CLDN18.2-targeted molecular imaging and precision therapy of gastrointestinal tumors
Claudin-18.2 (CLDN18.2) is a tight junction protein that is typically expressed only in normal gastric mucosal cells.When malignancy occurs,cell polarity is lost,intercellular adhesion structures are destroyed,and CLDN18.2 is therefore exposed to the surface of tumor cells [1].CLDN18.2 is specifically expressed in approximately 40% of human epidermal growth factor receptor 2 (HER2)-negative gastrointestinal cancers,making it a promising emerging therapeutic target.As recently reported,zolbetuximab,an CLDN18.2 targeting antibody,has shown amazing efficacy in patients with CLDN18.2-positive and HER2-negative gastric cancers[2].In clinical practice,the assessment of CLDN18.2 expression status is crucial to the determination of treatment regimen for patients.Molecular imaging can be used as a non-invasive test for diagnosis,staging,and efficacy monitoring.The results of molecular imaging of CLDN18.2 have been published continuously,and the development of CLDN18.2 molecular probe is of great significance for the development of tumor targeted therapy (Fig.1).
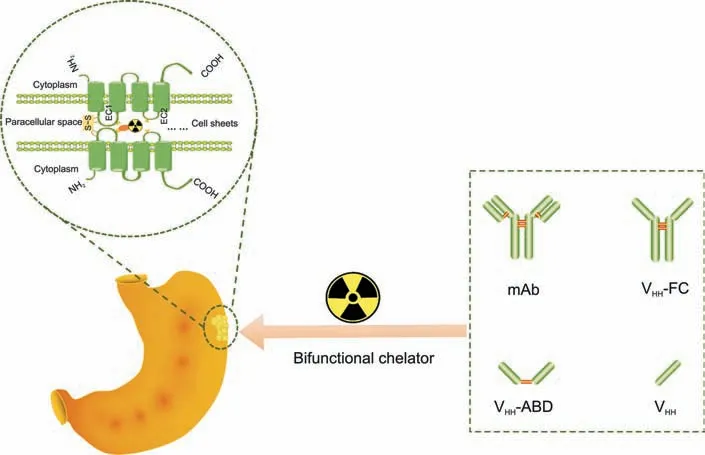
Fig.1. Construction of claudin 18.2(CLDN18.2)-targeted functional molecular probes.S-S:disulfide bonds;EC:extracellular loop;mAb:monoclonal antibody;VHH:variable domain of heavy-chain antibody;Fc: fragment crytallizable;ABD: albumin-binding domain.
1.The significance of the detection of CLDN18.2 expression status
In recent years,monoclonal antibodies,antibody-drug conjugates (ADCs),and chimeric antigen receptor-T therapies targeting CLDN18.2 have been used in clinical research.Both the SPOTLIGHT and GLOW studies of CLDN18.2 targeting antibody have achieved positive results,suggesting that zolbetuximab combined with chemotherapy in the treatment of gastric cancer with CLDN18.2 has a significant survival benefit advantage compared with patients with CLDN18.2 high expression of gastric cancer receiving chemotherapy alone.In addition,some early studies have evaluated other monoclonal antibodies and ADC drugs of this target,which also show good application prospects.FAST studies have shown that chemotherapy plus zolbetuximab improves progression-free survival and overall survival in patients with high CLDN18.2 expression compared with patients with lower CLDN18.2 expression.Therefore,the detection of CLDN18.2 expression status is a prerequisite for selecting patients with potential benefits of CLDN18.2-targeted therapy,but the accurate determination of its expression level is still one of the many challenges.Different detection methods lead to different cut-off values of protein expression,even if it is the same specimen of the same patient,and the detection results of different detection methods are also different,which brings great difficulties to the clinic.Due to the high heterogeneity of gastric cancer,immunohistochemistry is an invasive ex vivo detection method which cannot dynamically reflect the CLDN18.2 expression status of all lesions in patients in real time.
2.Molecular imaging for functional non-invasive detections of lesions
Nuclear medicine molecular imaging probe uses the radioactive signal emitted by the radioactive tracer to detect the expression and distribution of the target protein in the lesion,which can evaluate the efficacy and prognosis of targeted therapy,so as to guide the accurate diagnosis and treatment of tumors.Positron emission tomography (PET) imaging based on CLDN18.2-based functional molecular probes can obtain CLDN18.2 expression information of systemic lesions in tumor patients and tumor response to CLDN18.2-targeted drugs.In addition,the development of a functional molecular probe for CLDN18.2 may also be used in the study of adverse drug reactions in the future,and studies have shown that patients with previous gastrectomy are less likely to vomit when treated with zolbetuximab than patients without previous gastrectomy [2].This phenomenon may be due to CLDN18.2 monoclonal antibody non-specifically recognizing CLDN18.2 epitopes on gastric mucosal cells,resulting in damage to the gastric mucosa.The uptake of CLDN18.2 monoclonal antibody in tumors and gastric mucosa was visualized by the functional molecular probe of CLDN18.2,so as to provide imaging reference for the study of its toxic effects.Our group initially used124I-labeled murine monoclonal antibody 5C9 for preclinical studies,and the results showed that the probe could locate and guide surgery for CLDN18.2-positive tumors.More than that,as proof of the diagnosis and guided surgery concept,a near-infrared fluorescent II imaging probe FD1080-5C9 was also constructed [3].Both tracers provide a utility molecular imaging strategy for functional imaging and surgery guidance in cancers and further clinical validation is needed.Subsequently,a phase 0 clinical trial of124I-labeled humanized antibody 18B10 (10L) was carried out and the result showed that124I-18B10 (10L) could reflect CLDN18.2 overexpressed tumor lesions through PET functional imaging[4].During the imaging process,patients need to continue to take iodine to block the physiological uptake of iodine by the thyroid gland with a high degree of compliance.[89Zr]Zr-DFO-TST001 probe for the initial detection of CLDN18.2 expression level in gastrointestinal tumors was based on the renewed Good Manufacturing Practice (GMP) grade CLDN18.2 antibody TST001.It provides a feasible screening and efficacy evaluation of patients targeted for CLDN18.2 therapy [5].
3.The further of CLDN18.2 and other immune-therapy using functional molecular imaging
As may be known,probes are “soul” of the functional imaging.Even GMP grade TST001 radio-probe has high non-specific uptake of the liver and spleen,and it is believed that the fragment crytallizable(Fc)segment of the whole antibody binds to the Fc receptor of the liver,and the antibody can be engineered in the future to reduce its high background uptake in the liver.Also,smaller size,such as CLDN18.2 targeting probes which are based on Fab,nanobody,affibody,and peptides,may be developed.Smaller molecules have stronger tumor tissue penetration and faster blood clearance rates.The construction principle of CLDN18.2 functional molecular probe is:while retaining tumor specificity and affinity,minimizing the uptake of non-target organs and tissues,improving the contrast of imaging,and laying the foundation for the research and development of CLDN18.2-targeted radiopharmaceuticals and the integration of precision diagnosis and treatment of tumors.
Equipment is essential.In addition to the research and development of new nuclear molecular probes,the rapid development of nuclear medicine molecular imaging in disease diagnosis and treatment is inseparable from the upgrading and replacement of imaging equipment.PET imaging measurements are metabolically connected and therefore provide a quantifiable approach to pathophysiology.However,currently clinically widely available static PET acquisition devices produce only a single cumulative image per measurement.Traditional PET imaging focuses only on qualitative rather than quantitative assessment.After a patient is given radiopharmaceuticals intravenously,they usually need to wait some time before image acquisition.This uptake period is for radiopharmaceuticals to accumulate in the organ of interest and,in some cases,to wash the tracer out of the non-target organ.However,the distribution of radiopharmaceuticals is a dynamic process,with great differences between tumors and normal organs,as well as between patients.Dynamic PET acquisition is often limited to a single bed,limiting the coverage of the scanner's axial range.Benefits from the continued development of industry technology,a new framework for clinical dynamic whole body (DWB)PET imaging has emerged,where earlier PET systems needed to extend the scan duration to obtain sufficient count statistics,while DWB-PET systems can achieve the same quality in a fairly short duration as a time-off detector system.The generation of different kinetic data at the individual voxel level in dynamic images enables the generation of parametric images by kinetic modeling.
However,the application of CLDN18.2 functional molecular probe as a radiopharmaceutical CLDN18.2 in clinical practice also faces numerous challenges.The safe application of radiopharmaceuticals involves medical cyclotrons,GMP-compliant radiopharmaceutical manufacturing facilities,and dedicated treatment sites that meet relevant radiation safety standards.It is therefore critical to establish reliable distribution networks that can ensure the safe and timely delivery of these medicines.In contrast to conventional cancer treatment drugs,production cycles and logistics (delivery,application,and waste management)times of radiopharmaceutical must be adjusted to the half-life of radionuclides.
Clinical demands are ultimate goal: from molecular imaging to targeting therapy.Radionuclide drug conjugates (RDC) is similar to ADC in that they use antibody/Fab or small molecule (including affibody or peptides) mediated location to precisely destroy the lesion to avoiding the potential hazards of systemic exposure.The difference is that the RDC payload is a radionuclide (e.g.,α2+and β-radionuclide decay),which can be used for both diagnostic and therapeutic functions.In terms of ligand selection,targeted molecules of different molecular sizes can be tried,and ligands with better parameters can be selected for molecular probe construction based on tumor targeting and pharmacokinetic characteristics.Based on the high tumor specificity and affinity of CLDN18.2-positive molecular probes targeting CLDN18.2 in CLDN18.2-positive tumors,radionuclide therapy for gastric cancer can be carried out by therapeutic nuclide177Lu.
In summary,CLDN18.2-targeted drugs have shown significant efficacy in major clinical studies.With the dynamic imaging capability of whole-body PET imaging equipment,CLDN18.2 functional molecular probe is expected to accurately detect CLDN18.2-positive lesions in vivo and non-invasively,and has application prospects in efficacy evaluation,prognosis,treatment of multi-target co-expression population,and combination immunotherapy.
 Journal of Pharmaceutical Analysis2023年9期
Journal of Pharmaceutical Analysis2023年9期
- Journal of Pharmaceutical Analysis的其它文章
- Comment on: “Development of a CLDN18.2-targeting immuno-PET probe for non-invasive imaging in gastrointestinal tumors”
- JOURNAL OF PHARMACEUTICAL ANALYSIS BEST PAPERS 2022
- Targeted bile acids metabolomics in cholesterol gallbladder polyps and gallstones: From analytical method development towards application to clinical samples
- Characterization of natural peptides in Pheretima by integrating proteogenomics and label-free peptidomics
- Eight Zhes Decoction ameliorates the lipid dysfunction of nonalcoholic fatty liver disease using integrated lipidomics,network pharmacology and pharmacokinetics
- Rapid metabolic fingerprinting with the aid of chemometric models to identify authenticity of natural medicines: Turmeric, Ocimum,and Withania somnifera study
