Metabolomics: A useful tool for ischemic stroke research
Wento Li ,Chongyu Sho ,Chng Li ,Huifen Zhou ,Li Yu ,Jiehong Yng ,Hitong Wn ,**,Yu He ,*
a School of Pharmaceutical Sciences,Zhejiang Chinese Medical University,Hangzhou,310053,China
b School of Basic Medicine Sciences,Zhejiang Chinese Medical University,Hangzhou,310053,China
Keywords:Metabolomics Ischemic stroke Biomarker Precision medicine Single cell metabolomics
ABSTRACT Ischemic stroke (IS) is a multifactorial and heterogeneous disease.Despite years of studies,effective strategies for the diagnosis,management and treatment of stroke are still lacking in clinical practice.Metabolomics is a growing field in systems biology.It is starting to show promise in the identification of biomarkers and in the use of pharmacometabolomics to help patients with certain disorders choose their course of treatment.The development of metabolomics has enabled further and more biological applications.Particularly,metabolomics is increasingly being used to diagnose diseases,discover new drug targets,elucidate mechanisms,and monitor therapeutic outcomes and its potential effect on precision medicine.In this review,we reviewed some recent advances in the study of metabolomics as well as how metabolomics might be used to identify novel biomarkers and understand the mechanisms of IS.Then,the use of metabolomics approaches to investigate the molecular processes and active ingredients of Chinese herbal formulations with anti-IS capabilities is summarized.We finally summarized recent developments in single cell metabolomics for exploring the metabolic profiles of single cells.Although the field is relatively young,the development of single cell metabolomics promises to provide a powerful tool for unraveling the pathogenesis of IS.
1.Introduction
Metabolites stand for both the upstream input of the environment and the downstream output of the genome.Thus,metabolomics,the study of metabolites and metabolism,is the center of systems biology research and enables researchers to study how genes and the environment interact (Fig.1).Due to the development of analytical techniques and bioinformatics,metabolomics has become a new diagnostic tool in clinical and biological research.It is a relatively young omics approach and may be the most promising omics strategy because it directly reflects cellular phenotypes and aids in understanding the downstream effects of the central dogma (Box 1).Given these characteristics and the ease with which biological samples such as cells,tissues and blood may be studied by metabolomic analysis,metabolomics has great potential for the discovery of biomarkers [1].Metabolomics has several limitations,most of which relate to sample consistency,confounding factors,and the detection of low-abundance metabolites.Despite the limitations of metabolomics,new metabolic insights and advances in biological sample preparation and instrumentation that allow increasingly easy metabolomic analysis are leading to changes in the way that drugs are discovered,developed and treated [2,3].Metabolomics makes it possible to analyze individual metabolic phenotypes,which is believed to contribute to the development of precision medicine.
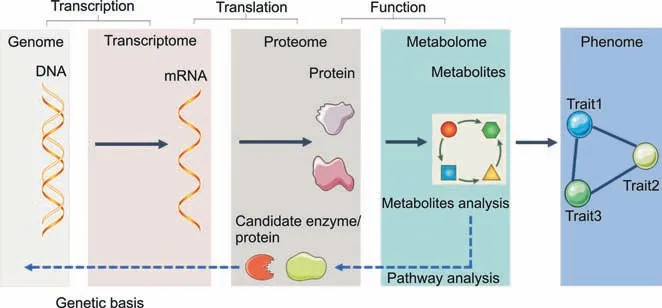
Fig.1. A systems biology approach with a focus on metabolomics.
Stroke is an acute cerebrovascular disease and a common cause of death in the elderly,affecting more than 15 million people worldwide [4-6].Stroke is the second most common cause of death[7].Ischemic stroke(IS)is a major disease type of stroke[8].Among elderly stroke patients,88.27% had an IS [9].At present,clinical treatment strategies for IS remain a great challenge [10].Only two available trials including intravenous tissue plasminogen activator and mechanical thrombectomy have been the currently authorized therapeutic options [11,12].However,these two treatments both have the limitation of narrow therapeutic window and several safety concerns [13].Therefore,the development of novel strategies is essential to save people with IS.
Ischemic brain injury is extremely complicated and involves a series of pathological processes [14].Metabolite disturbances are believed to be important factors in IS.The development of metabolomics makes it possible to discover these potential biomarkers with diagnostic and prognostic relevance.Recently,some metabolomics studies have also revealed the pathological process of IS.Because IS has a complicated pathogenic mechanism,it is difficult to develop an effective treatment method based on the strategy of“one drug,one target,”which leads to a subpar therapeutic outcome[15].Chinese herbal medicine(CHM)has been used for thousands of years in China to treat patients for stroke.In recent years,the metabolomics approach has been gradually applied to research on CHM,and attempts have been made to emphasize the critical role that metabolomics plays in resolving the numerous problems that CHM faces.It may scientifically express the meaning of syndromes and the efficacy of CHM treatment [16,17].The development of metabolomics provides a favorable opportunity for CHM research,clarifies the molecular targets and network regulations of the active compounds and CHM formulae,and helps people comprehend the fundamentals of CHM formulae to treating ischemic brain injury.
Box 1Features of metabolomics
▪Metabolism and metabolic phenotypes indicate what is currently happening compared to genes and genetic risk.
▪Metabolomics is the study of small-molecule metabolites and provides a functional fingerprint of the body's physiological and pathological conditions.
▪For metabolomics studies,pattern recognition and metabolite identification are two helpful methods.
▪Combined with drug metabolism,metabolomics is used for personalized medicine.
In this paper,we first look at the analytical platforms and recent advances in metabolomics,and discuss the advantages and disadvantages of each metabolomics approach.We review the application of metabolomics in IS research and analyze the main research information of metabolomics.Our goal is to find metabolic biomarkers that require further investigation to forecast the risk of IS,establish a clinical diagnosis,and recognize complications.Second,by analyzing the abnormal metabolic pathways in IS,we may gain a better understanding of the molecular processes underlying IS,which help develop new therapeutic strategies for IS disease.Additionally,we summarize representative natural ingredients and CHM formulae for the treatment of ischemic brain damage.Then,we discuss metabolomics-based research in personalized medicine and discuss the role of metabolomics in precision medicine and biomarker discovery.Finally,a higher resolution,single-cell level metabolomics analysis is introduced.It is evident that the field of metabolomics is advancing toward single cell analysis and discovery,which is undoubtedly one of the most powerful tools we presently have at our disposal.This will allow us to perform a more detailed and specific analysis of IS.
2.Search syntax and inclusion criteria
Relevant published studies were identified for the years 2005-2023 by means of Pubmed and Web of Science search in terms of the following general string with title: ‘‘Metabolomics’’or ‘‘Metabolomics and Cerebral ischemia’’ or ‘‘Metabolomics and IS’’ or ‘‘Metabolomics and biomarker’’ or ‘‘Metabolomics and Traditional Chinese Medicine’’or“Single-cell metabolomics”,and‘‘IS’’or‘‘Cerebral ischemia’’.The pre-set criteria for inclusion were(i) CHM or natural plants (e.g.Traditional Chinese Medicine formulas,herbal extracts): or individual compounds used for the prevention and treatment of IS;(ii)Only single-cell metabolomics studies were searched;(iii) Using the method of metabolomics research rather than using other methods;(iv) The study attempted to elucidate the underlying mechanism of CHM;(v)Only publication in English was included.
3.Analytical platforms in metabolomics
Modern analytical chemistry techniques and advanced computational tools are employed in metabolomics to characterize complicated biological products.The two analytical techniques that are utilized most frequently in metabolomics research are by far nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry(MS)[18].Currently,hundreds of metabolites are detected and quantified simultaneously in a single sample,depending on the analysis platform employed,particularly,the widely used chromatography-MS systems,such as liquid chromatography-MS(LC-MS)and gas chromatography-MS(GC-MS)(Table 1)[19-25].
3.1.NMR spectroscopy
NMR spectroscopy is used for quantitative analysis because its signal intensity is proportional to the number of1H,13C,15N,and31P in a single nucleus [26].In the field of NMR research,quantitative nuclear magnetic resonance (qNMR) is an important subfield focused on the precise and reproducible measurement of molecular concentrations [27].Utilizing qNMR,active chemicals incomplicated matrices have been extensively investigated and measured (e.g.,body fluids,natural product extracts) [28,29].In metabolomics,one of the main reasons for using1H NMR spectroscopy is that it is fast and the resulting spectra are not greatly affected by saline biological fluids.However,due to its low sensitivity,NMR is unable to measure low-abundance metabolites.Since there is no proper separation method,the signals from thousands of metabolites overlap,making precise structure identification challenging and time-consuming [30].However,technological advances have reduced these shortcomings and improved the sensitivity and resolution of NMR methods [31].For example,when compared to one-dimensional NMR spectra,two-dimensional NMR spectra allow for superior compound identification since crowding and overlap are reduced,and cross peaks exclusive to pairs of spin coupled nuclei are used to identify specific molecules [32].Identifying compounds is more challenging when there is a chance that the peaks in a NMR spectrum may shift due to changes in sample temperature or pH value.Heteronuclear single quantum coherence(HSQC)methods have improved spectral resolution and metabolite identification.By exploiting the larger13C chemical shift dispersion on one axis of the two-dimensional spectrum,HSQC spectroscopy could offer better resolution and greater metabolite selectivity.

Table 1 Comparison of metabolomics approaches.
3.2.MS-based spectroscopy
3.2.1.GC-MS
In contrast to NMR spectroscopy,MS provides a highly sensitive and selective way to quantify hundreds to thousands of metabolites based on their mass/charge ratio and signal strength.Although direct injection of biological samples is feasible,most MS platforms combine separation modes such as GC or LC [33].The analysis of naturally volatile or derivatized metabolites is done using GC-MS,and GC coupled to MS may be used for improved sensitivity and resolution [34].Because the13C-labeled metabolites are heavier than those containing the12C isotope,GC-MS is also used to track them [35].The advantages of GC compared to LC are that it has a strong chromatographic form with only insignificant changes during the analysis,and that since the analytes are volatile,they may be conveniently added to the mass spectrometer.
However,many metabolites are not volatile and need to be derivatizedfirst.For example,fattyacids require conversion to methyl esters,amino acids,and sugars,which can be analyzed by adding one or more trimethylsilyl groups [36].In addition,derivatization itself has the potential to destroy metabolic information.This process also makes it difficult to determine the source of the metabolites.
3.2.2.LC-MS
An alternative strategy to GC-MS is required to investigate intricate macromolecules while ensuring that metabolites are ionized without being destroyed.Due to its ability to detect and quantify a large number of metabolites without the need for derivatization,LC-MS has been widely employed for metabolite studies [37].Metabolite identification and chromatographic reproducibility are the two main obstacles hindering the development of LC-MS metabolomics techniques.Additionally,determining the structure of a metabolite becomes more challenging if multiple isomers are present,or “adducts” are created when molecules combine with other molecules.As analytical platforms and methods continue to improve and refine,some researchers have removed the chromatography step and used direct injection instead.To improve the ability to identify metabolites,highresolution instruments or triple quadrupole instruments are used to detect metabolites through their unique fragmentation patterns.Although matrix effects affect the accuracy of LC-MS results,this restriction may be circumvented by utilizing a variety of sample extraction processes and advanced tandem MS technique.
4.Pathophysiological signatures of IS
The pathophysiological effects of IS are caused by multiple complex molecular and cellular interactions that ultimately lead to brain damage and neurological dysfunction.The underlying pathophysiological processes of IS include genetic alterations,oxidative and nitrative stress,inflammatory,ferroptosis,and disturbance of the microbiota.
4.1.Genetic signature of IS
The development of cerebrovascular disease mainly involves genetic variables.IS is a multifactorial polygenic disease in which the role of environmental influences has been well studied,while the role of genetic factors needs to be further elucidated[38].With the advancement of whole-genome sequencing technology,it has been revealed that the bulk of transcripts are non-coding RNAs and that only approximately 2%of the human genome gets transcribed into proteins [39].Non-coding RNAs are discovered to perform housekeeping tasks in a variety of biological processes by participating in the transcriptional and post-transcriptional regulation of gene expression [40].Among them,long non-coding RNAs(lncRNAs)are of special importance in IS biology [41].Akella et al.[42] showed that numerous lncRNAs are abnormally expressed in the brain and blood after IS.Numerous of these lncRNAs were discovered to alter biochemical pathways,and these changes in expression displayed distinctive temporal and cell-type-dependent patterns.Therefore,it is impossible to overlook the involvement of lncRNA in pathogenesis following IS.LncRNA is expected to develop into a new model and target for regulating IS,as it is a significant endogenous regulatory mechanism (Fig.2).

Fig.2. The role of lncRNAs in the pathophysiology of ischemic stroke (IS).MALAT1:metastasis-associated lung adenocarcinoma tran 1;SNHG12:small nucleolar RNA host gene 12;ANRIL: antisense non-coding RNA in the INK4 locus;MEG3: maternally expressed gene 3;TUG1: taurine upregulated 1;KCNQ10T1: potassium voltage-gated channel subfamily Q member 1 opposite strand 1.
4.2.Oxidative and nitrative stress signatures of IS
Free radicals have a crucial part in the damage caused by brain ischemia-reperfusion,including reactive oxygen species(ROS)and reactive nitrogen species(RNS) [43].Acute IS is known to increase the production of free radicals,and both ROS and RNS molecules are confirmed to play a key role in tissue destruction [44,45].Free radical production during ischemia is caused by many processes,includingN-methyl-D-aspartate-mediated excitotoxicity,increased Ca2+influx,mitochondrial failure,and activation of neuronal nitric oxide synthase [46,47].Overproduction of ROS damages cellular macromolecules and contributes to the signaling mechanisms that lead to apoptotic cell death [48].Reactive radical overproduction has negative consequences that damage brain cells,but at homeostatic levels,these radicals are necessary signaling molecules that support proper neuronal activity [49].Normally,free radical scavengers and antioxidant enzymes prevent or mitigate the consequences of free radical damage[50].Redox homeostasis is kept in check by antioxidant and detoxifying enzyme activity.These enzymes include glutathione reductase,glutathione peroxidase,superoxide dismutase (SOD),and glutathione-S-transferase,which are being studied for their potential roles in IS [51].The SOD enzymes,such as extracellular SOD and manganese SOD,aid in the recovery of the brain after ischemia-reperfusion injury [52].
4.3.Inflammatory signatures of IS
Immunological and inflammatory cells play a major role in IS.Following cerebral ischemia,there is a significant inflammatory response that involves the activation of local inflammatory cells,the production of inflammatory mediators,and the recruitment and migration of leukocytes across the blood-brain barrier (BBB)[53].These cytokines are key players in the development of the immuno-inflammatory response induced by a stroke,which affects the severity and course of the disease [54].Cytokines with proinflammatory effects are involved in many processes in cerebral ischemia,and they directly activate endothelial cells,neurons and glial cells.For instance,endothelial nitric oxide synthase injury increases the level of ROS because nitric oxide (NO) synthesis is diminished [55].Increased ROS production causes proinflammatory genes to become active.Upon being activated,inflammatory cells,including microglia and astrocytes,release cytokines and chemokines,as well as matrix metalloproteinases(MMPs),NO,and even more ROS [56].Overall,these processes exacerbate cellular damage and further disrupt BBB,such as endothelial dysfunction due to endothelial cell apoptosis [55,57].
As early as 6 h into the process of ischemic brain damage,infiltrating neutrophils may appear in the ischemic penumbra region and create neutrophil extracellular traps [58].At 48 and 96 h following an IS,neutrophil infiltration increased further.Proinflammatory N1 neutrophils in ischemic brains promote the production of inducible nitric oxide synthase (iNOS),MMP-9 and myeloperoxidase(MPO),exacerbating oxidative stress-related brain damage.At the same time,neutrophil polarization into the N2 type provides neuroprotective benefits [59].The microenvironment through which neutrophils penetrate may explain their multiple functions.Oxidative/nitrogen stress and its associated redox signaling cascades in the microenvironment may play a crucial role in neutrophil polarization,particularly during ischemic injury.Therefore,inhibiting inflammatory factor-mediated neuronal cell death and enhancing the therapeutic result of IS may be achieved by targeting inflammatory cells and their surroundings.
4.4.Ferroptosis signatures of IS
The buildup of excessive intracellular iron ions,lipid peroxides and associated metabolites,as well as the peroxidation of polyunsaturated fatty acids,are the hallmarks of ferroptosis,a distinct kind of programmed cell death [60].Recently,it has been shown that individuals with ischemic cerebrovascular diseases have a remarkably increased amount of iron deposition in the brain tissue,and it is believed that this iron deposition may serve as an imaging marker of ischemic areas[61].According to epidemiological studies of population samples,iron deficiency anemia and IS have a high correlation [62].In addition,it has been demonstrated that IS patients have considerably higher serum iron ions,and that hepcidin plays a critical role in iron overload following cerebral ischemia[63].A middle cerebral artery occlusion model was utilized by Tuo et al.[64],who discovered considerable iron buildup in the ischemic half-dark zone of brain tissue and iron death in neurons.The results indicated that silencing Tau protein ameliorates this injury and may be involved in regulating the mechanism of ischemia-reperfusion-induced iron death.Ferroptosis and IS have received comparatively little scientific attention,and consequently the next line of inquiry for researchers should focus on the precise mechanism and therapeutic approach of ferroptosis in ischemia injury to brain tissue.
4.5.Gut microbiota signatures of IS
Recent studies have suggested that compromised gut microbiota may be a risk factor for IS and have an impact on the prognosis following IS [65].The gut microbiota is a microorganism that is dispersed throughout the digestive system and is primarily composed ofBacteroidetes,Firmicutes,Actinobacteria,ProteobacteriaandVerrucomicrobia[66].In particularly,theFirmicutes/Bacteroidetesratio was used as a predictor of health and disease[67].The IS alters the gut microflora's makeup.On the contrary,the gut microbiota influences the development of IS and regulates its outcome.The neuronal networks connecting the brain to the gut form a complex gut-brain or brain-gut axis with considerable bilateral connectivity.Growing evidence suggests that gut microbiota may be crucial to the development and management of IS[68,69].Three days after ischemia-reperfusion in mice,higher levels ofBacteroideteswere found,which was considered a marker of ecological dysbiosis after IS [70].On the other hand,a clinical study that collected stool samples two days after admission and examined the results showed that patients with acute IS and transient ischemic attacks had lower levels ofBacteroidetes[71].Relatively low concentrations ofOscillospira,Streptococcus,Lactobacillus,andFaecalibacteriumwere seen in monkeys following local cerebral ischemia [72].The principal sources of butyrate in host organisms are known to beFaecalibacteriumandOscillospiraspecies [73,74].Butyric acid,which reduces the production of proinflammatory cytokines and protects the integrity of the intestinal barrier,has been suggested as a potential treatment for brain disorders[75].It should be stressed thatLactobacillusis an essential host probiotic that has been shown to improve mood,cognition,and reduce inflammation associated with aging [72,76,77].Along with persistent systemic and brain inflammation,frequent stroke consequences include dementia and depression [78-80].Future clinical trials should examine the potential benefits oflactobacillussupplementation in patients with stroke.These findings suggest that in addition to gut microbiota dysbiosis,persistent systemic inflammation may emerge following brain infarction.Additionally,correlation studies demonstrated a strong relationship between excessiveBacteroidetesgrowth and elevated plasma lipopolysaccharide or inflammatory cytokine levels [72].As a result,pathological alterations in the brain are strongly correlated with the production of proinflammatory cytokines from the gut into the blood circulation [77].Therapeutic targets for IS may include the post-stroke gut microbiota and persistent systemic inflammation.
5.Metabolomics studies in IS
The metabolome of an organism is regulated by internal processes and the environment,and offers detailed information about the state of the organism.In this sense,metabolomics not only makes it feasible to find disease biomarkers in the form of endogenous (gene-derived) and exogenous (environment-derived) metabolites,but also provides novel insights into the underlying causes of disease [81,82].With these novel metabolic insights and the expanding availability of metabolomics research,there is little question that metabolomics will play a significant role in IS treatment strategies in the future.
Cerebral ischemia leads to local and systemic metabolic changes,such as changes in cellular energy metabolism pathways.Metabolomics studies are used to identify these metabolic changes to determine whether metabolites are used as biomarkers to conduct IS diagnosis and assess IS risk.Metabolites are made up of small molecules such as nucleotides,amino acids and lipids [83].Metabolites are the result of genetic,protein and environmental changes[84].Therefore,profiling of the metabolome might provide system-level information.
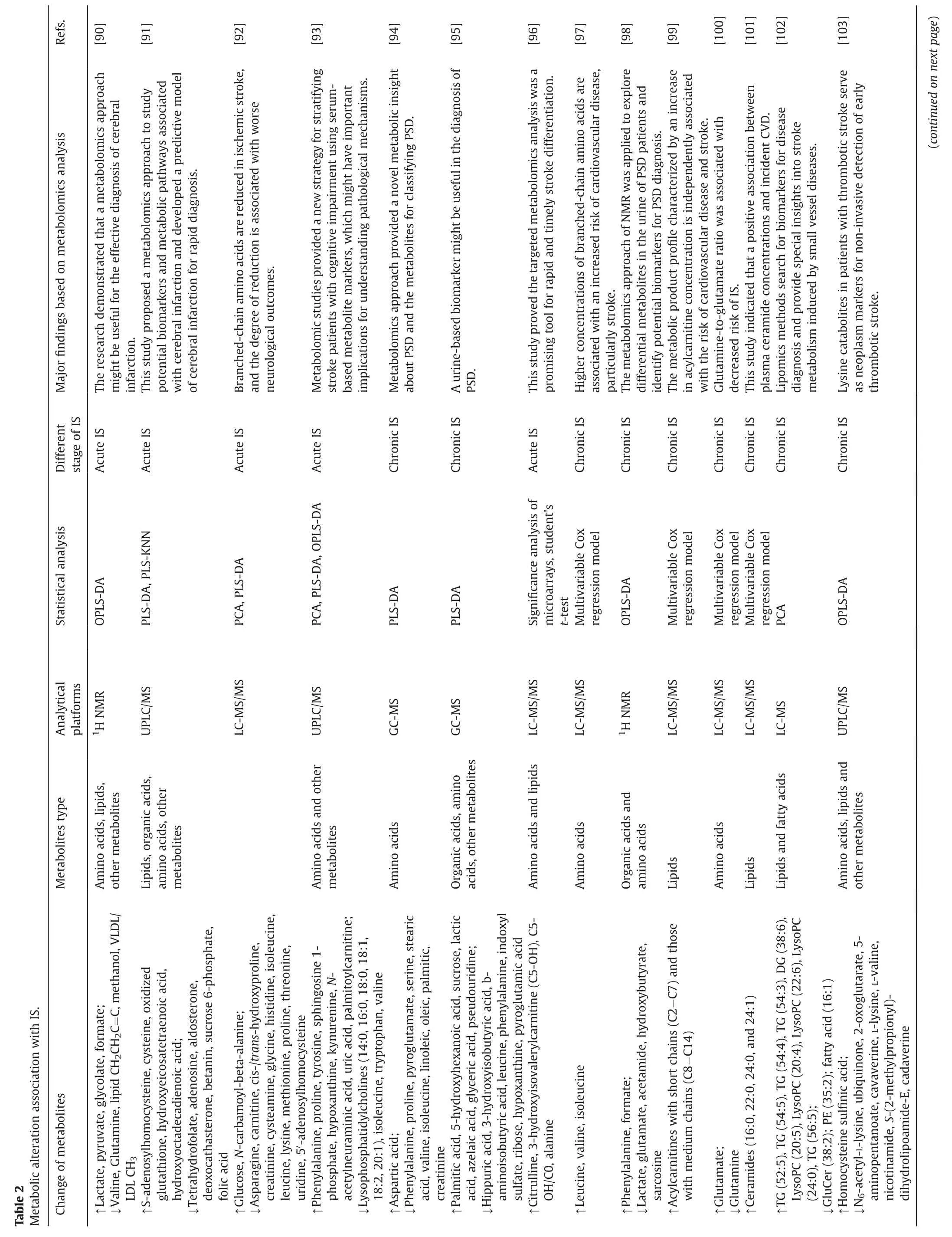
Metabolites associated with IS,such as amino acids,lipids,polyamines,and nucleotides,have been explored to understand the disease mechanisms of cerebral ischemia[85].Guo et al.[86]used LC-MS studies to find that serum concentrations of 37 metabolites were substantially different from those in a control group.These metabolites include sphingolipids,lysophosphatidylchoine,stearic acid,phosphatidylchoines,organic acids,amino acids,and carnitine derivatives,and pathway enrichment analysis has shown that key metabolites were involved in the biosynthesis of unsaturated fatty acids,ɑ-linolenic acid metabolism,fatty acid metabolism,ketone body synthesis and degradation,and the interconversion of pentose and glucuronide.Similarly,Wu et al.[87]employed LC-MS to find that there were reduced quantities of phosphatidylchoines,phosphatidylethanolamines (PEs),lysoPEs,and sphingosine-1-phosphate in the blood samples,showing perturbations in lipid metabolic pathways.In addition,blood concentrations of lactate,glucose,glucose 6-phosphate,succinate,malate,and citrate increased compared to control.Since these metabolites are associated with energy metabolism,this suggests that the glucose and anaerobic glycolytic pathways were disturbed [88].Luo et al.[89]found that the metabolites of lactate,creatine,glycine,alanine,leucine,and lysine in brain tissue,cerebrospinal fluid,and plasma of rats were changes,indicating disturbances in amino acid metabolism.Metabolic alterations associated with IS were summarized in Table 2 [90-104].
6.Metabolomics in the treatment of IS with CHM
The amount of metabolomics data has exploded dramatically in recent years,which has had an impact on all areas of life sciences,including the discovery and development of drugs [105].There is currently mounting evidence that CHM may regulate multiple targets with fewer side effects and lower toxicity,and that it is garnering more attention in the face of complex disease causes[106].For the creation of new drugs,CHM may be an inspiration.Numerous international investigations have recently been conducted to decipher the mechanism of action of CHM.Many metabolomic-based approaches have been used in these investigations to promote the exploration of CHM.Furthermore,metabolomics in conjunction with pharmacology is a reliable method for the study of novel biologically active substances and targets,as well as the presence of interactions[107].Metabolomics is expected to significantly advance CHM research and contribute to the modernization of CHM by expanding the use of modern means.
6.1.Extraction components from CHM for IS
CHM originates from natural sources and is used for clinical treatment in China.Chinese medicine plays a crucial role in the discovery and development of new drugs,such as,the discovery of artemisinin and its remarkable contribution in the fight against malaria.It includes effective extract ingredients and Chinese herbal formulas and preparations.Researchers find that some CHM extractions,such as sesamin,tanshinone IIA,salvianolic acid A,salvianolic acid B,6-paradol,baicalin,astragaloside IV,totarol,ginkgetin aglycone,naringin and 6′-O-galloylpaeoniflorin,have a potent neuroprotection effect against IS.As we all know,CHM extractions are frequently used in clinical for therapy,and most of their applications have been shown to be effective against IS.In addition to reducing oxidative stress,inflammation,and neuronal death,CHM extractions also promote angiogenesis and regulate autophagy to protect IS against a variety of threats.In a word,it is important to explore the mechanisms and effective molecular targets of CHM,which will provide new insights into the treatment of IS and facilitate the process of discovering new active drugs from CHM.Thus,we cover the effects of CHM extractions in ischemic brain damage in this part (Table 3) [108-118].
6.2.Metabolomics approach to explore the natural compounds and CHM formulae for IS treatment
Finding the metabolic biomarkers and signaling pathways,as well as understanding the therapeutic tenets of natural compounds and CHM formulations for IS,is made possible by the high throughput and high sensitivity of metabolomics.Numerous metabolites,including glutamate,isoleucine,leucine,valine,glycine,lysine,LysoPC (16:0),LysoPC (18:2),serine,uric acid,citrate,and palmitic acid,have the potential to act as biomarkers for ischemic brain injury.These metabolites have been linked to apoptosis,oxidative stress,excitotoxicity and inflammation [119].Comprehensive profiles of the molecular targets of natural compounds and CHM formulations for the treatment of IS are provided by combining advanced metabolomics and network analysis.
Famous Chinese herbal remedy Huang-Lian-Jie-Du Decoction(HLJDD) has been used to treat IS [120].HLJDD consists ofRadix Scutellariae,Cortex Phellodendri,Rhizoma CoptidisandFructus Gardenia[121].Its neuroprotective benefits against brain ischemiareperfusion damage are aided by HLJDD's antioxidant and antiinflammatory properties [122,123].Zhu et al.[124] used a metabolomics approach to identify 19 biomarkers associated with metabolic stress,glutamate/GABA-glutamine cycle,and enhancement of cholinergic neuron function.The disturbance of amino acid metabolism in MCAO ischemic rats was also reversed by HLJDD,as demonstrated by global and amino acid-targeted metabolomics combined with correlation network analysis [125].In addition,berberine,baicalin,and jasminoidin,which are the main active ingredients in HLJDD,were indicated to have neuroprotective benefits.A recent study looked at the effects of berberine,baicalin and jasminoidin on neuroprotection.1H NMR metabolomics indicated that these three components may regulate oxidative stress,aberrant metabolism,neuron autophagy and inflammatory response in cerebral ischemia-reperfusion damage [126,127].
A well-known CHM formula called Angong Niuhuang Wan(AGNHW)is frequently applied as emergency care in China for stroke and traumatic brain injury [128].Clinical research suggests that AGNHW may reduce brain damage in IS patients [129,130].The protective effects of AGNHW against cerebral ischemia-reperfusion injury included maintaining BBB integrity,reducing infarct size,and improving neurological function by inhibiting ROS/RNSmediated MMP activation and maintaining tight junction proteins[131].Utilizing ultra-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry (UPLC/Q-TOF MS) and pattern recognition technique,the detoxicant components of AGNHW was discovered.According to the results,36 of the 41 endogenous metabolites associated with liver toxicity in cinnabar and realgar might be regulated by the herbal components of AGNHW[132].Additionally,Zhang et al.[133] used network pharmacology and molecular docking methods to study AGNHW,which identified quercetin,β-estradiol,berberine,baicalein and β-sitosterol as the main active ingredients and identified the key targets,including interleukin 6 (IL-6),AKT1,mitogen-activated protein kinase 3(MAPK3),PIK3CA and tumor necrosis factor(TNF).Until now,the use of metabolomics to study the active ingredient of AGNHW and its application in ischemic stroke has not been reported.
When treating cerebral infarction,the CHM formulae Naodesheng (NDS) is frequently utilized and has positive therapeutic results [89].In rats with localized cerebral ischemia,NDS was reported to significantly reduce the area of infarction and improve the impairment of neurological function [134].NMR-based metabolomics approach to determine the protective effect of multiple components from NDS has been shown to regulate oxidative stress,energy metabolism,lipid metabolism,amino acid metabolism,and inflammation in cerebral ischemia-reperfusion injuries [89].Inanother study,it was also shown that a bioactive extract of NDS had protective effects in rats with ischemic stroke,with potential mechanisms involving multiple metabolic pathways,like energy metabolism,amino acid metabolism,oxidative stress,and inflammatory damage [135].Other CHM formulas,such as Naoshuantong Capsule [136],Fu-Fang-Dan-Zhi Tablet [137] and Mi-Jian-Chang-Pu Decoction [138] were used to understand the molecular mechanisms of CHM in the treatment of IS through metabolomics methods.However,there is still a need to use metabolomics approaches to explore the molecular mechanisms of the active ingredients in CHM and CHM formulations for the treatment of IS.
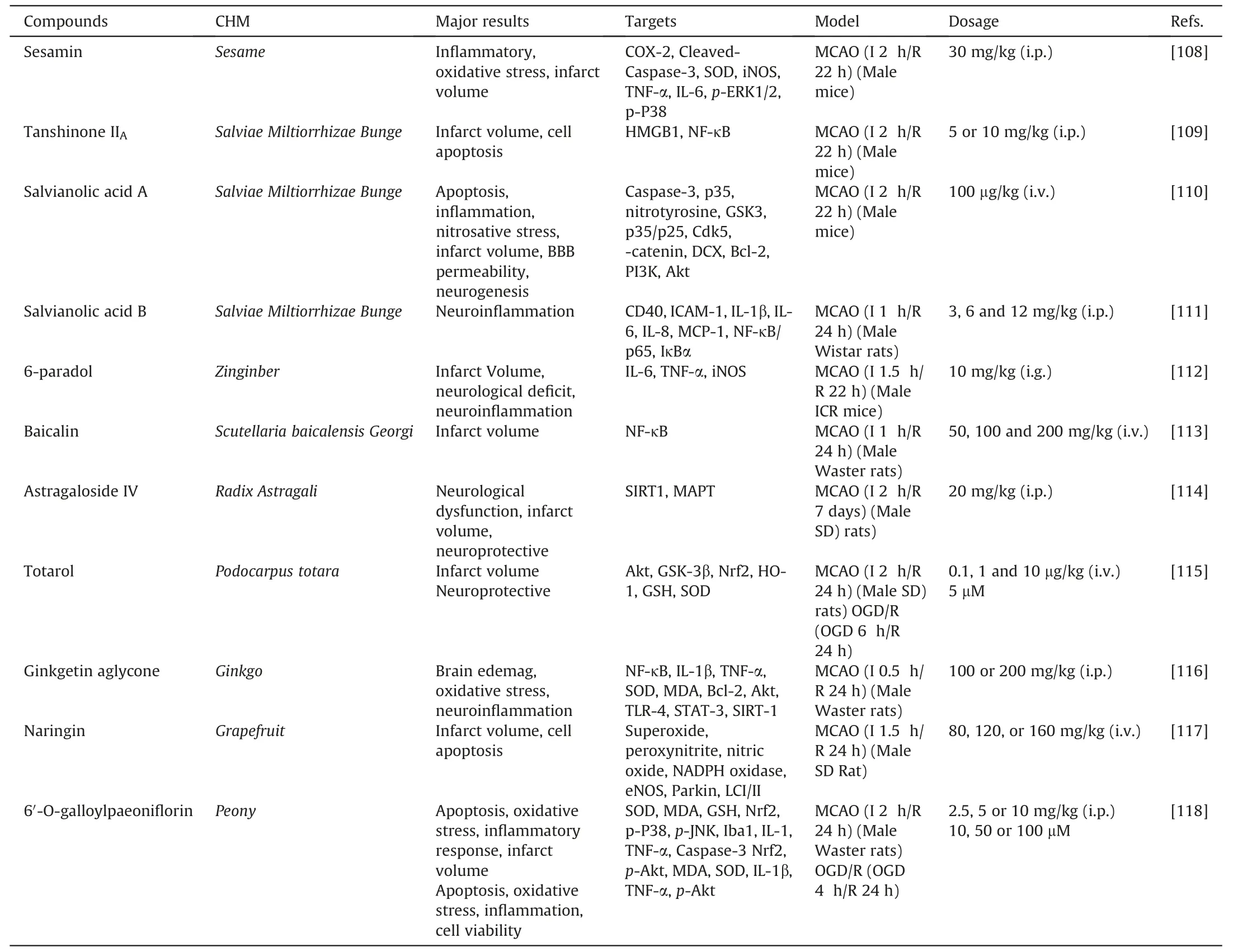
Table 3 The role of natural bioactive compounds in ischemic brain injury.
7.Data analysis and processing methods for metabolomics
Metabolomics is a omics approach that aims to qualitatively and quantitatively cellular metabolites [36].Along with other omics such as genomes,transcriptomics,and proteomics,metabolomics provides essential insights into the make-up of organisms.Research on metabolomics has so far been conducted using a range of methods,mostly concentrating on the two popular platforms,NMR and MS.Although non-destructive and high reproducibility are demonstrated using NMR[21,139],MS-based metabolomics is a common technique because of its broad analytical coverage,high sensitivity,high throughput,and capability of molecular structure identification.Because metabolomics raw data is complicated,ongoing improvements to the analytical process are required for the best possible information retrieval.The complexity stems from the structure of the spectrographic data as well as the linear and nonlinear interactions between metabolites.There are some challenges with the data structure due to high noise levels,batch effects,and missing findings.The metabolomics community has thus always been keen to adopt fresh computational and statistical techniques to improve data processing.
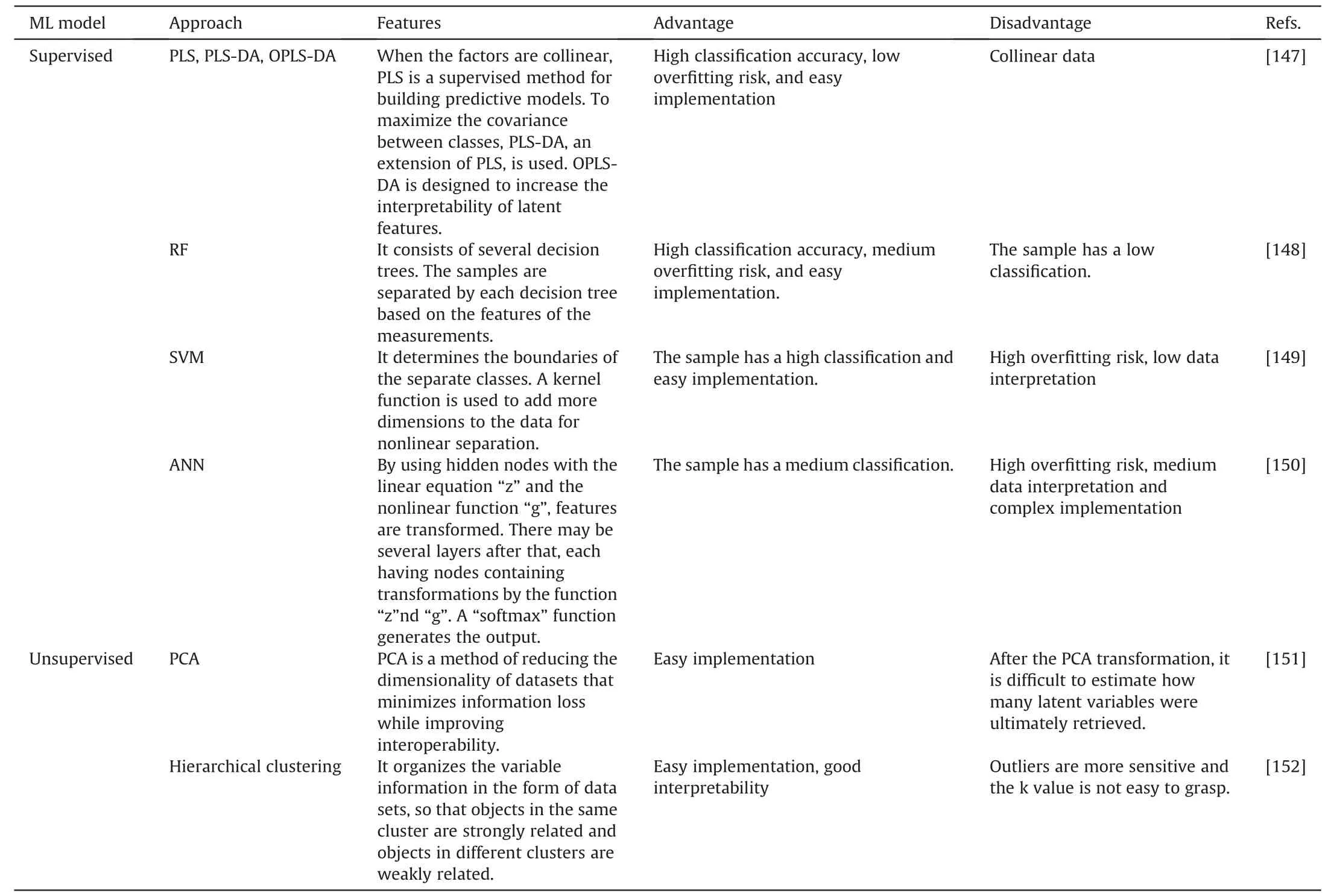
Table 4 Description of statistical models in machine learning (ML).
Machine learning (ML) is a computer method to finding meaningful patterns in data[140].For instance,assume that we get genome-wide gene expression measurements from the blood of individuals who have received a certain medication.Forecast whether a new patient would be receptive or unresponsive to the therapy based on his or her gene expression profile would be a typical issue that may be handled using a ML method [141].To make credible predictions about unknown data,statistical models are trained on data in ML.Artificial neural networks (ANNs) were among the first ML methods to be used;in 1990,Curry and Rumelhart [142] released “MSnet” to discern between different classes of metabolite composition and ANNs were continuously used and improved[143,144].A few years later,random forest and support vector machines were introduced into the field of omics[145,146].The common supervised and unsupervised ML models are summarized in Table 4 [147-152].
ML has advanced quickly and now offers multiple algorithms.The vast of ML solutions feature intuitive user interfaces that let chemometricians evaluate different ML strategies and improve applications for spectrum analysis.Utilizing the proper statistical techniques,metabolic data is evaluated to identify biomarkers and determine connections between various factors [153].In general,univariate statistics are used to analyze only one variable,whereas multivariate statistics are used to analyze many variables.Skewed or jagged datasets are utilized for nonparametric testing,whereas normally distributed datasets are used for parametric tests [154].Unsupervised multivariate statistical analysis techniques include Kmeans,hierarchical clustering,self-organizing maps,and principal component analysis (PCA).These approaches make the data less dimensional and provide clusters based on sample-to-sample data similarity [155-157].Using supervised multivariate statistical analysis techniques,the relationship between a given etiology and metabolites was investigated.The methods for regression and classification are included in the supervised multivariate statistical analysis.Regression between response and predictor variables was performed using partial least squares(PLS)and partial least squares discriminant analysis (PLS-DA) [158].Orthogonal projections to latent structures (OPLS),an improved PLS method,employs an orthogonal signal correction filter to eliminate uncorrelated variables and reduce model complexity[159].Different algorithms are used to simultaneously create classifiers,such as discriminative analysis,support vector machine (SVM),K-nearest neighbor classification,and soft independent modeling of class analogies[160,161].
8.Metabolomics and precision medicine
The goal of precision or customized medicine is to use advanced omics testing to customize a patient's medical care to their specific profile.Using high-throughput omics approaches such as genomics,proteomics,and metabolomics in conjunction with computational techniques such as bioinformatics,we may be able to learn more about specific targets in biological systems [162].Among them,metabolomics involves the identification and quantification of small molecules,and the resulting network interactions,representing the functional genome of an individual.The entire collection of metabolites in a biological sample is called the “metabolome” and is highly dynamic.Significant changes in multiple metabolic pathways essential for maintaining cellular homeostasis may be evaluated at the metabolic level.Metabolomics combined with clinical chemistry actually provides over 190 clinically approved metabolite biomarkers[163].The success of metabolomics in biomarker translation relative to other omics approaches appears to be that the underlying instrument(tandem mass spectrometer) is robust,greatly quantitative,readily adaptable to different detection methods and has been used in numerous clinical tests laboratory [163,164].In addition,a lot of clinical chemistry laboratories have the necessary expertise,regulatory clearance and personnel qualifications to perform accurate chemical biomarker validation.Future clinical approval of several more drugs is expected,in line with further recognition of the inherent advantages of metabolomics.This is due to continued advances in metabolomic automation and quantification,as well as improvements in software and protocols for biomarker selection and validation.
Drug response and monitoring is another area where metabolomics has an impact on precision medicine.The individual's capacity to metabolize or utilize the medicine has a significant impact on drug response,which is very diverse.Despite the discipline of pharmacogenomics,which has genotyping as its major focus,is widely known,the developing of pharmacogenomics demonstrates how metabolomics may be utilized to augment this genetic information [165].Measurements of medication dosage and response have been made possible by pharmacometabolomics,which has produced several exciting and beneficial findings.For instance,to optimize patient dose,fast MS-based monitoring of immunosuppressant medications and their metabolites is being used [166].In addition,MS-based assays have been shown to be slightly faster,more sensitive and more robust than conventional immunoassays[167].In drug dose determination,the metabolomics approach allows one to phenotype an individual's likely drug response prior to administration[168].This metabolomics approach avoids the timeconsuming and occasionally risky approach that many physicians employ when figuring out specific dosages for medications with constrained therapeutic windows.Recently,pharmacometabolomics has begun to benefit from several large-scale genome-wide association studies and metabolome-wide association studies[169,170].Such studies not only help explain individual differences in metabolite levels,but also highlight how endogenous metabolites combined with single nucleotide polymorphism typing can be used to predict or better understand an individual's response to a drug or therapeutic intervention [170].
9.Single cell metabolomics: the future approach in IS research
“Metabolome” refers to the collection of all small molecule metabolites from a single cell to a whole organism [171].These metabolites represent the physiological state of the sample and are necessary ingredients for many intracellular processes and activities.Cells are the basic components of organisms [172-174].The downstream products of the genome,transcriptome,and proteome are the cellular metabolomes,and the results of these studies provide crucial biological information [175].Due to their inherent complexity as biological systems,cells are frequently used as model systems in a wide range of metabolomics studies [176,177].As a result,quantitative metabolomics has long been a potent analytical tool in many fields.Due to sensitivity issues,current metabolomics approaches often require a high number of cells per sample,especially for certain methods such as NMR[178].Although the fact that multicellular samples enable efficient detection of chemical compositions,cellular heterogeneity is neglected.Differences between individual cells may be the result of genetic,epigenetic,developmental and environmental factors.There is growing evidence that cells are highly heterogeneous,and that they are incredibly diverse,even when they come from the same tissue[179,180].Single cell analysis enables the investigation of cell-tocell differences within a cell population and may provide crucial clues to identify disease causes that are hidden in bulk analyses[181,182].Single cell genomics,transcriptomics,proteomics and metabolomics have experienced a boom in recent years due to advances in single cell isolation techniques and analytical tools.Single cell omics provides insights into the heterogeneity of cell phenotypes and genotypes [183-185].Particularly,single cell metabolite profiling helps us better understand how different types of cells respond to environmental cues and may also provide us with crucial data for the diagnosis of diseases and the development of new drugs [173,174,186].
9.1.Methods for single cell metabolomics
Due to the small cell size (usually many tens of micrometers in diameter for a mammalian cell) [171,187],intrinsic complexity of intracellular species [188],and rapid turnover of cellular metabolites,sampling analysis is one of the most challenging steps in single cell studies [189,190].Thus,an efficient sampling strategy often requires a high magnification microscope to watch cells,a finely controlled mechanical system to move the sample,and an effective extraction or desorption procedure to acquire data on cellular content.
To present,a variety of approaches have been described for sampling and ionizing molecules from individual cells in both vacuum and natural environments.Ion beam[191],laser[192]and probe [193] sampling approaches are among them (Fig.3).The method used for single cell separation is arguably the most crucial stage,as improper operation may disturb the surrounding environment and lead to inaccurate metabolic profiles [171,185].Now,the two most common methods for sampling and ionization in vacuum settings are ion beam and laser techniques.For these methods,the sampling and ionization processes occur concurrently.The ion-beam based methods,such as nanoscale secondary ionization MS (SIMS) and time-of-flight SIMS,may achieve high resolution (100 nm-1 μm) [194].These techniques provide high spatial resolution and outstanding sensitivity,making them effective tools for single cell imaging and intracellular studies[195,196].
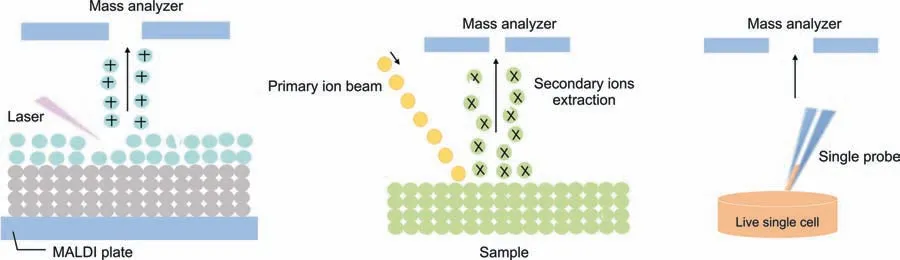
Fig.3. Examples of single cell metabolomics techniques.(A) matrix-assisted laser desorption-ionization mass spectrometry (MALDI MS),(B) secondary ionization mass spectrometry (SIMS),and (C) single probe mass spectrometry.
Since SIMS-based approaches are generally more energetic,they often produce a high number of fragments,which makes data processing of most biomolecules challenging.The main laser-based technique for single cell research is matrix-assisted laser desorption-ionization (MALDI) MS,which relies on interactions between the laser beam and organic matrix molecules to start analyte desorption and ionization.This method offers great measurement sensitivity and throughput while having exceptional salt tolerance [197,198].Additionally,when used in conjunction with a flash-freezing sample preparation technique,MALDI MS may deliver high-fidelity findings that accurately reflect the native distribution of cellular analytes [199].Despite their advanced equipment and wide variety of applications,SIMS and MALDI based techniques require rigorous sample preparation due to vacuum working conditions,which may alter the chemical composition of the cells compared to those in the original biological environment.Contrary to vacuum-based methods,ambient sampling techniques may successfully preserve many the cellular microenvironment while sampling in situ.For metabolites with a high turnover rate,this distinctive feature becomes increasingly advantageous [187].Numerous creative approaches have been created due to the flexibility of the work environment.These procedures typically involve distinct sampling and ionization phases,with nanoelectrospray ionization serving as the primary basis for ionization.
Monosaccharides,disaccharides,and trisaccharides are among the cellular metabolites fromA.cepacells discovered and identified using laser-ablation electrospray mass spectrometry(LAESI-MS)by Shrestha et al.[200].There are several designs for probe-based approaches.For instance,Pan et al.[193] and Liu et al.[201]exploited a microscale liquid connection between two fused silica capillaries to detect lipids and amino acids from single cells using nano-desorption electrospray ionization.In addition,other tools such as single probes and T probes are used to detect intracellular metabolites as well as drug compounds from the natural microenvironment of single cells.Microfluidic-based technologies provide a more thorough inspection of microscale devices by using microfabrication techniques.To identify diverse cellular lipids and better distinguish variations in cellular metabolic profiles across cell types,a flow-assisted cellular classification system has been created [202].
9.2.Single-cell metabolomics for metabolic analysis of IS
Single-cell measurements advance our understanding of the function and microstructure of highly complex biological systems[203].Brain cells exhibit significant heterogeneity,including neuronal,glial cells,and epithelial cells of cerebral blood vessels,even cells that are close to each other may exhibit significant differences in synaptic connections and metabolic product profiles.The study of cell heterogeneity is of great significance for elucidating neurochemical pathways,to better understand relevant mechanisms,and further improving the diagnosis and treatment of brain diseases [204].
Single-cell metabolomics with mass spectrometry enables a large variety of metabolites to be simultaneously detected from individual cells,without any preselection or labelling,to map phenotypes on the single cell level.Although the field is relatively young,it is steadily progressing with an increasing number of active research groups,techniques for cell sampling and ionization,tools for data analysis,and applications to answer important biomedical and environmental questions.The research strategies for single-cell metabolomics include cell handling,sampling,detection,quantification,and data analysis[205].For example,the strategies for processing and sampling individual neuronal metabolites in the single-cell metabolomics of IS are as follows.Firstly,primary neuronal cells were obtained from neonatal rats using a culture method based on the previous description[206].Secondly,neuronal cells induced by oxygen/glucose deprivation and reoxygenation(OGD/R)were used to simulate the pathological process of cerebral ischemia/reperfusion.Thirdly,optical imaging,sample preparation,MALDI matrix application,and MS analysis are performed sequentially.Fourthly,conduct data analysis and statistics.
10.Conclusion and prospective
IS is a multi-faceted,complex disease that causes many deaths and cases of severe impairment worldwide.Current diseasespecific diagnostics and treatments are urgently needed to facilitate the development of more precise and personalized care.
In systems biology research,metabolomics is a potent tool that offers fresh perspectives on the pathological state of disease.Measuring the metabolites in an organism is part of the developing subject of metabolomics,which has enormous potential in the field of IS,whether for disease early detection or for tailored medication.Currently,metabolomics has provided a novel approach for the diagnosis,prediction and prognosis of cancer,cardiovascular diseases,neurological disorders diseases.Studying abnormal metabolic changes in IS and finding the potential metabolic biomarkers of IS provides a deeper understanding of the IS pathogenesis.A clinical biomarker has not yet been found,despite the vast number of IS-related metabolites have so far been found.Diet and comorbidities,which have a large impact on metabolomic profiles and biomarker discovery,have also been confounding factors in studies.Due to variations in the experimental setup and the methods used for metabolic analysis,the results are occasionally conflicting.Thus,the results are far from real-world practice.
CHM is widely used in China and Southeast Asia,and there are a variety of herbal medicines used to treat diseases in Europe,North America and Australia.Many compounds have been isolated from CHM,and most of these compounds have not yet been used in pharmacological studies aimed at the development of new drugs.The discovery of lead compounds from CHM faces several challenges.In addition,due to the lack of scientific research methods,the true value of CHM has not been fully recognized globally.Fortunately,metabolomics has become a hot topic in the world of life sciences research and is also extensively employed in research on CHM as the post-genome age approach.Metabolomics has been developed in recent years for innovative drug discovery and provides powerful tools to understand the nature and function of herbal compound formulations.Its characteristics are in concert with the holistic of CHM use,indicating that it is seen to have the potential to improve the study of CHM.The introduction of the concept of metabolomics,which enables the study of living systems from a holistic perspective,has opened a unique and novel opportunity to investigate CHM.The use of metabolomics strategies in CHM has started to offer fresh perspectives into the essence and molecular underpinnings of the system of CHM.
The heterogeneity of IS is highly influenced by the complexity of its etiology and pathophysiology.IS remains a challenging and deadly disease,in part due to its heterogeneous nature,despite the ongoing development of novel therapies and treatments.Thus,we need to conduct studies that look at the heterogeneity of IS tissues to better understand the pathophysiology of IS and create more effective treatments.Because one of the hallmarks of IS is its metabolic changes,metabolomics has become a promising research direction.Particularly,the development of spatial metabolomics has given the opportunity to identify molecular localizations in addition to their relative abundances.In this way,small-molecule alterations may be directly correlated with anatomical features.This may lead to the invention of personalized medicine and faster diagnostic methods,as well as further understanding of heterogeneous diseases.
As the most fundamental structural and functional units found in biology,cells may be affected by a variety of factors that can alter their proliferation,differentiation,and metabolism,leading to a wide range of heterogeneity between each individual cell,even within the same organism.Despite being genetically identical,the composition and concentration of chemical substances found in two homologous cells can differ.Population cell analysis provides only averaged results,and information regarding cellular individuality is often lacking.Bulk analysis of many cells has more material available for analysis.Single-cell analysis is required to identify phenotypic heterogeneity among individual cells and find subpopulations of seemingly similar cells to decipher the origin of drug resistance,differentiation,and disease progression.However,there are many further requirements for single cell analysis to be truly useful in systems biology and medical diagnosis.Challenges in single-cell analysis include the low picoliter volume of material available for analysis,the rapid shift,properties,and concentrations,which range from nanomolar to millimolar[207].Despite the challenges,single cell analysis can be successfully achieved through the development of a range of technologies.Studying cell morphology and composition at the single cell level provides accurate information about the specific microenvironment in a single cell and applications to answer important biomedical questions.
Metabolomics research is clearly moving towards single cell analysis.Due to its use as an effective tool for determining the precise mechanisms behind cellular and molecular activity,single cell analysis has attracted increasing interest from researchers working in a variety of biological domains.MS methods are highly sensitive and selective,enabling the analysis and measurement of substances in individual cells.The variety of MS methods available for single cell analysis has grown over the years of research.Since each method has advantages and disadvantages of its own,the variety of different approaches is advantageous for researchers working on single cell analysis.In addition,the study of cellular environments and individual cells is accompanied by higher technical requirements,thus requiring continuous improvement of MS systems,especially in terms of detection sensitivity and temporal and spatial resolution.As technologies develop,MS will become increasingly crucial in single cell analysis with greater spatial resolution and sensitivity,which will facilitate biological research.Single cell metabolomics is expected to be a powerful tool for early detection of IS in the future and provide valuable insights into the pathogenesis of IS and single cell metabolism.
CRediT author statement
Wentao Li: Writing-Original draft preparation;Chongyu Shao,Chang Li,andHuifen Zhou: Project administration;Li Yu and Jiehong Yang: Formal analysis;Haitong Wan: Supervision,Funding acquisition;Yu He: Conceptualization,Supervision,Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 81873226,and 81874366),and the Zhejiang Provincial Science and Technology Innovation Leading Talent Project of “Ten Thousand Talents Plan”(2019).
 Journal of Pharmaceutical Analysis2023年9期
Journal of Pharmaceutical Analysis2023年9期
- Journal of Pharmaceutical Analysis的其它文章
- CLDN18.2-targeted molecular imaging and precision therapy of gastrointestinal tumors
- Applications and safety of gold nanoparticles as therapeutic devices in clinical trials
- 1,8-cineole ameliorates colon injury by downregulating macrophage M1 polarization via inhibiting the HSP90-NLRP3-SGT1 complex
- Ginsenoside Rk2,a dehydroprotopanaxadiol saponin,alleviates alcoholic liver disease via regulating NLRP3 and NLRP6 inflammasome signaling pathways in mice
- Quantification of soluble epoxide hydrolase inhibitors in experimental and clinical samples using the nanobody-based ELISA
- Gut microbiota-based pharmacokinetic-pharmacodynamic study and molecular mechanism of specnuezhenide in the treatment of colorectal cancer targeting carboxylesterase
