauge length of 15 mm.
The interfacial shear strength (IFSS) was calculated by the Kelly-Tyson Equation[24]:
whereτis the interfacial shear strength,σ(lc) is the fiber strength at the critical length,dis the fiber diameter,lcis the critical fragment length of the fiber.
The fiber strength at the critical fragment length was calculated using the Weibull weakest-link theory[25].
whereσ(l0) is the fiber strength at the gauge length ofl0(20 mm) andmis the Weibull modulus.
2.5 Characterization
The UV measurements were performed in 10 mmwided quartz cells by a UV spectrophotometer (Shimadzu UV-2600, Japan). The absorbance peak of FDDA was determined at 323 and 432 nm, consistent with literature reports[19]. The morphologies of CFs were observed by a field emission scanning electron microscope (FE-SEM, JEOL, JSM-7001F) at an accelerating voltage of 5 kV. The surface chemistries of the fibers were studied by an X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI) with AlKα (1 486.6 eV) X-ray source operated at 12.5 kV.
3 Results and discussion
3.1 SEM observations
The surface morphologies of the CFs with and without FDDA adsorption are shown in Fig. 2. The surface of the as-received CFs were generally smooth,with some shallow grooves (Fig. 2a). With an increase in the FDDA concentration after adsorption,the grooves were gradually disappeared, indicating the FDDA was attached onto the CF surfaces (Fig. 2b-c).But after an electrochemical oxidation, the grooves on the CFs reappeared again (Fig. 2d), suggesting that the attached FDDA was removed from the CFs. In addition, no obvious damage to the CFs was observed.
3.2 X-ray photoelectron spectroscopy analysis
The survey XPS results are listed in Table 1.There was no ferrum species detected on the CF surface, but it suddenly appeared after FDDA adsorption,and the relative amount reached 1.5%. However, the ferrum concentration decreased to 0.88% after electrochemical oxidation, indicating that the adsorbed FDDA could be reversibly desorbed. The residual ferrum species on the surface were attributed to the insufficient oxidation for FDDA. It is worth noting that the oxygen content was significantly increased, possibly due to the partial oxidation of CFs during the electrochemical desorption.
3.3 FDDA adsorption isotherm
The FDDA adsorption isotherm on CFs is plotted in Fig. 3. With an increase in the FDDA concentration, the adsorbed amount of FDDA on the CFs increased significantly, and then reached a plateau at concentration above 1.4 mmol/L (close to the CMC value of 1.36 mmol/L), This is a typical Langmuir adsorption isotherm[26]. The saturated adsorption amount of FDDA could be determined to be 0.15 mmol/g.
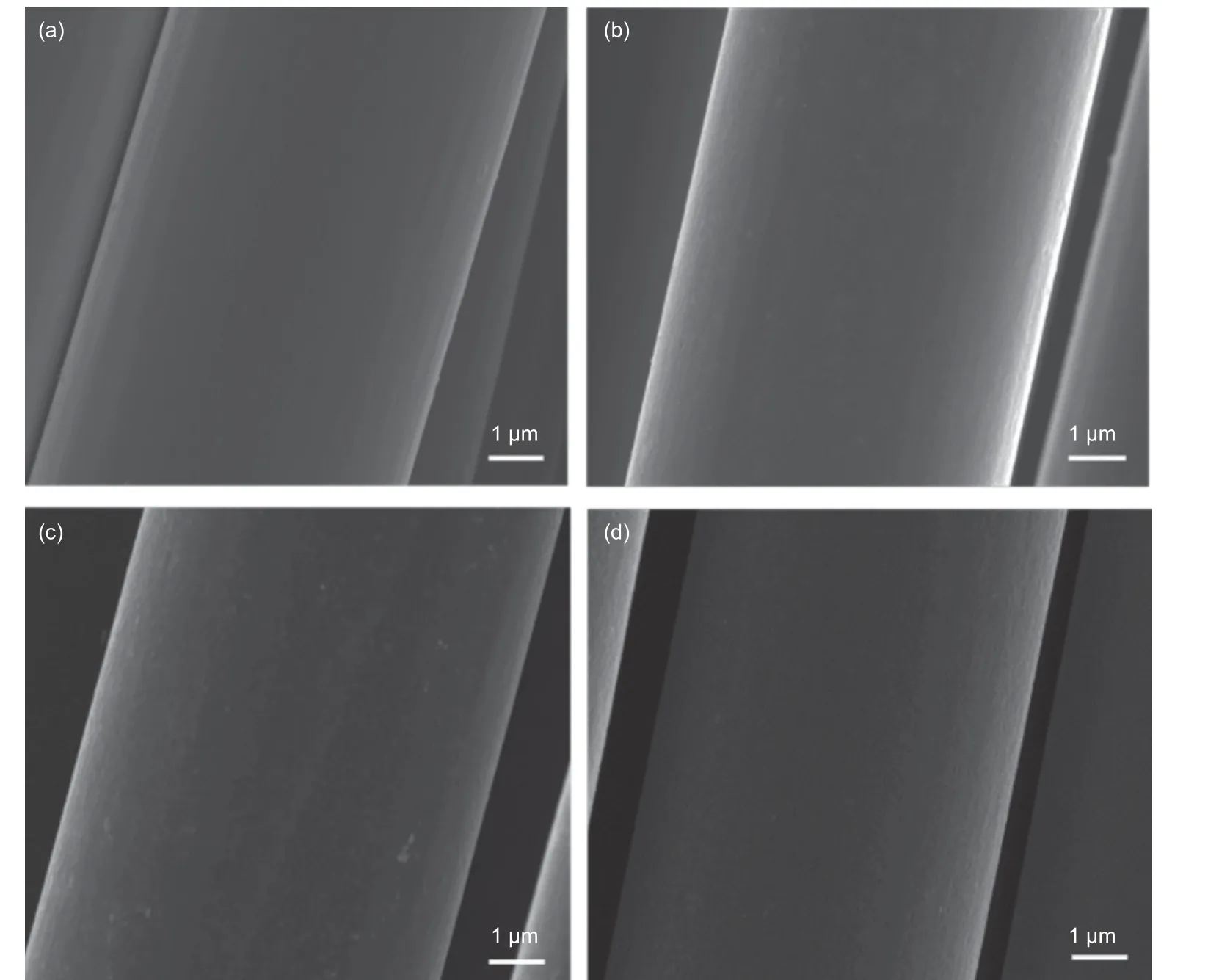
Fig. 2 SEM images of CFs: (a) before adsorption, (b) after adsorption (0.8 mmol/L, lower than CMC), (c) after adsorption (1.6 mmol/L, higher than CMC),(d) after adsorption and electrochemical desorption
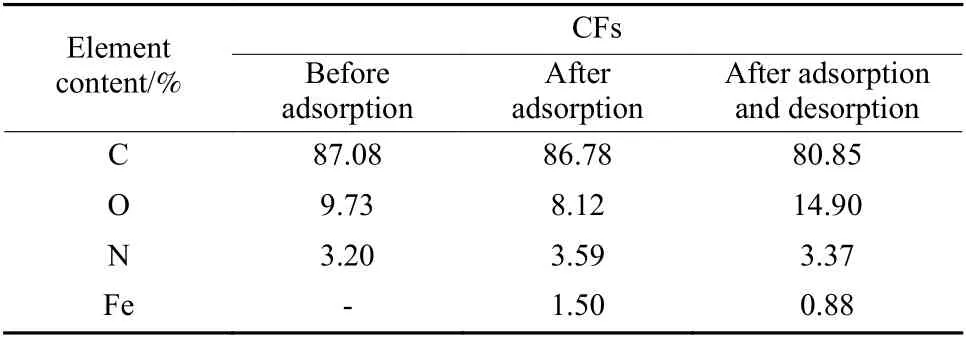
Table 1 The relative contents of surface elements on the CFs
Both the Langmuir and Freundlich models were employed to fit the obtained FDDA adsorption results.The fitting parameter (R2) is a statistical measure of how closely the data are fitted to the regression line.TheR2for both models were highly close to 1, indicating that both models were suitable for describing the FDDA adsorption isotherms on CFs. The Freundlich model is an empirical equation usually used for the multi-layer adsorption[27], suggesting that the FDDA adsorption on the CFs was very possibly in multi-layers.
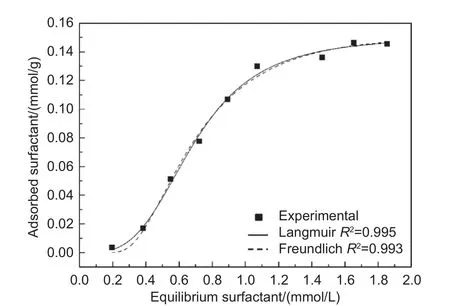
Fig. 3 FDDA adsorption isotherm for CFs and the fitting curve with the Langmuir (solid line) and Freundlich (dotted line) models
In order to further understand the FDDA thermodynamic adsorption on the CFs, the Gibbs adsorption isotherm (Equation 6)[28]was used to estimate the occupied area by one FDDA molecule,
whereΓ1is the excessive amount of the FDDA,Γ1is the maximum adsorption capacity,γis the surface tension, and NAis Avogadro’s constant. From the slope of the linear fitting at low FDDA concentrations (from 0 to CMC), the ∂γ/∂(lgC) could be obtained for calculating theΓ1value in Equation 6.
The calculated results showed that the occupied area by one FDDA molecule was about 0.68 nm2. Assuming that the process is a monolayer adsorption, the saturated adsorption capacity should be 7.76×10-4mmol/g (the surface area of 1 g CFs is about 3 178.33 cm2). Comparatively, the saturated adsorption capacity obtained by the experimental adsorption isotherm was 0.15 mmol/g, almost 200 times the calculated values based on the monolayer adsorption model. Thus, it is reasonable to conclude that the FDDA should be in multi-layers around the CF surfaces, at least in part,during the adsorption process.
3.4 Interaction between FDDA and CFs
Three possible FDDA-CF interaction modes should be considered, electrostatic attraction or repulsion, π-π interaction, and hydrophobic interaction.Fig. 4 shows the temperature-dependence of the adsorption capacity of FDDA on the CFs. The adsorption amount decreased significantly with increasing the temperature, implying that the FDDA adsorption was thermodynamically dominated. Thus, the adsorption thermodynamics was considered as follows[29]:
whereKcis the thermodynamic equilibrium constant,Qeis the equilibrium adsorption capacity,Ceis the equilibrium concentration, and Δandare correspond to the entropy, enthalpy and Gibbs free energy, respectively.
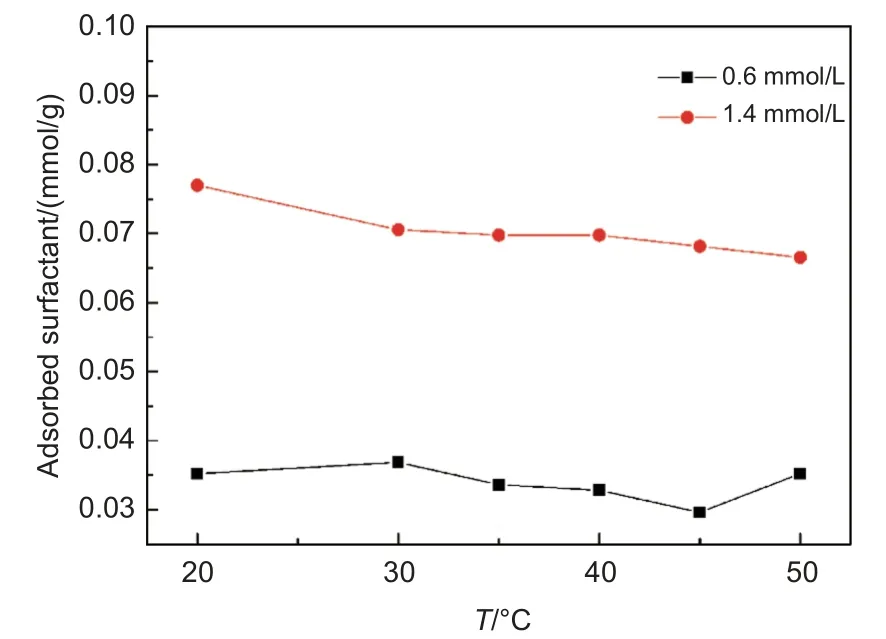
Fig. 4 The adsorbed amount of FDDA on CFs under different temperatures
The calculated thermodynamic parameters are summarized in Table 2. The values of the enthalpy changes, Δ, were negative, indicative of an exothermic process. The positive entropy changes,ΔSθmcorresponded to an increase in the degrees of freedom at the solid-liquid interface during FDDA adsorption.In all the investigated temperatures, the obtained Gibbs free energy changes, Δ, were negative, indicating that the FDDA adsorption on the CFs is spontaneous in thermodynamics. The ΔGvalues were in the range of -9.91~-10.51 kJ/mol, the typical values of physisorption (typically from -20 to 0 kJ/mol), further confirming that the physisorption should be dominant in FDDA adsorption on the CFs.
Fig. 5a shows the pH value dependence of saturated FDDA adsorption amount on CFs. The saturated FDDA adsorption amount changed only slightly in the pH value range from 2 to 12, suggesting that the electrostatic attraction may not be the dominant interaction between FDDA and the CFs.
To further explore the binding model and interaction mechanisms between FDDA and CFs, the surface chemistries of the FDDA, bare (only desized) and FDDA-absorbed CFs were subjected to X-ray photoelectron spectroscopy analysis (Fig. 5b). The XPS spectra of the bare CFs was characterized by the presence of a weak N1s line located at around 401.0 eV,from the N species in the received CFs[30]. But after the FDDA-adsorption, this weak N1s line was almost overlapped by a new strong line at about 402.5 eV, a typical N1s line from the FDDA. This further confirmed the presence of FDDA on the surface of CFs.
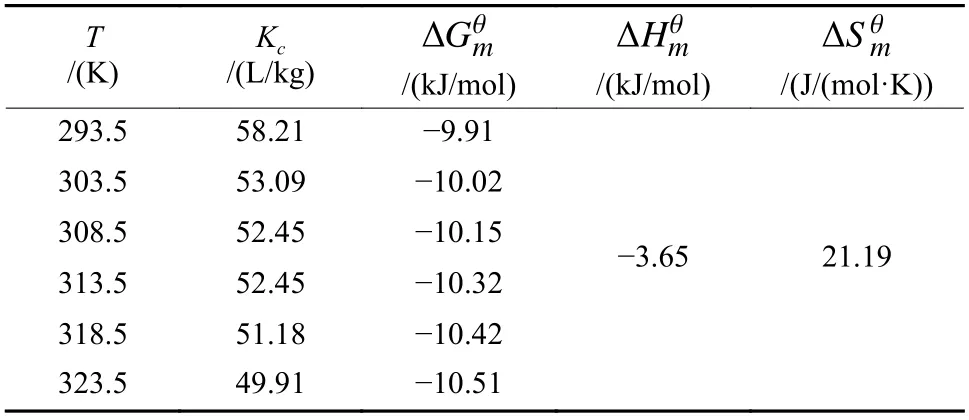
Table 2 Thermodynamics parameters for the adsorption of FDDA on the CFs
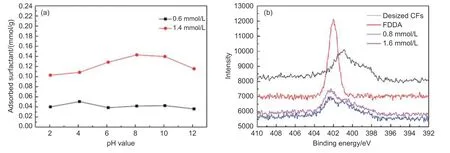
Fig. 5 (a) Effect of pH value on the adsorption of FDDA on the CFs and (b) Narrow spectrum of N1s region
In general, the non-electrostatic adsorption should be the major interaction modes for FDDA adsorption on CFs, possibly the hydrophobic interaction,π-π interaction, or both.
3.5 Changes in the tensile strength of the FDDAadsorbed CFs
Fig. 6 shows the tensile strength (σ) and Weibull modulus (m) for the bare CFs, FDDA-adsorbed CFs and the FDDA-adsorbed and electrochemically desorbed CFs. The tensile strength of the CFs changed slightly after the FDDA adsorption and electrochemical desorption, indicating that the adsorption and desorption have little damage to the CFs. This was in consistent with the SEM observation (Fig. 2).
The Weibull modulus is an indication of scattering degree for the strength data. In this study, themof the CFs before and after adsorption were very close,further indicating that the adsorption and desorption process did not cause significant damage to the CFs.
3.6 Changes in the CF/epoxy interfacial properties induced by FDDA adsorption
The IFSS of untreated CF composite was 19.34 MPa, and the IFSS of the FDDA modified CF composite was 30.82 MPa, which was increased by 59.3%. It can be seen that the interfacial shear strength between the CFs and the epoxy resin was improved by the surface modification of CFs with FDDA. Fig. 7 shows that the contact angle of epoxy resin on the adsorbed-FDDA CFs was 8.5% lower than that of as-received CFs, indicating that the wettability between the CFs and epoxy resin was improved by the adsorbed FDDA on CF surface. Therefore, it can be concluded that the improvement of wettability is important to enhance the interfacial adhesion in the composite.
Fig. 8 shows birefringence patterns of the fragmented CFs embed in epoxy resin, observed by a polarizing microscope. The birefringence diffraction pattern reflects the interfacial adhesion properties[31]. The shorter the break-point intervals, the stronger the CF/epoxy interfacial adhesion[32]. Compared with the bare CF/epoxy specimen, the birefringence patterns of the FDDA-adsorbed CF/epoxy specimen were significantly shorter, more distinct and concentrated, indicating stronger interfacial adhesion induced by FDDA adsorption.
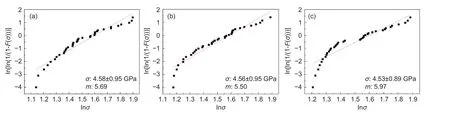
Fig. 6 The tensile strength Weibull distribution curves for (a) bare CFs, (b) FDDA-adsorbed CFs,(c) FDDA-adsorbed and then electrochemically desorbed CFs
Fig. 7 The optical photos and corresponding contact angles of epoxy resin droplets on (a) the as-received CFs and (b) the FDDA-adsorbed CFs
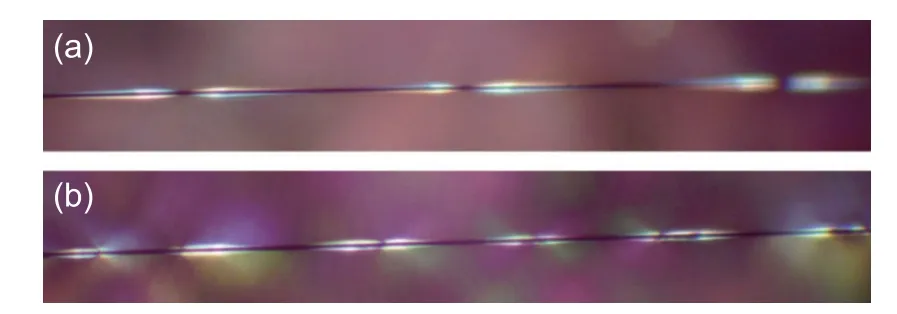
Fig. 8 The birefringence patterns of the unidirectional single-CF model composites after single-filament tensile fragmentation tests: (a) the as-received CFs and (b) the FDDA-adsorbed CFs
4 Conclusions
The CFs were reversibly surface-modified by adsorption/desorption of FDDA in multi-layers, without causing significant damages to the CFs. The adsorption isotherms showed that the non-electrostatic interaction modes were primarily responsible for adsorption, including π-π interaction and hydrophobic interaction. Furthermore, compared with the bare CFs, the FDDA-adsorbed CFs were able to afford significantly improved CF/epoxy interfacial adhesion properties.This method provides a potential approach for withdrawing CFs from a recycled composite if necessary.
Data availability statement
The data supporting the findings of this study are openly available in Science Data Bank at https://cstr.cn/31253.11.sciencedb.10400 or https://doi.org/10.57760/sciencedb.10400.
Acknowledgements
Major Science and Technology Projects of Shanxi Province (20181101020), Independent Innovation Fund Project of Shanxi Institute of Coal Chemistry-Basic Research Project Supported by ICC CAS(SCJC-HN-2022-15).
- 新型炭材料的其它文章
- Large-scale synthesis of 3D ordered microporous carbon at low temperature using cobalt ions exchanged zeolite Y as a template
- Highly efficient Co—N—C electrocatalysts with a porous structure for the oxygen reduction reaction
- Recent advances in 3D interconnected carbon/metal high thermal conductivity composites
- Development of biochar electrode materials for capacitive deionization: preparation, performance, regeneration and other challenges
- Synthesis and electrochemical properties of nano-Si/C composite anodes for lithium-ion batteries
- Factors that influence the performance of hydrogen detectors based on single-wall carbon nanotubes

