Factors that influence the performance of hydrogen detectors based on single-wall carbon nanotubes
ZHANG Zhi-feng, YANG Ye-xin, ZHU Song-lin, SHI Yan, SONG Jiang-feng,*,REN Guang-kun, DENG Shun-jie, TIAN Xiao-feng,*, ZHENG Zhe,*
(1. The College of Nuclear Technology and Automation Engineering, Chengdu Unversity of Technology, Chengdu 610059, China;2. Institute of Materials, China Academy of Engineering Physics, Jiangyou 621908, China)
Abstract: Single-wall carbon nanotubes (SWCNTs) have been used to fabricate hydrogen gas (H2) detectors for several decades.It has been proven that they barely interact with H2 so that numerous modifications are used to assist this function. Additives include metals, metal oxides, polymers etc. Previous research suggests that the presence of functional groups on the SWCNTs may improve the response by several orders of magnitude. Recently, many different novel structures have been exploited, and structural parameters of the SWCNTs, such as diameter and chirality, also influence the performance of the detectors. Modifications of the SWCNTs are classified and other factors that influence the performance are also discussed, with the aim of accelerating the manufacture of detectors with a high responsivity and low limit of detection.
Key words: Gas sensors;Single-walled carbon nanotubes;Modification;Self-characteristic;Air monitoring
1 Introduction
As one-dimensional nanomaterials, carbon nanotubes (CNTs)[1], especially single-walled carbon nanotubes (SWCNTs) have attracted much attention due to their porous structure, large surface area, and excellent properties like electrical[2-3], mechanical[3-4],thermal conductive[5-6], adsorptive field[7]and gas sensors[8-9]. Numerous applications based on CNTs or SWCNTs show great performance. For example, gas sensors based on SWCNTs show faster response at room temperature with smaller size and lower energy consumption, compared with traditional metal oxide sensors. As a result, they are considered among the best options for creating high-performance gas sensors. Notable advancements have been made in CNTs-based gas sensors since Dai and colleagues[10]initially fabricated a sensor with SWCNTs in 2000.Researchers prefer Pd/SWCNTs to other hydrogensensitive materials so far. However, the research pointed out that SWCNTs rarely interacted with H2so that the modifications on SWCNTs would be crucial for the performance. The candidates can essentially be divided into metals, metal oxides, and polymers. Additionally, it suggests that performance can be significantly improved by combining functional groups on SWCNTs with additives. Thus, the key to fabricate sensors with great response lies in the selection and preparation of suitable sensitive materials.
This Review provide a thorough summary of modification and performance in the SWCNTs-based H2sensor. First, various hydrogen-sensitive materials,including metals, metal oxides and polymers, are introduced. Then, the influence factors are discussed,like diameters and chirality of SWCNTs, which significantly affect the performance. Finally, a summary of the difficulties and potential development of SWCNTs hydrogen sensors are provided.
2 Discussion
2.1 Functionalization of SWCNTs
Covalent functionalization and non-covalent functionalization are 2 types of modifications that have been suggested for SWCNTs to enhance their hydrogen sensing capabilities. Covalent functionalization shows detrimental effects on the inherent electrical properties of SWCNTs, despite the fact that it was proven to be reliable and long-lasting. Non-covalent functionalization has the drawback of instability,which restricts the sensor's operating environment.
2.1.1 Noncovalent functionalization on SWCNTs
In general, non-covalent alteration is typically less stable than covalent functionalization. However,SWCNTs modified by nanoparticles, particularly Pd,are preferred by researchers for their exceptional sensitivity, the LOD, and response time to target gas characteristics. The interaction between bare SWCNTs and many kinds of gases have been researched by both theoretical calculations and experiments. Lack of active sites on the surface makes it difficult for SWCNTs to adsorb H2. Therefore, the unmodified SWCNTs have low sensitivity to H2and hardly achieve sensitive monitoring[11]. The hydrogen-sensitive material on the surface of the SWCNTs plays a vital role in the adsorption of H2. The active site of the hydrogen-sensitive material can adsorb more H2and improve the sensitivity of H2detection. Owing to great electron conductivity and density, metals act as one of the most powerful materials in the scientific research and industry. In the last few decades, many kinds of metals especially transition metals have been employed in sensors to assist SWCNTs in detecting H2.The reports include metals such as Pd[12-14], and Pt[15].In addition, metal oxides[16-19]and metal-organic frameworks (MOFs) are also been introduced to assist SWCNTs in the detection. Some results regarding the sensors’ performances are showed in Table 1.
The performance of the sensor was altered by particle position in modified SWCNTs. In the work reported by Kong et al., both individual SWCNTs and its film worked well with the modification of Pd particle. The results showed that the individual SWCNTs performed slightly better than the film[20](Fig. 1a), with responsivity of 2 and 1.5 respectively.Particle size in modified SWCNTs affected the sensor performance. Pd particles modified by electrodeposition showed great effect on the H2sensor, especially at increased Pd deposition time, an Ohmic behavior was observed[21](Fig. 1b). It was proven that the performance depends on the surface treatment of SWCNTs and deposition size of Pd. The defect of SWCNTs also affected the sensor performance, which will be referred in the next chapter.
Metal oxide nanoparticles play a significant impact in the reaction to H2. The impacts of temperature,deposition time, and composite materials are blamed for the performance of the metal oxide/SWCNTs sensors. For instance, Dojin Kim et al. stated that painting CuO was done by first depositing Cu on a porous SWCNTs substrate, then oxidizing it thermally. The sensor response time was determined to be 1.2 min at 250 °C in 6% H2[16](Fig. 2a). As can be observed, sensor performance improves with temperature, which is positively connected with and significantly influenced by temperature. Chen et al. created SnO2/SWCNTs composites utilizing a spin-on sol-gel technique in conjunction with purified SWCNTs. At 200 °C , the SnO2/SWCNTs sensor had the fastest reaction and recovery times[22](Fig. 2b).
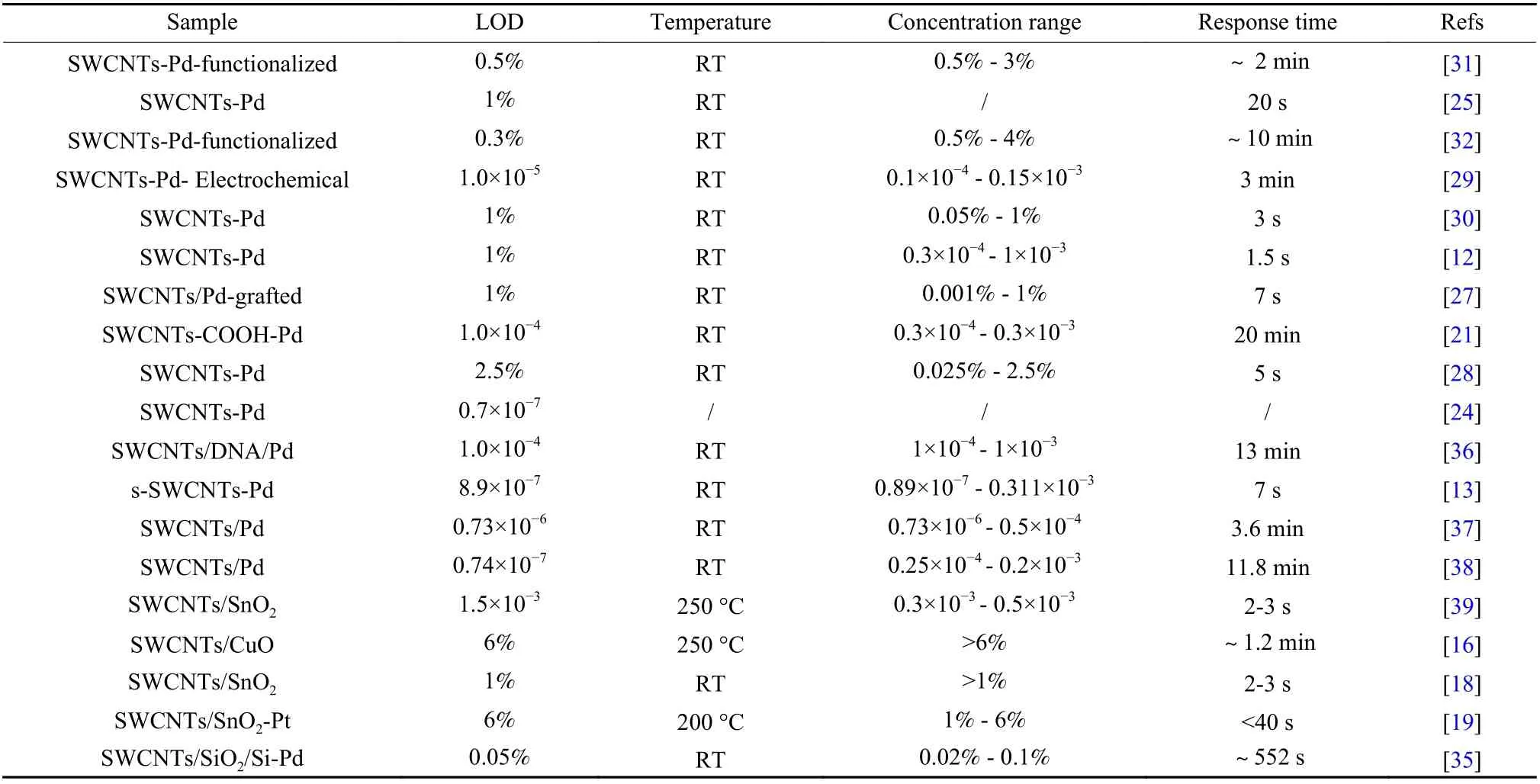
Table 1 Characteristics of various SWCNT-Based H2 Sensors
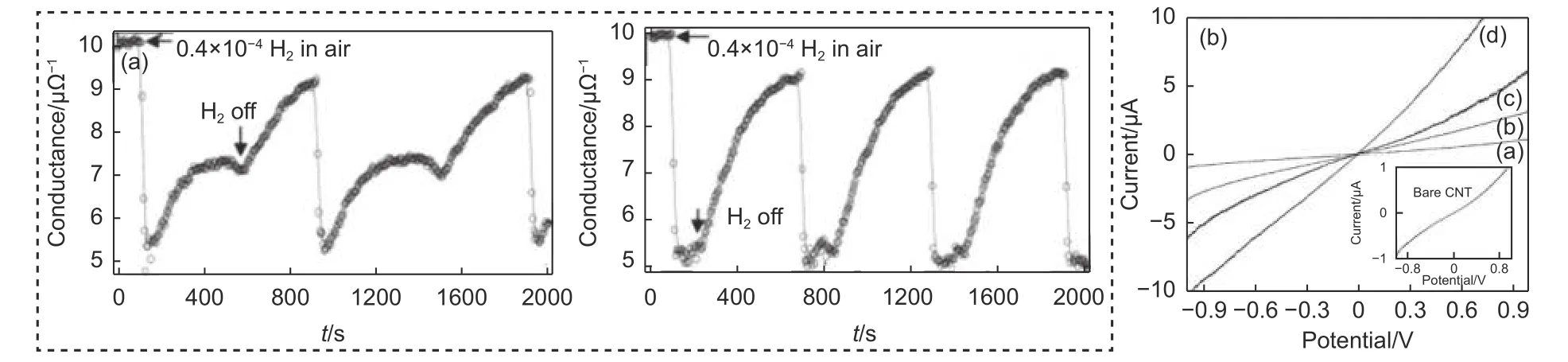
Fig. 1 (a) Response curve of an individual SWCNTs coated with Pd particles to 4.0×10-4 H2 on and off cycles in air (left) and to 0.4×10-4 H2 self-recovery in air (right). Reproduced with permission from Ref[20], Copyright from WILEY-VCH Verlag GmbH, D-69 469 Weinheim, 2001. (b) I-V characteristics showing the resistance change with increase in amount of charge used for Pd of electrodeposition. Reproduced with permission from Ref[21],Copyright from 2007 American Chemical Society
Yang et al. coated SnO2on SWCNTs network sensors by magnetron sputtering. The findings indicate that SnO2/SWCNTs will transition from ap-type ton-type semiconductor with an increase in SnO2deposition time, and hydrogen will cause a decrease in SnO2/SWCNTs resistance[17].
In order to get a quick reaction in 2-3 s in the presence of 1% H2at room temperature, Mao et al.used an electrostatic force directed assembly (ESFDA) procedure to deposit SnO2(3-6 nm) onto SWCNTs[18]. It was suggested that ESFDA is a promising method for making H2sensors based on materials composed of SWCNTs. For the efficient detection of H2, Nguyen Duc Hoa et al. introduced Pt-decorated bead-like SnO2nanowires.
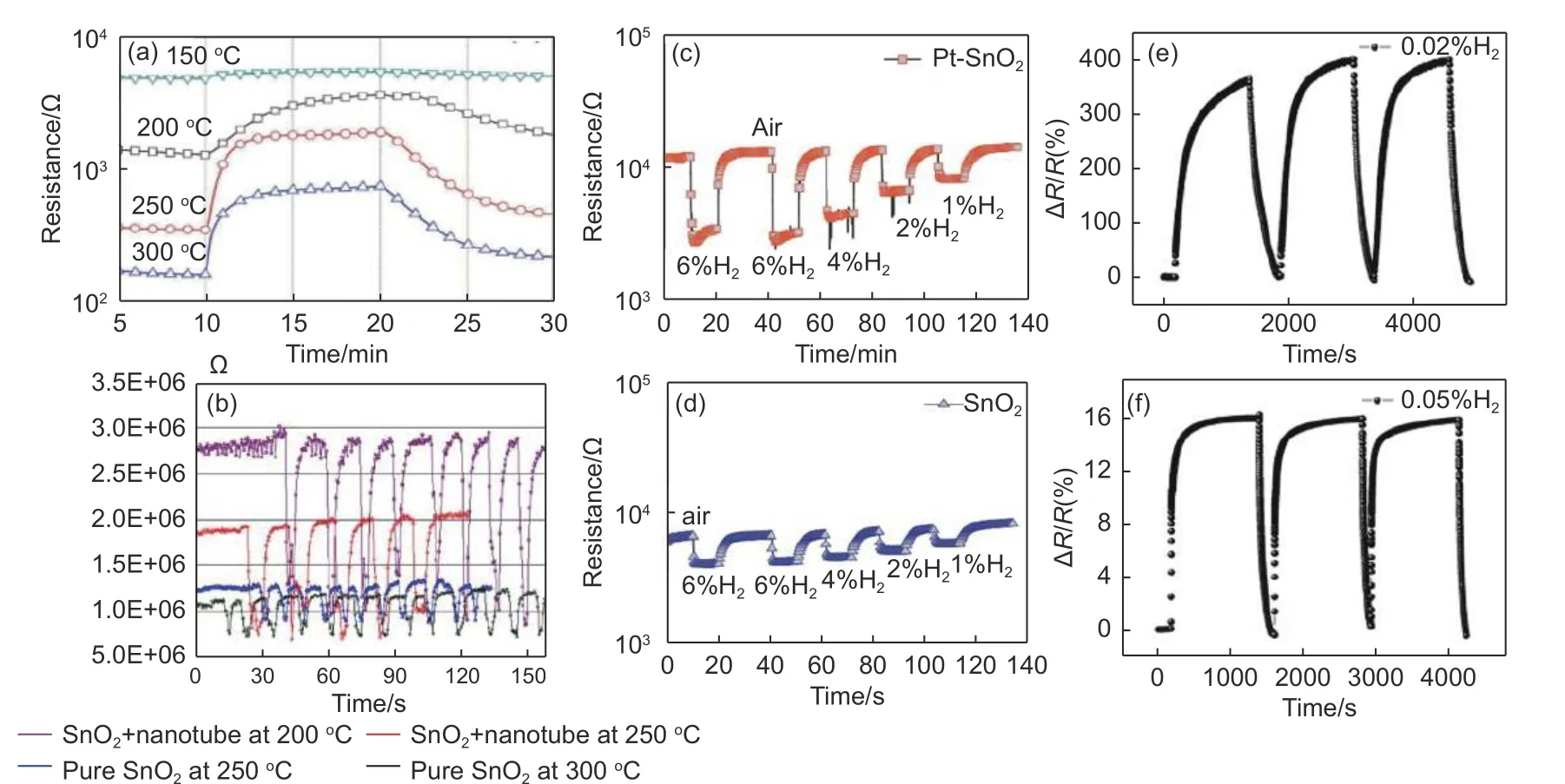
Fig. 2 (a) Response curves of for 6% H2 at different temperatures. Reproduced with permission from Ref[16], Copyright from 2010 Elsevier B.V. (b) Response curves of 0.15×10-2 H2 for SnO2 and SWCNTs/SnO2 at different temperatures, respectively. Reproduced with permission from Ref[22], Copyright from 2007 Elsevier B. Response curves of (c) SnO2-SWCNTs and (d) Pt-SnO2-SWCNTs films with different transparencies in 1%-6% H2. Reproduced with permission from Ref[19], Copyright from 2012 Elsevier B.V. Response curves of (e) the Pd-SWCNTs film/SiO2/p-Si heterostructure for 0.02% H2 and (f) the Pd-SWCNTs film resistance-type device for 0.05% H2. Reproduced with permission from Ref[34], Copyright from 2015 Author (s)
In the experiment, Sn and Pt were sequentially placed on single-walled carbon nanotubes in the experiment, followed by oxidation. The hydrogen-sensitive material loaded with Pt in SnO2/SWCNTs reacts at a rate that is approximately 5.7 times that of SnO2/SWCNTs, efficiently detecting low H2concentrations with a reaction time of less than 1 min[19](Fig. 2c-d). Further evidence that metal-metal oxide composites perform better than single materials provides a new perspective for creating H2-sensitive materials based on SWCNTs in the future.
In order to improve the sensing performance,novel sensing materials based on SWCNTs have been designed to improve sensing capabilities. Maxim Polyakov et al. fabricated the novel hybrid nanomaterial SWCNTs/SiPc using axially substituted silicon(IV) phthalocyanine to cross-link SWCNTs (SiPc).The LOD of SWCNTs/SiPc H2sensor can reach 0.7×10-4[23]. In recent times, MOFs with large specific surface area and excellent H2adsorption capacity have received considerable attention as promising materials for H2storage applications. Alexander Star et al.discovered that Pd cooperated with copper(II) benzene-1,3,5-tricarboxylate (HKUST-1) showed greater hydrogen-absorption capacity. The theoretical LOD of s-SWCNTs/Pd-/HKUST-1 can reach 0.7×10-7[24],which is the highest theoretical response to date for resistor-based sensors.
In addition to the different hydrogen-sensitive materials mentioned above, the method in which SWCNTs are modified also has an impact on the sensor performance. SWCNTs can be modified with either chemical deposition or physical deposition, which include popular chemical deposition methods such as chemical reduction[25-27]or electrochemical techniques[28-30]and physical deposition methods like electron beam evaporation[12], sputtering[31-32]and evaporation deposition. At present, physical deposition methods such as electron beam evaporation and sputtering are the most commonly used methods.
By exhibiting strong interactions across densely packed interfaces, heterostructured nanomaterials can enhance sensor performance much more than a single material. The way of contact between SWCNTs and metal oxides must be taken into consideration. Chun Joong Kim et al. fabricated PN-junction type composite sensors by SWCNTs and zinc oxide (ZnO) and thoroughly investigated the H2sensing properties at various temperatures. At room temperature, SWCNTs adsorbed H2and further electrons that released from H atoms recombined with holes in the SWCNTs, leading to the increase of resistance. At high temperature,H2reacted dominantly with oxygen on ZnO. The electrons were firstly released on the ZnO particles and were subsequently transferred to the SWCNTs[33]. Furthermore, Du et al. prepared Pd-SWCNTs film/SiO2/Si heterostructures by a simple and practical filtration method. This structure can detect about 552 s at 0.05% H2concentration at room temperature[34](Fig. 2e-f). Moreover, Wongwiriyapan et al. utilized silicon dioxide as a protective layer to improve the stability, wherein the response fluctuated in the range of 20% while the fluctuation can reach 80% without SiO2[35]. In this work, another mechanism was proposed. Different from the field-effect transistor (FET)structure, the carrier direction is controlled by adjusting gate voltage. The chemical gating effect of the dipole layer on SWCNTs is formed using H atoms trapped by partial hydrogen atoms at the Pd-SiO2interface[35]. The dipole layer induces electron carriers in thep-type SWCNTs on the SiO2side, however, there are more hole carriers inp-type SWCNTs. The hole carriers are reduced, thus lowering the conductance.
2.1.2 Covalent functionalization on SWCNTs
Common covalent functionalization uses strong oxidation to introduce carboxylic acid (—COOH) and hydroxyl (—OH) groups. Although covalent functionalization is essential to achieve higher sensor selectivity, over-functionalization can disrupt the π-electron system and reduce sensitivity. With the progress in this direction, the researchers found that different oxidant modifications showed significantly different properties and speculated that this difference was due to different functional groups interacting with SWCNTs. The researchers found modification exhibited wildly different performance and speculated that the difference could be attributed to the interaction with functional groups on SWCNTs. Theoretical calculations further confirmed that the O or N of the surface functional group such as hydroxyl (OH), carboxyl(COOH), and carbonyl (C=O), can enhance binding force between sensitive materials and SWCNTs[40].The oxygen-containing functional group can provide additional active sites[41]. The principle is based on change in electrical properties induced by charge transfer between the gas, SWCNTs walls and some attached functional groups[42].
It is noteworthy that if the Pd could be precisely loaded on the functional groups, the H2response may be better. DNA was employed by Su et al. as a dispersant on SWCNTs and then Pd2+was deposited followed by electrochemical reduction to form Pd particles on the DNA/SWCNTs. The sensor’s response time was about 10.6 min for 0.5% H2concentration at room temperature[43].
—OH and —COOH groups improve the hydrophilicity of SWCNTs and enable metal particles to be better loaded on the wall, in order to improve the adsorption capacity of SWCNTs. Al-Diabat et al.[44]produced electrophoretic deposition to create 2 different kinds of CNT sensors, each with a different sensitivity material (CNT or CNT—OH). As a result, the CNT—OH sensor had higher H2sensitivity than one that used a nonconditioned —OH. A naturally occurring class of SWCNTs wraps that has received a lot of attention is conjugated polymers. Even though polymer encapsulation is a non-covalent method for dissolving and functionalizing SWCNTs, we rationally include it in the chapter on covalent functionalization.Dojin Kim et al. fabricated a composite sensor of SWCNTs with wrapping chitosan (CHIT). The CHIT conjugate is a porous insulating polymer material. It contains many functional OH and NH3groups. H2can adsorb onto the CHITviaa dipole-induced-dipole interaction between the non-polar H2and polar functional groups[36](Fig. 3a-b). However, Pd/SWCNTs sensor performs better than SWCNTs—COOH if the SWCNTs—COOH is not loaded with Pd. Chen et al.prepared 3 different gas sensors of unloaded SWCNTs, SWCNTs—COOH, and 3.07% (mass fraction)Pd-doped/SWCNTs, and found that Pd-doped/SWCNTs had the best performance, which was capable of detecting 0.5×10-4in air at 275 °C. The response time and recovery time were about 100 and 150 s[45]. This may be because the number of active sites increased after SWCNTs modification, but H2adsorbed on the surface of functional groups could not be dissociated.

Fig. 3 (a) A schematic illustration of a film of SWCNTs-based on a glass substrate. (b) Interactions of H2 with the Type III sensor. Reproduced with permission from Ref[36], Copyright from 2010 Elsevier B.V. (c) Response of Pd-SWCNTs and Pd-exfoliated SWCNTs thin films during repeated H2 on and off with an H2 concentration of 4% in air. Reproduced with permission from Ref[46], Copyright from 2007 Elsevier B.V. (d) The image of Pd random decoration of the s-SWCNTs at different position, response of the defective device. Reproduced with permission from Ref[47]. Copyright from 2010 American Chemical Society
M. Krishna Kumar et al. dispersed SWCNTs with COOH and C=O groups in gum arabic (GA)solution to obtain a single stable dispersion, then decorated Pd on them. It showed better response to 4% H2in air at room temperature[46](Fig. 3c). The sensing performance is further impacted by SWCNTs defect in addition to the previously mentioned particle location. In Khalap’s other work, when the particles precisely combined with the defect of SWCNTs, the increment of performance could reach three orders of magnitudes while the normal decoration only brings about 50% to several times improvement[47](Fig. 3d).
2.2 Chirality of SWCNTs
With further development in understanding in this area, the factors influencing the performance of SWNT-based sensors have become clearer. Both diameter and chirality of SWCNTs significantly affect the responsivity. It has been reported that the LOD could decrease to 10 ppm by depositing sub-6 nm Pd nanoparticles on the (6,5) SWCNTs ropes[48](Fig. 4ab). Several works have proven that the influences were are not simply due to the chirality of SWCNTs(Fig. 5a-e). The response of SWCNTs-based sensors with diameter of 1.4 nm or chirality of (9,7) were found to be 3 orders of magnitude higher than unsorted materials[37](Fig. 5b,e). The work also pointed out that smaller diameter gives less variation. This is attributed to the offset of change of work function caused by spilling of H atoms from Pd to SWCNTs,which is related to curvature of SWCNTs.
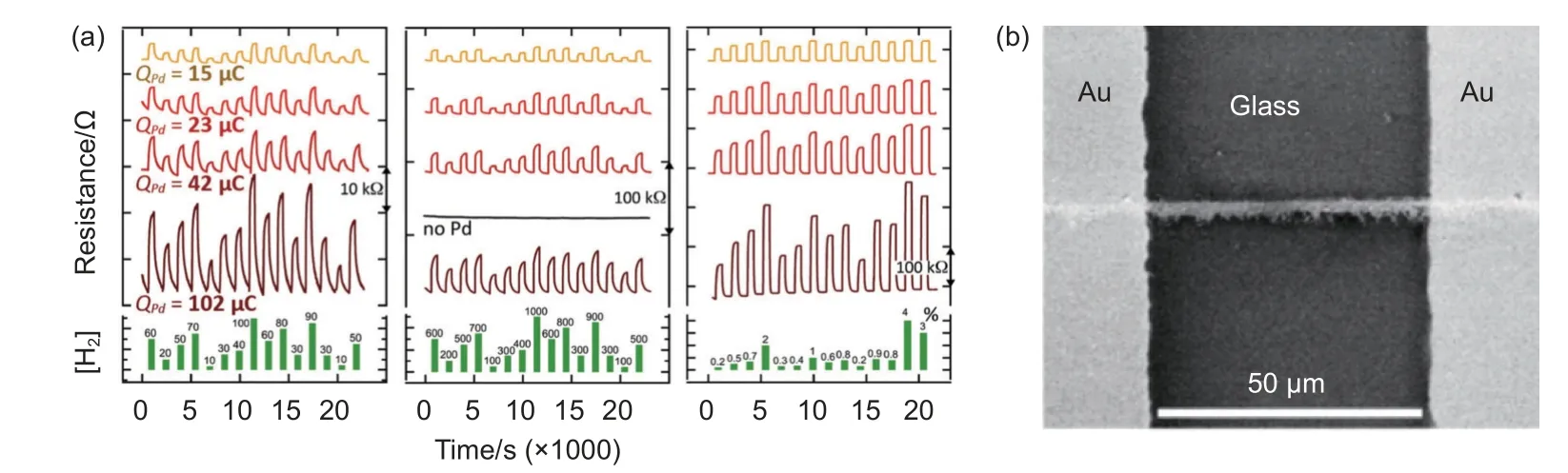
Fig. 4 (a) The response of CNT-Pd nanoparticles H2 sensor with the deposition of four QPd values: showing three different of H2 concentration: Left, 0.1×10-4< [H2] < 0.1×10-3; Middle, 0.1×10-3 < [H2] < 0.1×10-2, Right, 0.2×10-2 < [H2] < 0.4×10-1. (b) The image of a single CNT rope decorated with Pd nanoparticles.Reproduced with permission from Ref[48], Copyright from American Chemical Society
However, another research showed that SWCNTs with larger diameters, such as 3 nm, exhibited only a 2 times change in conductivity when exposed to H2/N2atmosphere. The change in the Schottky barrier when a metal and SWCNTs are in ohmic contact is often insignificant. However, the work speculated that low performance of sensor based on SWCNTs with large diameters was limited by small bandgap and Schottky barrier of sensors. The influence of Schottky barrier has been taken into account for improving the performance[49-51](Fig. 5a,c).
Even though SWCNTs-based sensors that have been modified with various materials have excellent H2detection capabilities, the sensor structure will also have an impact on the performance of the sensor. In general, a resistive sensor and a field-effect transistor(FET) make up the most typical structure. Only two electrodes are utilized in the resistive sensor construction. The FET is made up of a gate electrode, which is typically covered behind an insulating gate oxide substrate and provides a gate voltage to modify the channel current, and two electrodes (source and drain),joined by a semiconductor(s-SWCNTs) as a channel.FET-structure SWCNTs-based sensors are more sensitive than resistive sensors. Bongsik Choi et al. used the semiconductor single-walled carbon nanotube(s-SWCNTs) network channel with Pd electrodes to prepare a FET sensor, which can achieve H2detection at a low concentration of 0.02% - 0.1%. The sensor utilizes the Schottky barrier formed by Pd and s-SWCNTs to detect the change of work function. The formation of Pd-H will reduce the work function of Pd when H2is adsorbed, and the height of the Schottky barrier will change. The response of different H2concentrations is observed by adjusting the gate voltage values(VG)[50]. Using s-SWCNTs and Pd particles to form Schottky contact with Ti/Au electrodes, the response time was as fast as 7 s at 0.311×10-3H2and the LOD can reach 0.89×10-6[13](Fig. 5f-h). Moreover,Liu et al.[52]fabricated an H2sensor utilizing semiconducting CNTs. The fastest reaction time for resistorbased sensors has been as fast as 9 s, and the recovery time was 50 s at 1×10-5H2. By adjusting a voltage on the back gate, gas molecule desorption was sped up.Similarly, Alamri M. A et al.[53]improved the sensor response by employing C-band ultraviolet (UVC) radiation in addition to gate compression for quick recovery times. The H2gas response was increased by up to 4.3 fold at the ideal UVC irradiation of 10 min,along with a speed increase of 3.6 times, compared to the Pt-NPs/SWCNTs/Gr sensors as-made prior to the UVC irradiation. The cause was linked to the desorption of leftover molecules that were adsorbed on the surfaces of the SWCNTs and graphene during the manufacturing of the sensor using UVC radiation.Zhou et al. fabricated floating-gate (FG) FET transistor. The constructed FG FET-type H2sensors have the highest response to date for H2sensors, with a record LOD of 0.9×10-7at room temperature and as low as 0.5×10-8at ~100 °C[54]. The SWCNTs sensor’s performance can be significantly enhanced by using FETs in its fabrication. However, the fabrication of FETs requires advanced techniques, such as the preparation of high-quality s-SWCNTs. Devices fabricated with this structure require a device of SWCNTs,which requires a strong electron transfer capability of SWCNTs when gate voltage is applied, resulting in improved sensor performance.
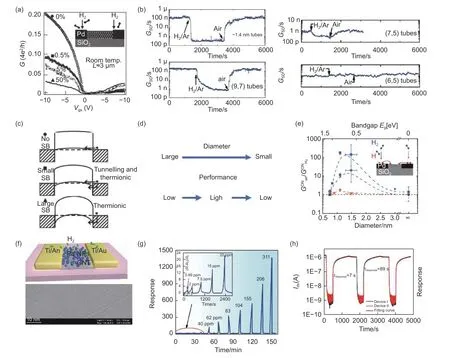
Fig. 5 (a) Conductivity to different H2 concentrations. Reproduced with permission from Ref[49], Copyright from 2003 Nature Publishing Group. (b) Response curves of at different diameter and chirality SWCNTs/Pd FETs on Si/SiO2. Reproduced with permission from Ref[37], Copyright from 2011 American Chemical Society. (c) Changes of the valence band of Schottky barrier with various H2 concentrations. Reproduced with permission from Ref[49], Copyright from 2003 Nature Publishing Group. (d) The diameter of SWCNTs increases from large to small, but the performance increases from low to high to low. (e) The relationship between the diameter and conductivity of different chiral carbon nanotubes in three s-SWCNTs FETs. Reproduced with permission from Ref[37], Copyright from 2011 American Chemical Society. (f) The image of the Pd-decorated SWCNTs film H2 sensor. (g) Real time response to different H2 concentrations at room temperature. (h) Response time and decay time can be defined by fitting portions of the curves. Reproduced with permission from Ref[13], Copyright from American Chemical Society
The diameter and chirality of the SWCNTs have a big impact on H2transport. The potential benefit of this one chemical is its ability to execute directed functionalization using only pure material, allowing for the creation of extremely thick sensors that can work independently. There is currently a lack of studies that clearly demonstrate which diameter, chirality SWCNTs are best for enhancing sensing performance.The best range of SWCNTs sizes and band gaps to offer the best sensing performance, however, is evident.The ability of SWCNTs to transmit electrons is significantly diminished by an extremely broad band gap,which lowers sensor performance. For best performance, a compromise between the diameter and the intrinsic electrical characteristics of SWCNTs must be found. They are thought to be the best option for producing high-performance H2sensors. Therefore, it is essential to continue to prepare and explore the influence of SWCNTs diameter and chirality on the mechanism.
3 Mechanism
The CNT gas-sensitivity mechanism primarily relies on the adsorption and desorption of gases onto the material’s surface. A change in resistance will occur as a result of an electron transfer occurring with the dissociated H2on the surface of SWCNTs. Yet, research has revealed that SWCNTs scarcely absorb H2.Charge transfer between carbon nanotubes and H2can be facilitated by the functionalization of carbon nanotubes. The detection of sensors rely on charge transfer, by which the metals or metal oxides have improved. The effect of functional groups is believed to change the electron structure of SWCNTs. Apart from metal particles enhancing interaction, the polymer encapsulation increases the sensitivity by increasing concentration of CNTs or holes.
The gas-sensitive mechanism can be broken down into 2 parts. For sensors based on s-SWCNTs,H2molecules adsorbed on the surface of Pd dissociate into hydrogen atoms, and the generated PdHxwork function decreases. Electrons are transferred from Pd to SWCNTs, and the number of hole carriers inp-type SWCNTs decreases, leading to an increase in resistance. The process can be reversed because hydrogen atoms dissolved in Pd can combine with O2in the air to form OH, which in turn will combine with hydrogen atoms to form H2O. After the H2O leaves the Pd/SWCNTs system, the sensor returns to its initial resistance value. Another is that the contact method between SWCNTs and metal electrodes also has an impact on device performance (Schottky barrier). The Schottky barrier generated between SWCNTs and metal electrodes increases with the work function of the electrode’s electrode, and as the Schottky barrier increases in height, the tunneling rate of the electron decreases noticeably, sharply amplifying the resistance. The Schottky barrier variations in ohmic contact between metal electrodes and SWCNTs are typically quite small. The 2 models mentioned above are the most popular ones used to interpret gas sensing mechanisms. There are variations in the recognition of the gas-sensitive mechanism, nevertheless, because of the complicated structure of SWCNTs. For example,the difference between m-SWCNTs and s-SWCNTs was not observed in the model that Pd is modified on the defects of CNTs. It is believed the change of resistance came from the scattering of defects so that band gap influenced little in this way. On the other hand, by using experiment and theoretical calculations, the substantial impacts of functional groups[55]and doping[56]on the electronic structure and band gap of SWCNTs have been investigated. It is considered that the band gap has an effect on the change of resistance.
4 Summary and outlook
This work provides a comprehensive review of the recent research progress on gas sensors based on SWCNTs. Given their distinctive structure and electrical properties, SWCNTs-based gas sensors have been extensively studied. Various mixed materials, including metal/SWCNTs, metal oxide/SWCNTs, hydrogen storage material/SWCNTs, and oxygen-containing functional group/SWCNTs, have been designed to enhance the performance of carbon-based sensors. However, there are still huge unexplored void surrounding the design and mechanism of hydrogensensitive materials as well as the development trend of smart gas sensors. Only by addressing these challenges can SWCNTs-based gas sensors be successfully employed in practical application.
(1) Materials improvement
Although some lab tests show that certain sensor performances meet or exceed practical application requirements, the production of sensors on a large scale often results in a decline in performance and quality.Currently, commercial gas sensors made of nanocarbon are still in the development phase due to the significant hurdle posed by the chirality, structure, and purity of SWCNTs in achieving commercialization. The production of SWCNTs on a large scale may no longer be the primary challenge thanks to the advances in macro-preparation technology. However,controlling the fine structure of SWCNTs remains a difficult task. As a result, fabrication strategies combining large scale production with fine-tuned control is still a challenge.
It should be noted that SWCNTs rarely interact with H2. To enhance the performance of SWCNTs sensors, various materials including metals, metal oxides, and novel composites with oxygen-containing functional groups have been utilized on the surface of SWCNTs. The modification of SWCNTs can be achieved through non-covalent and covalent functionalization methods. The sensors’ performance varies with different metal modifications, with Pd-modified SWCNTs sensors exhibiting better response to H2compared to other sensors. Metal oxides, polymers,and functional groups improve performance by raising the number of active sites on SWCNTs, the amount of gas adsorbed, and the effectiveness of electron transport.
(2) Mechanism
The main driver of gas adsorption is typically atoms cannot meet the demands of valence electrons or ionic unsaturated oxygen ligands[57]. Transition metals like Pd have vacant d orbitals that are easily activated by gas absorption. The catalytic activity will be impacted by the d orbital hole. However, too many holes will cause inadequate dissociation. It is crucial to find an optimal value of hole and to balance adsorption-desorption capacity.
Unsaturated oxygen coordination is present in the suspended bonds created by Zn2+in the case of the metal oxide ZnO[58], making these sites favorable for the adsorption of oxygen anions and target gases once the gas-sensitive reaction has begun. The performance of the reaction is impacted by the fact that the majority of metal oxides cannot undergo electron transition at ambient temperature. Future studies must therefore concentrate on the unsaturated oxygen coordination concentration of the metal oxides that are susceptible to hydrogen at room temperature.
The degree and kind of functionalization of SWCNTs with functional groups depend on the production temperature and the type of oxidant.
Due to the different gases and materials used in sensing, charge transfer trends during detection reveal variances. When materials absorb H2, electrons move in SWCNTs to combine with holes, which decreases the conductivity.
(3) Intelligent SWCNTs-based gas sensors
In recent years, intelligent gas sensors have made significant advancements. When combined with wireless modules, these sensors can send messages directly to remote receivers[59-60]. Moving forward, it is expected that SWNT-based gas sensors will become more intelligent[61], digitalized, and capable of detecting multiple gas mixtures simultaneously[62]. Currently, SWCNTs-based gas sensors are unable to detect the target gas in a mixed atmosphere. Through selection of materials and design of sensor structure, the interference can decrease. The response from interfering gases can also be eliminated by deep learning and machine learning[63], which lowers the sensor’s rate of misreading to an incredibly low level. Therefore, if the excellent detection sensitivity of s-SWCNTs is combined with the interference reduction characteristics of smart sensors, s-SWCNTs could become superior potential candidates for the next generation of smart hydrogen sensors with elimination of signal drift.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(52002362, 51902298), Foundation of President of China Academy of Engineering Physics(YZJJLX2020007), Institute of Materials, China Academy of Engineering Physics program(TP02201907), Sichuan Science and Technology Program (2020JDRC0002).
- 新型炭材料的其它文章
- Guide for Authors
- 《新型炭材料(中英文)》征稿简则
- Insights into the carbonization mechanism of bituminous coal-derived carbon materials for lithium-ion and sodium-ion batteries
- Large-scale synthesis of 3D ordered microporous carbon at low temperature using cobalt ions exchanged zeolite Y as a template
- Development of biochar electrode materials for capacitive deionization: preparation, performance, regeneration and other challenges
- Recent advances in 3D interconnected carbon/metal high thermal conductivity composites

