Synthesis and electrochemical properties of nano-Si/C composite anodes for lithium-ion batteries
YUAN Li-ye*, LU Chun-xiang*, LU Xiao-xuan YUAN Shu-xiaZHANG Meng3, CAO Li-juan YANG Yu
(1. National Engineering Laboratory for Carbon Fiber Technology, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;3. University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: Phenolic resin was coated on the surface of nano-Si by a microencapsulation technique, and then carbonized under Ar protection to prepare a nano-Si/C composite. The composites were first prepared using 4 different mass ratios (1∶2, 1∶4, 1∶6,1∶8) of phenolic resin to nano-Si. The obtained average thicknesses of amorphous carbon coating were 7, 4.5, 3.7, 2.8 nm, respectively. By comparing the cycling and rate capability, the best electrochemical performance was obtained when this ratio was 1∶4,with a 4.5 nm amorphous carbon coating. The electrochemical properties of this material were then comprehensively evaluated,showing excellent electrochemical performance as an anode material for Li-ion batteries. At a current density of 100 mAg-1, the material had a first specific discharge capacity of 2 382 mAhg-1, a first charge specific capacity of 1 667 mAhg-1, and an initial coulombic efficiency of 70%. A discharge specific capacity of 835.6 mAhg-1 was retained after 200 cycles with a high coulombic efficiency of 99.2%. In addition, the nano-Si/C composite demonstrated superior rate performance. Under current densities of 100, 200,500, 1 000 and 2 000 mAg-1, the average specific discharge capacities were 1 716.4, 1 231.6, 911.7, 676.1 and 339.8 mAh g-1, respectively. When the current density returned to 100 mA g-1, the specific capacity returned to 1 326.4 mAh g-1.
Key words: Microencapsulation technology;Lithium-ion batteries;Silicon anode;High capacity;Low-cost
1 Introduction
Lithium-ion batteries (LIBs) have been widely used in portable electronic products and electric vehicles[1], attributing to their high energy density, operating voltage, low self-discharge characteristics and maintenance requirements[2]. Currently, graphite is the most widely used anode material for commercial LIBs because of their excellent electronic conductivity,high coulombic efficiency and low charge-discharge electrochemical over-potential[3]. However, its theoretical specific capacity is only 372 mAh g, which hardly meets the increasing requirement of anode materials for high-capacity and high-power LIBs[4]. Owning to high theoretical specific capacity (3 579 mAh g-1),low discharge platform (~0.45 Vvs. Li/Li+), limited reactivity with electrolytes, abundant earth reserves and environmental friendliness, silicon (Si) is one of the most promising candidates that could replace graphite as the next-generation anode material for LIBs[5-7]. However, silicon-based anode materials also have serious disadvantages. For example, considerable volume change is usually accompanied with the charging and discharging process. This huge volume variation could lead to the pulverization and shedding of silicon particles and the rupture of electrode coating, resulting in rapid capacity attenuation and poor cyclic stability[8-13].
In order to overcome the above shortcomings, a number of methods have been proposed to optimize the Si-based anodes. One typical method is designing nanoscale silicon materials, such as silicon nanoparticles, silicon nanofibers and silicon nanotubes. In this way, the absolute volume change of silicon particles could be reduced during the charge and discharge process, alleviating the induced stress from volume expansion[14-20]. Moreover, the nanoscale anode materials could also significantly increase the surface area and grain boundary of silicon anodes, which can effectively shorten the diffusion path of lithium ions, and promote the rapid formation of silicon-lithium compounds from lithium ions with silicon[21]. Another classic approach to restrain the capacity attenuation of silicon anodes is to coat a carbon layer on the surface of silicon nanoparticles to build a silicon/carbon (Si/C) nanocomposite[22-26]. During the chargedischarge process, the outer carbon coating would not merely act as a buffer layer to limit the volume expansion of silicon nanoparticles[27-29], but also prevent the reaction between silicon nanoparticles and electrolyte.Therefore, nano-Si/C nanocomposite is considered as one of the most promising anode materials for highcapacity and high-power lithium-ion batteries[30].
At present, numerous methods have been reported to prepare nano-Si/C nanocomposites, such as mixing the nano-Si with graphite[31-33], combining nano-Si with various carbon precursors[34-37], and combining graphite, carbon precursors with nano-Si[38-39].In this paper, a fast, efficient and cost-effective method is reported to synthesize a new nano-Si/C nanocomposite with superior electrochemical performance.Briefly, quantitative phenolic resin was first coated on the nano-Si evenly as carbon precursorviamicrocapsule technology. The obtained core-shell structure was then calcinated in the argon atmosphere to prepare nano-Si/C nanocomposite anode material. Benefiting from the hard carbon gained by carbonizing phenolic resin, the electrical conductivity of nanocomposite was significantly improved, leading to the enhanced electrical contact of electrode. More importantly, the volume change of nano-Si could be alleviated attributing to the mechanical strength of hard carbon. The electron microscopy characterization showed that the surface of nano-Si is uniformly coated with hard carbon, while the electrochemical tests of the prepared nano-Si/C nanocomposite anode material demonstrated that its discharging specific capacity could reach as high as 2 382 mAh g-1, and after 100 cycles,1 035 mAh g-1capacity could be retained. Moreover,the nano-Si/C anode also exhibited excellent rate performance.
2 Experimental
2.1 Materials
Commercial nano-Si was purchased from Shanghai McLean Biochemical Technology Co. Ltd. (100 nm, 99%). Phenolic resin was obtained from Shandong Balun New Material Technology Co. Ltd. Silicon oil was purchased from Dow Corning Co. Ltd.(AR). Ethyl alcohol was purchased from Tianjin Tianli Chemical Reagent Co., Ltd. (AR). Carbon tetrachloride was purchased from Tianjin FengChuan chemical reagent Technology Co., Ltd. (AR). All the chemical reagents were directly used without any special handling.
2.2 Material characterization
The morphologies as well as particle size of nano-Si/C nanocomposite were observed by the field emission electron microscopy (FE-SEM, JSM-7900F)and transmission electron microscopy (TEM, JEM-2100F). The crystalline structure was determined by X-ray diffraction (XRD, D8 ADVANCE A25) and micro-Raman spectroscopy (Raman, HR800-632.8).The thermal decomposition of the nano-Si/C nanocomposite was studied by thermal gravimetric analyzer (TG, TGA 2) from 60 to 700 °C at 10 °C/min under the airflow.
2.3 Electrochemistry characterization
The working electrode was prepared by mixing the nano-Si/C nanocomposite as active material, carbon black as conductive additive with PVDF as the binder at the ratio of 8∶1∶1, using NMP as the solvent. The above mixture slurry was casted at a thickness of 100 μm and pressed on copper foil as the current collector. The collector was then dried at 80 °C under vacuum for 24 h. The prepared collector worked as working electrode and pure lithium metal used as reference electrode were assembled as CR2032-type cells in an Ar-filled glovebox.Celgard2500 polyethylene was used as the separator.Commercial electrolyte of 1 mol L-1LiPF6dissolved in a mixture of ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC) with a ratio of 1∶1∶1, and 5% fluoroethylene carbonate (FEC)was added as additives. Galvanostatic charge and discharge (GCD) cycling and rate performance were measured in a voltage range from 0.01 to 3.0 V by LANDCT 2001A battery test system. Cyclic voltammetry (CV) was measured using an electrochemical workstation (Chenhua, CHI 660E) at a scanning rate of 0.1 mV s-1. Electrochemical impedance spectroscopy (EIS) tests were performed within the frequency range of 100 kHz to 0.01 Hz with an AC-amplitude of 10 mV.
3 Results and discussion
Fig. 1 schematically illustrates the synthesis process of nano-Si/C nanocomposite. Firstly, phenolic resin was dissolved in ethyl alcohol to form a transparent solution, and the concentration of the phenolic resin solution was 1%. Nano-Si which was 4 times the mass of phenolic resin was then dispersed in the solution (Fig. 1a). The dispersion was added into the silicon oil which worked as continuous phase, and the whole system was stirred to form a stable emulsion(Fig. 1b). Next, the emulsion was heated to cure phenolic resin (Fig. 1c). The nano-Si microcapsules coated with phenolic resin were obtained after being filtered,washed and dried in vacuum (Fig. 1d). Finally, the nano-Si microcapsules were carbonized in a tube furnace under an argon atmosphere to obtain an even coating of hard carbon on nano-Si particles, denoted as nano-Si/C nanocomposite (Fig. 1e).
Fig. 2a shows the Raman spectroscopy analysis of nano-Si/C nanocomposite and nano-Si as reference sample. The nano-Si showed 3 peaks at low frequency (i.e., 297, 507 and 927 cm-1) that are derived from silicon lattice stretching. For nano-Si/C nanocomposite, the same 3 low frequency peaks could be identified, indicating the crystal structure of nano Si was preserved. Moreover, two other high frequency peaks (i.e., 1 362 and 1 594 cm-1) could also be observed. These two peaks originated from the characteristicD(disordered) andG(graphitic) peaks of carbon materials. The relatively higherD/Gratio indicate the existence of amorphous carbon in nano-Si/C.Fig. 2b shows the X-ray diffraction patterns of nano-Si and nano-Si/C nanocomposite. Both of the patterns exhibited the same sharp diffraction peaks of crystal silicon, corresponding to the lattice plane of (111),(220), (311), (400) and (331), respectively. This consistence of silicon pattern demonstrates that the crystal silicon was not oxidized to SiOxduring the preparation. Moreover, a broad band at 20°-27° could be observed at low intensity from nano-Si/C, which could be attributed to the amorphous carbon from the carbonization of resin, implying the the existence of carbon coating in the nano-Si/C nanocomposite.
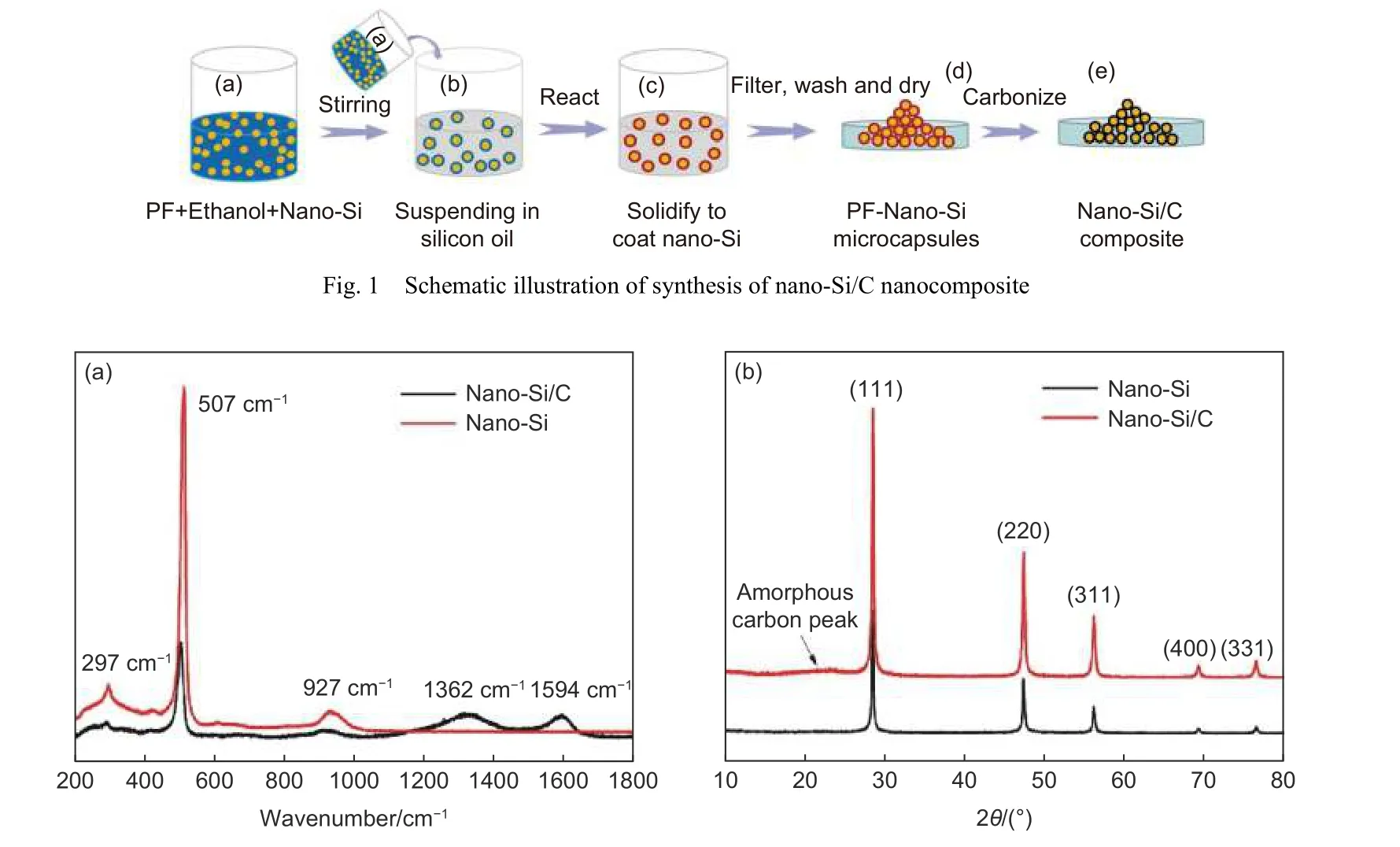
Fig. 2 (a) Raman and (b) XRD spectra of nano-Si and nano-Si/C samples
Fig. 3 shows the SEM images of nano-Si and nano-Si/C nanocomposite. It could be observed that both the nano-Si and nano-Si/C nanocomposite presented spherical shape (Fig. 3a-b). Nano-Si/C nanocomposite exhibited rough surface, owing to the carbon coating around the surface of nano-Si particles.
Transmission electron microscopy (TEM) and corresponding high-resolution transmission electron microscopy (HRTEM) images of nano-Si/C are shown in Fig. 4. Clearly, all the nano-Si particles were encapsulated by a carbon coating (Fig. 4a, c) 4.5 nm in thickness. As shown in Fig. 4d, the well-ordered lattice spacing of 0.316 nm corresponds to the (111)plane of cubic Si, demonstrating that crystal silicon was not oxidized to SiOx. The SAED image of nano-Si/C in Fig. 4b demonstrates that the nano-Si in nano-Si/C nanocomposite retained a spherical monocrystalline structure.

Fig. 4 (a, c) TEM images of nano-Si/C; (b) SAED image for the region highlighted by red square in (a); (d) HRTEM image of nano-Si/C
In order to investigate the influence of carbon coating thickness on the electrochemical performance of nano-Si/C nanocomposite, four different mass ratios of phenolic resin and nano-Si (1∶2, 1∶4, 1∶6 and 1∶8) were employed (Fig. 5). As shown in the TEM images, when the mass ratio of phenolic resin to nano-Si was 1∶2, 1∶4, 1∶6 and 1∶8, the average thickness of amorphous carbon coating was 7 (Fig. 5a),4.5 (Fig. 5b), 3.7 (Fig. 5c) and 2.8 nm (Fig. 5d), respectively. This thickness variation suggests that a smaller ratio of phenolic resin to nano-Si results in thinner carbon coating.
Thermogravimetric analysis was performed on amorphous carbon obtained by direct carbonization of phenolic resin, nano-Si, and nano-Si/C nanocomposite prepared by 4 different mass ratios of phenolic resin to nano-Si (Fig. 6). The temperature range was 60-700 °C, and the heating rate was 10 °C min-1. The analysis revealed that a significant weight loss of 93%for amorphous carbon was observed at high temperature, attributed to the oxidation of carbon to CO2in the air flow. Comparatively, the weight of nano-Si slightly increased about 1.63%, owning to the formation of a silica layer under oxidative atmosphere. The weight losses of nano-Si/C nanocomposites were 12.27%, 7.31%, 5.16% and 3.80%, corresponding to different mass ratios of phenolic resin to nano-Si,1∶2, 1∶4, 1∶6 and 1∶8, respectively. Therefore,the weight loss of nano-Si/C nanocomposite could mainly be attributed to the oxidation of surface amorphous carbon coating. Moreover, it is shown that with the decrease of thickness of carbon coating, the weight loss also decreased accordingly.
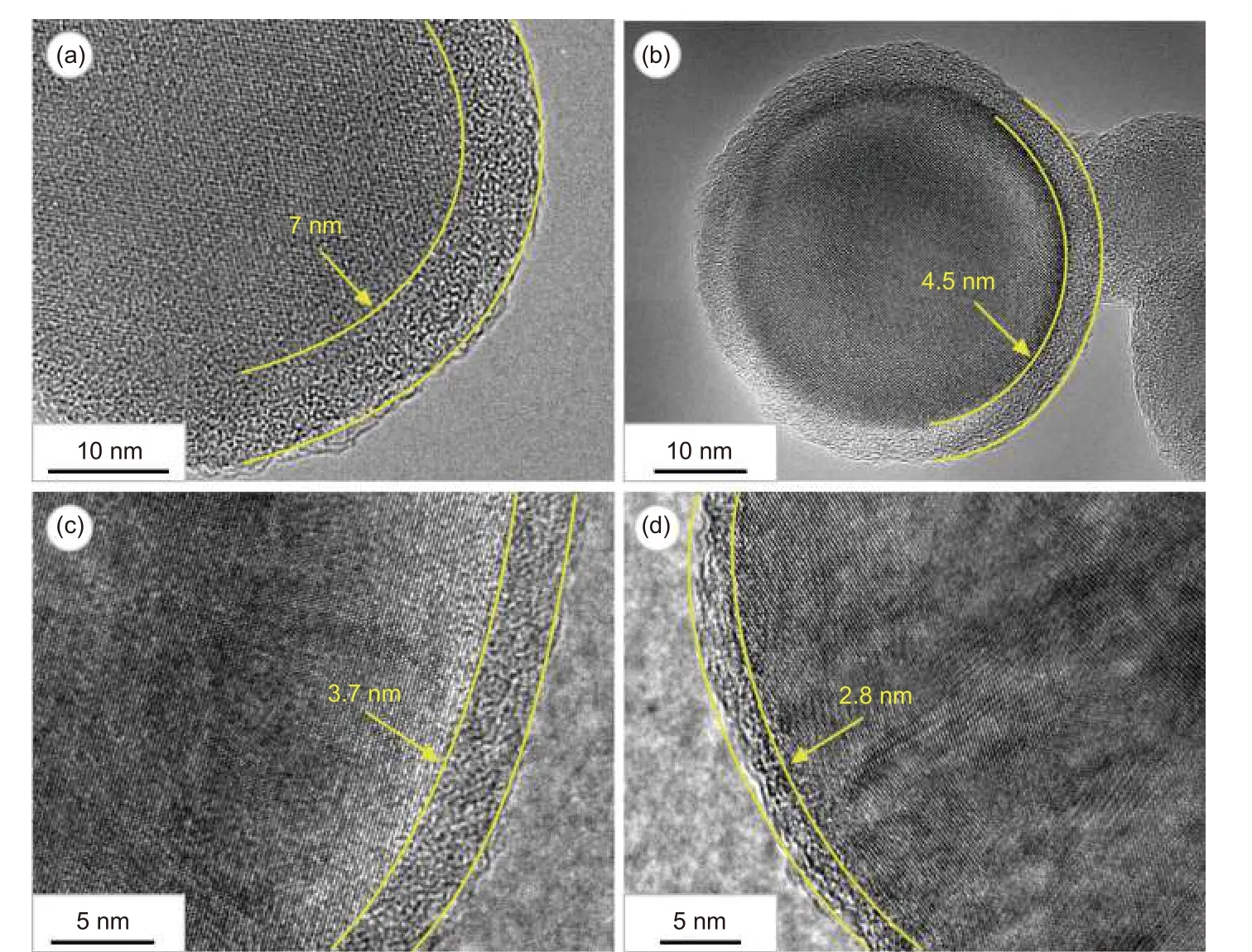
Fig. 5 TEM of nano-Si/C composite obtained by different mass ratios of phenolic resin to nano-Si: (a) 1∶2, (b) 1∶4, (c) 1∶6 and (d) 1∶8
Fig. 7a shows the cycling performance of four nano-Si/C nanocomposites obtained by different mass ratios of phenolic resin to nano-Si. When the ratio was 1∶2, the first charging specific capacity was 1 317.2 mAh g-1at the current density of 100 mA g-1. After 50 cycles, the capacity retention was about 352.2 mAh g-1. For the ratio of 1∶4, the first charging specific capacity was 1 667 mAh g-1, after 50 cycles of charging and discharging process, the remaining specific capacity was 1 253.7 mAh g-1. When the ratio was increased to 1∶6, the first charge specific capacity of prepared nano-Si/C nanocomposite was 1 232.2 mAh g-1. And the charging specific capacity retained of 947.4 mAh g-1after 50 cycles. When the ratio was 1∶8 for nano-Si/C nanocomposite, the first charge specific capacity and the reserved specific capacity after 50 cycles were 1 117 and 626.3 mAh g-1, respectively. As shown in Fig. 5, the thickness of amorphous carbon coating was 7.5, 4.5, 3.7 and 2.8 nm, respectively, corresponding to different mass ratios of phenolic resin to nano-Si. When the carbon coating was extremely thin, its mechanical strength was too weak to sustain the swelling of nano-Si during the charging and discharging process. With the increase of cycles, the carbon coating would suffer fatigue damage, and fall off from the inner silicon particle. Upon collapse, this carbon coating loses its protective effects, thus compromising the cycling performance. However, when the carbon coating was too thick, it would also negatively impact the cycling performance. This could be explained by considering that with the increase of carbon coating thickness, the migration channel of lithium ions became longer, making the insertion and extraction of lithium ions to silicon anode much difficult. Some lithium ions would also block the channel and turned into dead lithium,resulting in reduced capacity and poor cycling performance. According to the cycling results, the optimal mass ratio of phenolic resin to nano-Si was determined as 1∶4, and the corresponding carbon coating thickness was 4.5 nm.
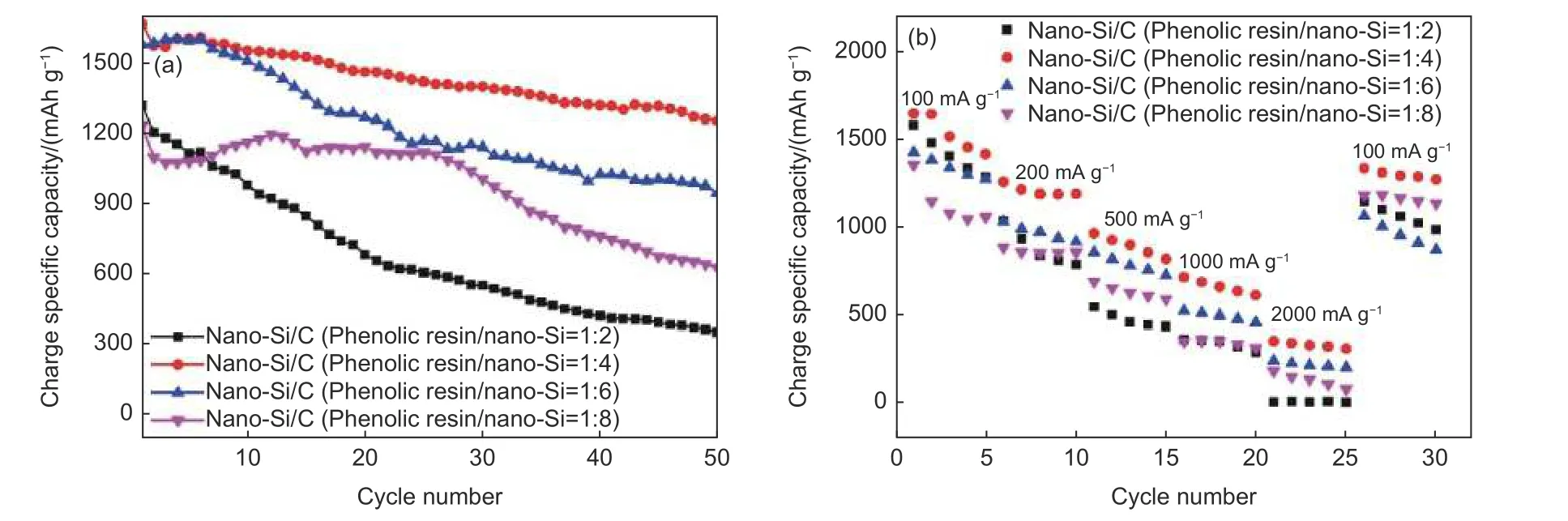
Fig. 7 Comparison diagrams of electrochemical performance of nano-Si/C nanocomposites obtained by different mass ratios of phenolic resin to nano-Si and (a) cycle performance and (b) rate performance
Fig. 7b shows the rate performance of nano-Si/C nanocomposite obtained by different mass ratios of phenolic resin to nano-Si. For ratio of 1∶2, the average specific capacity of nano-Si/C nanocomposite was 1 420.1 mAh g-1at the current density of 100 mA g-1.While at current density of 200, 500, 1 000 and 2 000 mA g-1, the average specific capacities were 882.48,478.52, 337.3, and 8.1 mAh g-1, respectively. When the current density returned to 100 mA g-1, the specific capacity could be restored to 1 064.5 mAh g-1. For ratio of 1∶4, at the current density of 100, 200, 500,1 000 and 2 000 mA g-1, the average specific capacity were 1 536.9, 1 209.7, 893.8, 663.2 and 332.6 mAh g-1, respectively. When the current density returned to 100 mA g-1, the specific capacity could be restored to 1 301.6 mAh g-1. When the ratio was 1∶6,the average specific capacity of nano-Si/C nanocomposite was 1 346.1, 971.7, 788.9, 494, 221.1 and 962.8 mAh g-1at current density of 100, 200, 500,1 000, 2 000 and 100 mA g-1, respectively. However,when the ratio was 1∶8, the average specific capacity of nano-Si/C nanocomposite was 1 137.7, 861.6,634.7, 343.8, 132.7 and 1 164.4 mAh g-1, respectively. It is shown that nano-Si/C nanocomposite at phenolic resin to nano-Si ratio of 1∶4 exhibited the highest specific capacity at both large and small current density. The excellent reversibility of the capacity could be attributed to the much improved electrical conductivity, with enhanced electric polarization reaction of nano-Si and its maximized utilization during the cycling process, leading to the higher specific capacity in the large current density. Moreover, the good mechanical properties of carbon coating could restrain the expansion of nano-Si, protecting the structural integrity of nano-Si, so the specific capacity of nano-Si/C nanocomposite could be restored to a higher level. However, as mentioned above, when the carbon coating was too thick, the lithium ions migration channel became longer. The electric polarization reaction was sped up when the current density was increased, congesting the ions migration channel and leading to a sharp drop in specific capacity. Therefore,4.5 nm carbon coating obtained from phenolic resin to nano-Si ratio of 1∶4 was the optimal thickness for nano-Si/C nanocomposite.
In order to observe the surface morphology changes of active substances after charge and discharge cycles, the nano-Si and 4 different electrodes mentioned above after 50 cycles were observed by FE-SEM. As shown in Fig. 8a, spherical nano-Si particles are barely visible since the structure undergoes pulverization and disintegration after the charge and discharge cycles. Fig. 8b-e are the morphology images of nano-Si/C electrodes after cycles, whose mass ratios of phenolic resin to nano-Si are 1∶2,1∶4, 1∶6 and 1∶8, respectively. When the mass ratio is 1∶2 and 1∶4 (Fig. 8b-c), the thicknesses of carbon coating are larger in this 4 kinds of electrodes,therefore their mechanical strengths are high enough to effectively confine the volume expansion of nano-Si particles in the cycling process, helping them retain a good spherical shape. In Fig. 8d, cracks and pulverization begin to appear on the surface of the electrode, it is due to the smaller thickness of amorphous carbon coating which does not have a mechanical strength strong enough to withstand the volume expansion of nano-Si particles inside. With the decrease in mass ratio of phenolic resin to nano-Si, cracks and pulverization become more serious (Fig. 8e).
The following measurements were further employed on the optimal nano-Si/C nanocomposite (carbon coating of 4.5 nm) to comprehensively evaluate its electrochemical performance. Fig. 9a shows the charge and discharge curves of nano-Si/C nanocomposite at 1st, 2nd, 50th, 100thand 200thcycles, respectively. The discharge curve had a long plateau below 0.1 V, corresponding to the alloying process of crystalline silicon and lithium. The inclined voltage platform on the charge curve corresponded to the lithium escaping process to form amorphous silicon. The initial discharge specific capacity was 2 383 mAh g-1,and the first coulombic efficiency was 70%. This efficiency loss was mainly due to the decomposition of electrolyte to form SEI membrane during the first charge and discharge process. The 2nd, 50th, 100thand 200thdischarge specific capacity was 1 932, 1 227.4,1 035 and 835.6 mAh g-1respectively. Moreover, a stable charging plateau at 0.5 V could be observed from the first to the 200thcycle, indicating the excellent cycling performance of nano-Si/C anode composite.
Fig. 9b shows the cyclic voltammetry (CV)curves of nano-Si/C nanocomposite after different cycles. The CV curves were measured by the half-cell test at a voltage of 0.05-1.5 V and a sweep rate of 0.1 mV s-1. In the first cycle, a reduction peak appeared during the cathode scanning around 0.67 V,corresponding to the decomposition of electrolyte and the formation of SEI. It disappeared in the subsequent scanning process, indicating the formation of stable SEI during the 1st cycle. The cathode peak at 0.2 V was ascribed to the amorphization of crystalline silicon for lithiation[40-44]. During the first anodic scanning,the oxidation peak at 0.33 and 0.52 V corresponded to the phase transition reaction from amorphous LixSi to amorphous Si[45-46].
Fig. 9c shows the cycling performance of nano-Si/C nanocomposite. It is well known that pure nano-Si has a high first reversible specific capacity(2 793 mAh g-1), but declines rapidly. After 80 cycles,only 8.4 mAh g-1specific capacity could be retained.However, the core-shell structural nano-Si/C nanocomposite featuring pyrolyzed carbon coating exhibited excellent cycling stability. At 100 mA g-1, the first discharge specific capacity is 2 383 mAh g-1, the charging specific capacity was 1 667 mAh g-1, and the first coulombic efficiency was 70%, with the capacity retention as high as 835.6 mAh g-1after 200 cycles.Moreover, during the cycling process, the coulombic efficiency could be maintained above 94%, demonstrating its outstanding cycling stability. This boost to stability could be attributed to the unique core-shell structure of nano-Si/C naocomposite and carbon coating, which buffered the volume expansion of active silicon during the cycling process and maintained the electrical contact within the battery.
In Table 1 some recent works on Si-based composites as anodes for LIBs are quantified. Du et al.[47]prepared a novel Si-based composites (Si@C-AL-azo-NO2) which exhibited a high reversible specific capacity of 882 mAh g-1at a current density of 200 mA g-1over 150 cycles and an initial coulombic efficiency(CE) of 65%. Chen et al.[48]synthesized a new nanofiber of silicon/carbon with hollow core-shell structure, whose reversible capacity was 1 020.7 mAh g-1after 100 cycles at 0.2 A g-1and initial CE was 53.4%.Fang et al.[49]prepared a unique core-shell structure of silicon@titania (Si@TiO2) composites, which had ahigh capacity of 804 mAh g-1and a initial CE of 51.3% after 100 cycles at 0.1 C. It is evident that the initial CE of the nano-Si/C nanocomposite in this work is better than most of the previously reported results, while the cycling stability and capacity retention of nano-Si/C nanocomposite are apparently not so high. For the nanocomposite prepared with 1∶4, the capacity is 1 667 mAh g-1at the current density of 100 mA g-1, and reaches a value of 1 253 and 834.6 mAh g-1after 50 and 200 cycles, respectively. In the first 50 cycles, the attenuation rate of single cycle is 0.49%, while in the following 150 cycles, it decrease to 0.22%. It demonstrates the structure of nano-Si/C nanocomposite become increasingly stable and the capacity attenuation tends to flatten out. There is room for improving the capacity retention of nano-Si@C nanocomposite with further research.
Fig. 9d shows the rate performance of optimal nano-Si/C nanocomposite. Under the current density of 100 mA g-1, the average specific capacity of nano-Si/C was 1 716.4 mAh g-1. While at the current densities of 200, 500, 1 000 and 2 000 mA g-1, the average specific capacities were 1 231.6, 911.7, 676.1 and 339.8 mAh g-1, respectively. When the current density returned to 100 mA g-1, the specific capacity was restored to 1 326.4 mAh g-1. Comparatively, the average specific capacities of bare nano-Si were about 2 300, 700, 250, 40 mAh g-1at current densities of 100, 500, 1 000, 2 000 mA g-1. When the current density returned to 100 mA g-1, only 1 000 mAh g-1specific capacity could be restored[53]. This excellent reversibility demonstrates that the core-shell structure was beneficial for the transportation of Li+and electrons, thus suggesting that carbon coating could significantly improve the conductivity of the silicon material.
Fig. 10 shows the electrochemical impedance spectra (EIS) of nano-Si and nano-Si/C electrodes. As shown in Fig. 9, the EIS curves were fitted by an equivalent circuit model, whereRs, CPE,R1(Rr) andW1represent Ohmic impedance, double-layer capacitance, charge transfer impedance and Warburg impedance tail, respectively. The electrochemical impedance spectra were composed of a semicircle in the high frequency region, a semicircle in the intermediate frequency region and an inclined line in the low frequency region. The semicircle in the high frequency region corresponded to the interface impedance of electrolyte and electrode surface, namely the SEI film impedance. The semicircle of the intermediate frequency region corresponded to the charge transfer impedance of lithium-ion embedded active substance. The inclined line in the low frequency region corresponded to the diffusion impedance of the ions in the electrode. It was found that for nano-Si/C electrode the semicircle radius in the high frequency was much smaller than that of nano-Si electrode, which proved that the core-shell structure of nano-Si/C and carbon coating could significantly facilitate the charge transfer process, provide effective ion transfer path,and generate uniform SEI film on the electrode particles. Therefore, the SEI film impedance and the charge transfer impedance were reduced in the battery.
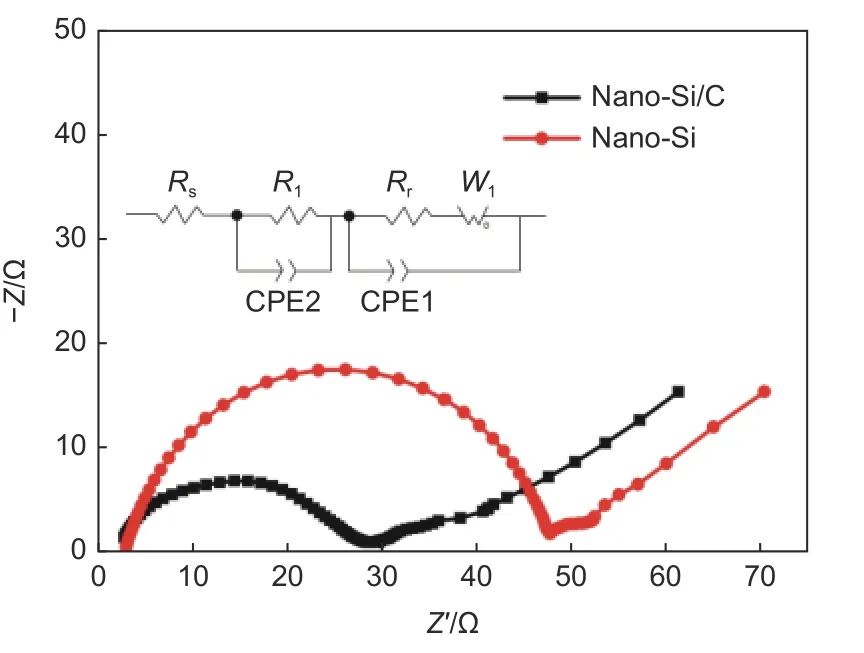
Fig. 10 Nyquist plots of nano-Si and nano-Si/C electrodes
4 Conclusion
In summary, phenolic resin was coated on the surface of nano-Si particlesvia in-situpolymerization using a microencapsulation technique. The nano-Si/C nanocomposite was then obtained by carbonization under the Ar atmosphere. The nano-Si/C nanocomposite presented a core-shell structure, featuring nano-Si inner core and amorphous carbon coating with controllable thickness. When the mass ratio of phenolic resin to nano-Si was 1∶4, the prepared nano-Si/C nanocomposite exhibited the best electrochemical properties, and its outer carbon coating had an uniform thickness of 4.5 nm. The optimized carbon coating would not only effectively alleviate the volume expansion of nano-Si during the cycling process, but also improve the conductivity of the anode material.The lithium-ion battery electrode prepared by the nanocomposite demonstrated excellent cycling stability. At the current density of 100 mA g-1, the specific capacity is 835.6 mAh g-1after 200 cycles with a high coulombic efficiency (99.2%). Moreover, the superior rate performance is also promising for application in LIBs. Therefore, the reported novel core-shell structural nano-Si/Cviamicroencapsulation technique could provide a new simple, efficient and costeffective pathway for providing environmentally friendly anode materials.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://cstr.cn/31253.11.sciencedb.09527 or https://doi.org/10.57760/sciencedb.09527.
Acknowledgements
This work is funded by “Supported by Fundamental Research Program of Shanxi Province(20210302124312)”. We would also like to thank Shiyanjia Lab (www.shiyanjia.com) for the XRD analysis.
- 新型炭材料的其它文章
- Guide for Authors
- 《新型炭材料(中英文)》征稿简则
- Insights into the carbonization mechanism of bituminous coal-derived carbon materials for lithium-ion and sodium-ion batteries
- Factors that influence the performance of hydrogen detectors based on single-wall carbon nanotubes
- Large-scale synthesis of 3D ordered microporous carbon at low temperature using cobalt ions exchanged zeolite Y as a template
- Development of biochar electrode materials for capacitive deionization: preparation, performance, regeneration and other challenges

