Stages of care for patients with chronic hepatitis C at a hospital in southern Brazil
Manoela Badinelli Vaucher,Camila Ubirajara Silva,Ivana Rosangela Santos Varella,Arthur Yu-Shin Kim,Dimas Alexandre Kliemann
Abstract
Key Words: Cascade of care; Elimination; Hepatitis C virus; Sustained virologic response
INTRODUCTION
The World Health Organization (WHO) set the goal of diagnosing 90% of cases of viral hepatitis and treating 80% of diagnosed cases with the aim of reducing the incidence by 90% and mortality attributable to hepatitis by 65% by 2030[1].The hepatitis C virus (HCV) care cascade represents the care that patients receive in the respective health services and consequently illustrates the basic indicators of the WHO targets[2].In the first stage are people with HCV infection, in the second are the patients aware of the diagnosis of HCV, in the third, those who underwent treatment, and in the fourth stage, those who achieved cure with viral suppression from 12 wk to 24 wk after the end of treatment[2-4].Other stages can be added, such as retention in care and after cure monitoring, but it is difficult to standardize the criteria, hindering the possibility of later comparison[3].
The elaboration of the treatment cascade facilitates the identification of barriers and groups of risks which we must work with[3,5].The correlation between sociodemographic variables and results between stages is an important tool for the analysis of the HCV cascade of care[6].Mental health problems, change in follow-up location, and restriction of information about the disease were detected as causes of failures in the stages of chronic HCV treatment in a study by health professionals[7].Therefore, the construction of local cascades is necessary for the understanding of gaps in current practices and the elaboration of changes[8].
Brazil joined the Hepatitis C Elimination Plan in 2017[9].Since then, the country has been developing and implementing guidelines of care and prevention to guarantee a greater access to diagnostic tests and the treatment for all patients with chronic HCV infection and acute hepatitis C and children with infection by HCV[9].Retreatment is also possible, especially after the availability of new direct-acting antivirals (DAAs)[9].Despite the measures instituted in Brazil, there are no data available for the elaboration of a local cascade of care, which determines the need to qualify national databases to monitor the hepatitis elimination policy[10].
The South region of Brazil is responsible for the highest detection rate of confirmed HCV infection in the country and also for the highest mortality rate, with higher rates than national data[11].Within this region, the city of Porto Alegre, in 2020 was the second capital with the highest HCV detection rate, and in 2021 the first, even higher than the national rate[11].The Hospital Nossa Senhora da Conceição (HNSC) in Porto Alegre is a tertiary hospital that has an infectology service which is a reference in treatment services for patients with human immunodeficiency virus (HIV) infection and viral hepatitis[12].The objective of this study was to define the continuity of care or treatment cascade for patients with chronic HCV infection at the HNSC and to define sociodemographic variables that influence follow-up between each step of the cascade.
MATERIALS AND METHODS
This referred retrospective cohort study included patients diagnosed with chronic HCV infection between 2015 and 2020 at the HNSC.All hospitalized patients and outpatients above 16 years of age notified by the HNSC epidemiological center for viral hepatitis with positive anti-HCV or HCV-polymerase chain reaction (PCR) results detectable during this period were analyzed.Patients under 16 years of age were excluded, as they would be followed up at a pediatric hospital attached to the HNSC.Patients who died, who were not connected with the HNSC, or who were not located in the electronic medical records of the hospitals were excluded.
The Information System for Notifiable Diseases (SINAM) for Viral Hepatitis, electronic medical records from the HNSC, computerized Laboratory Environment Management System (GAL), and Medicine Administration System (AME) provided data such as age, gender, race, education level, history of pregnancy and drug use, profession, city of origin, institutionalization situation, and co-infection with HIV and hepatitis B virus (HBV).Specific data of the disease under study were also obtained, such as date of diagnosis of HCV infection (anti-HCV test), history of consultation with a specific outpatient clinic for the treatment of hepatitis, initial quantitative HCV-PCR and final quantitative HCV-PCR (after the 12thwk since the end of treatment), genotyping, history of cirrhosis or hepatocellular carcinoma, specific regimen, and date of treatment prescribed for HCV.Data were collected in 2022 and all information up to that period was considered.
Regarding the HCV cascade of care, five exposure columns were built according to the stages of HCV care.The first stage covers all people diagnosed with chronic HCV infection, that is, patients with positive anti-HCV or detectable quantitative HCV-PCR in the analyzed period.The second includes patients who underwent some quantitative PCR collection, and the third includes patients who underwent consultation at a specialized outpatient clinic for monitoring hepatitis C.The fourth step integrates all who underwent treatment with specific antivirals for chronic HCV infection according to the established protocol.The fifth step ends with all patients who achieved a sustained virological response, that is, those who obtained an undetectable quantitative HCV-PCR test after the 12thwk since the end of treatment.Patients who did not collect this exam after treatment were not included in the fifth step, being presented in the cascade as “missing” and subsequently analyzed within the group of those who were not cured.The percentages were then calculated using the “n” of the first step and the “n” of the previous step as denominator, thus obtaining two percentages for analysis, being represented using a series of unidirectional columns.
Using the IBM SPSS version 25 program, Poisson regression with robust simple variance was performed to estimate the incidence ratio (IR) at a 95% confidence interval (95%CI) for the variables of gender, age group, race, education, city of residence, place of follow-up, presence of cirrhosis, institutionalization, year of diagnosis, and co-infection with HIV/HBV related to each step of the cascade: PCR-HCV collection, bond, treatment, and sustained virologic response (SVR).All variables that had a value ofP< 0.20 in the simple analyzes were included in the multivariable model, and in this model only variables withP< 0.05 were considered significant.
Two more cascades were also built, discriminating between patients who underwent treatment at the HNSC and those who underwent treatment at other locations after the diagnosis.The comparison of the sociodemographic characteristics between the groups, HNSC and external, was performed using Pearson’s chi-square test and results withP< 0.05 were considered significant.The study was approved by the research ethics committee of the Hospitalar Conceição Group, under number 51462421.8.0000.5530, and informed consent was waived, subject to the patient’s commitment to confidentiality.
RESULTS
By searching the HNSC viral hepatitis notification database between 2015 and 2020 at the HNSC, 2498 patients were identified.A total of 487 patients who died, with decompensated cirrhosis, hepatocellular carcinoma, renal failure, and sepsis as the main etiologies reported, were excluded.Another 1232 patients were also excluded because they had a diagnosis of other viral hepatitis, an HCV diagnosis prior to 2015, a false anti-HCV test result, no attachment to the HNSC, or being younger than 16 years old.A total of 779 patients diagnosed with HCV infection during the analyzed period were included, but of these 70 had spontaneous cure and, as they did not require treatment, were disregarded for further analyses.
For the HCV cascade of care, 709 patients were analyzed, showing a sociodemographic profile of being predominantly male (54.3%), white (76.6%), and from Porto Alegre (44.7%), and just having had completed elementary education (67.4%).The mean age of the patients was 53 years.Only 22 patients had a history of pregnancy, 13% co-infection with the HIV, 10.3% co-infection with HBV, 17.8% had a history of cirrhosis, and only 2% a diagnosis of hepatocellular carcinoma.It was identified that 24.9% had a history of drug use and 33 patients of institutionalization.Regarding the genotype, 44.5% of the patients did not have an identified genotype and, among the available genotypes, genotype 1 was the most prevalent (60%), followed by genotype 3 (34.6%) and finally genotype 2 (5.3%).
Regarding the total of 709 patients, 534 (75.3%) collected quantitative PCR, 461 (65%) consulted at a specialized clinic, 344 (48.5%) underwent treatment for HCV infection, and 204 (28.7%) reached SVR.When considering the previous column as the denominator, the percentages would be, respectively, 75% with RT-PCR collection, 86% consulted, 75% treated, and 59% with SVR confirmed by post-treatment examination.Both percentages are represented in Figure 1.The results of the simple and multivariate analyzes of the variables in relation to each step of the cascade are described in Tables 1 and 2, respectively.
Patients with incomplete primary education had lower rates of HCV-PCR collections after the diagnosis of HCV infection in the simple analysis (P< 0.20), as well as patients who underwent diagnosis in 2018; however, these variables were not significant in multivariate analysis.Patients living in the metropolitan area and countryside regions, who consulted at an outpatient clinic at the HNSC, co-infected with HIV and HBV, with a history of cirrhosis, and who were diagnosed in 2016 and in 2020, had higher collections of PCR-HCV in the first analysis (P< 0.20).In the second analysis, patients from the metropolitan area and countryside regions, patients from the HNSC, and those diagnosed in 2020 were more likely to obtain HCV-PCR (P< 0.05).
Despite linkage being the subsequent step in the cascade, there were 22 patients who consulted and did not collect any HCV-PCR test.The variables that showed a difference in favor of creating a link to a specialized outpatient clinic were female gender, living outside the city of Porto Alegre, having cirrhosis, and consulting at the HNSC.The diagnoses in 2018 and in 2019, as well as having only elementary school, were factors contrary to consulting with specialists.In the multivariate analysis, only being from the countryside or metropolitan area and consulting the HNSC were significant (P< 0.05).Through the records in the GAL and AME, we identified that 121 patients were followed up in other places after the diagnosis in the hospital.
Being female, having a history of cirrhosis, living outside the city of Porto Alegre, and consulting at the HNSC were protective factors in the simple analysis for undertaking the treatment.As risk factors for not undergoing HCV treatment, co-infection with HIV, being institutionalized, being non-white, having had only completed elementary school, and having been diagnosed in 2019 were identified.Consulting at the HNSC and being from the countryside remained significant protective factors (P< 0.05).The mean time between diagnosis and initiation of treatment was approximately 2 years.The DAAs most used in treatment were sofosbuvir and ledispasvir (26.0%), sofosbuvir and velpatasvir (22.0%), sofosbuvir and daclatasvir (17.0%), and sofosbuvir, daclatasvir, and ribavirin (15.0%).Only eight patients (1.1%) underwent more than one treatment.Among the 22 pregnant women in the study, only nine underwent treatment, but timing of treatment before or after pregnancy was unknown.No history of vertical transmission was found in these cases, since the GAL system evaluated the newborns of the respective pregnant women.
Out of all 344 patients who underwent treatment, only 204 reached SVR; however, 136 patients had no record in the GAL of collection of HCV-PCR control after the 12thwk since the end of treatment.Only four had SVR failure, resulting in a documented DAA response rate of 98%.The risk factors in the simple analysis for not achieving documented SVR were being of non-white race and having only elementary education.As variables favorable to SVR, living in the metropolitan area, in the countryside, and consultation at the HNSC were found, in addition to having been diagnosed with HCV infection in 2015 and in 2016.In the multivariate analysis, only the places of residence (countryside and metropolitan area) and consultation at the HNSC were significant (P< 0.05).
Because significance was found in all stages of HCV care regarding the place of follow-up, we constructed two separate cascades of care: One for patients who remained under follow-up at the HNSC and another for patients who chose to be assisted in other places.As previously mentioned, 340 patients remained at the HNSC, of whom 70% underwent treatment and 49% achieved SVR, 1% showed failure, and 20% did not collect a PCR test after treatment.Among 121 outpatients, 88% underwent treatment and 31% had SVR, but 55% did not perform a control PCR collection and 2% had SVR failure (Figure 2).As variables favorable to SVR, living in the metropolitan area, in the countryside, and consultation at the HNSC were found, in addition to having been diagnosed with HCV infection in 2015 and in 2016.In the multivariate analysis, only the places of residence (countryside and metropolitan area) and consultation at the HNSC were significant (P< 0.05) (Table 3).
DISCUSSION
We conducted this study in our local hospital to identify the HCV care cascade and relevant characteristics for progressing from diagnosis to successful cure, to provide representative data from Rio Grande do Sul and mainly from the city of Porto Alegre[11,13].The highest prevalence of HCV infection was found in patients who are male, white, and aged over 40 years, consistent with both data from the state of Rio Grande do Sul and national statistics[11,14].Also, higher prevalence of HCV was observed in patients with only elementary education[11].Regarding co-infection with HIV, we present data that are very close to those of the South region in 2021 (10.1%)[11].Groups most vulnerable toinfection by the HCV, such as people living with HIV, institutionalized people, and drug users, were considerably represented, corroborating the importance of focusing on testing and preventive actions for these particular subpopulations[9,14].Genotypes 1 and 3 were the most prevalent, as expected according to national and local data[14].

Table 1 Analysis of variables with absolute number and relative percentage (%) and Poisson regression with robust simple variance of each variable in relation to steps of cascade of care of patients diagnosed with hepatitis C virus infection at the Hospital Nossa Senhora da Conceição between 2015 and 2020
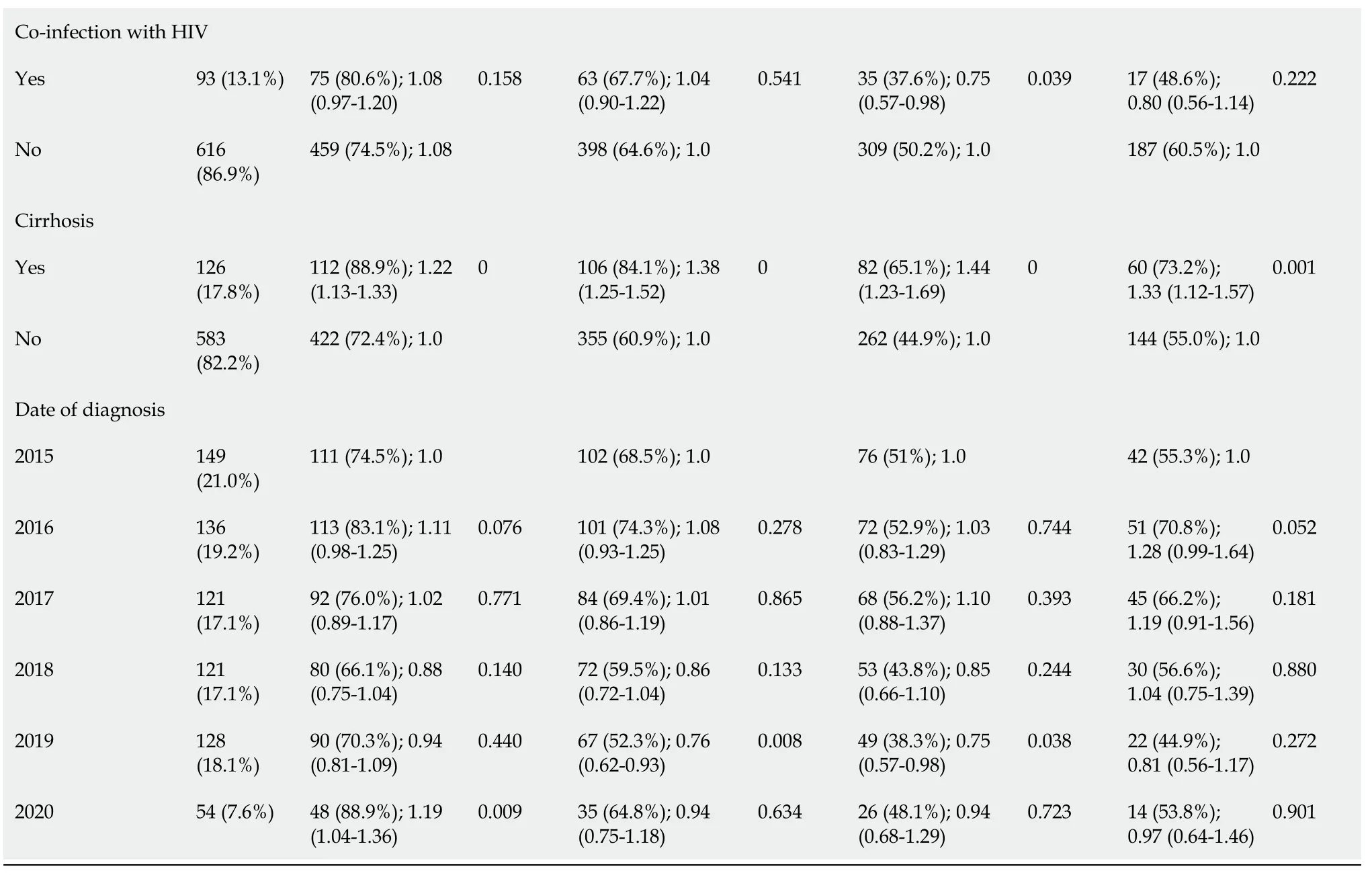
P value < 0.20 was considered significant.HNSC: Hospital Nossa Senhora da Conceição; HCV: Hepatitis C virus; HBV: Hepatitis B virus; SVR: Sustained virologic response.

Figure 1 Cascade of care of hepatitis C virus infected patients diagnosed at the Hospital Nossa Senhora da Conceição between 2015 and 2020.HCV: Hepatitis C virus; SVR: Sustained virologic response.
According to the WHO, the second step of the HCV cascade of care would be represented by patients aware of their diagnosis, with a target of 90%[1].However, in this study, we did not carry out this estimate and included patients from the diagnosis of chronic HCV infection which we performed at the HNSC.Overall, the WHO target of patients on treatment (80%) was not reached.But when analyzing the cascade broken down by place of follow-up, patients being followed up outside the HNSC reached the goal.This can be explained by a possible data collection bias, where all patients with prescriptions for treatment in the AME system from other locations were linked to other services.In addition, HNSC patients may have even started treatment due to the lack of clinical conditions, such as neoplasms orserious comorbidities.Not having genotyping or HCV-PCR test within a year are also bureaucratic reasons that interfere with the delay in starting HCV therapy, which is unfortunate given the possibility of simplified protocols with pangenotypic regimens[15,16].

Table 2 Poisson regression analysis with multivariable robust variance of significant variables in relation to steps of cascade of care of patients diagnosed with hepatitis C virus infection at the Hospital Nossa Senhora da Conceição between 2015 and 2020
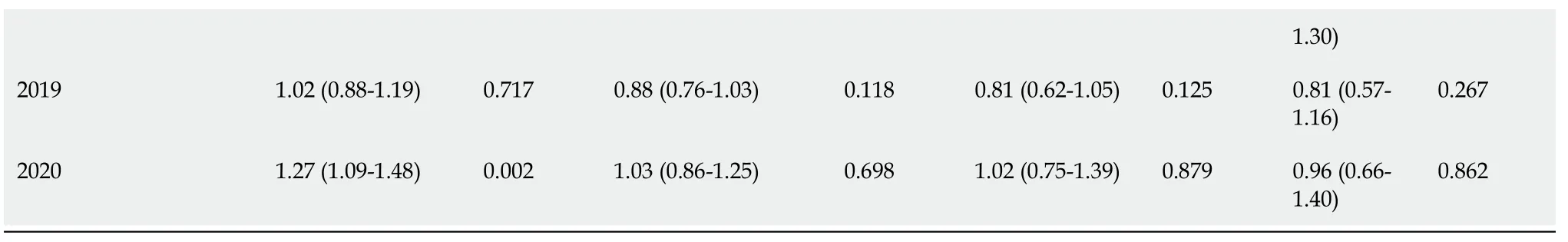
P value < 0.05 was considered significant.HCV: Hepatitis C virus; SVR: Sustained virologic response; HNSC: Hospital Nossa Senhora da Conceição.

Table 3 Comparison between patients with hepatitis C virus infection who underwent follow-up at the Hospital Nossa Senhora da Conceição and external ones using Pearson’s chi-square test
About 90% of patients with HCV infection are cured with the new DAAs, resulting in a markedly decreased risk of liver-related morbidity and mortality and also a drastic reduction of onward transmission[5].Despite the high response rate to DAAs, the SVR percentages are surprisingly low in this study, which represents the biggest “gap” among all the cascade steps.This is due to the large number of patients with missing data after treatment, making it impossible to confirm SVR.In addition to the collection of HCV-PCR control outside the Brazilian Unified Health System (SUS), another reason for the ignored data would be the loss to follow-up of the patients after the completion of the treatment, identifying the importance of implementing a strategy to enhance the rate of return of the patients after the exams[17].The coronavirus disease 2019 pandemic had an important impact on the follow-up of these patients after treatment[18].HNSC patients had a higher SVR and a lower number of ignored HCV-PCR tests, possibly due to less loss to follow-up.
RT-PCR collection

Figure 2 Cascade care of hepatitis C virus infected patients diagnosed between 2015 and 2020 at the Hospital Nossa Senhora da Conceição and who underwent follow-up at the Hospital Nossa Senhora da Conceição and elsewhere (external).HNSC: Hospital Nossa Senhora da Conceição; HCV: Hepatitis C virus; SVR: Sustained virologic response.
National data and data from the state of Rio Grande do Sul have shown a progressive decrease in reported cases of viral hepatitis with anti-HCV reagents and concomitant HCV-PCR reagents, which may demonstrate an increase in notifications of cases of serological cure, or even less access to confirmatory HCV-PCR tests[11,13].Patients who were connected to the hospital collected significantly more PCR-HCV, which may have been facilitated by the logistics of collecting the test inside the hospital under study, after the diagnosis was made.This can also be explained by the greater severity of the patients, as patients with comorbidities, such as HBV and HIV infections and cirrhosis, were shown to have greater access to the test.Living in the countryside and in the metropolitan area were also significant protective factors in this analysis for HCV-PCR collection, as most of these patients have consultations at the HNSC, possibly due to less access to confirmatory HCV-PCR tests outside the capital Porto Alegre[13].Patients ended up collecting more exams in 2020, demonstrating more accessibility to the exam that year or even greater concern for investigating the disease of patients throughout the pandemic.
Bond
Gaps occur at all stages of HCV care, with dropouts in care occurring before and after linking to specialized care[19].Consultation at a specialized outpatient clinic is recommended in the treatment lines established in the country; however, more recent guidelines describe the intention of training non-specialist physicians[10,14].HNSC patients may have found it easier to create a bond with an infectology or gastroenterology outpatient clinic because they were diagnosed in the same hospital.On the other hand, patients from the countryside and metropolitan area, who mostly have consultations at the HNSC, sought this service possibly due to a shortage or lack of specialized professionals in their cities of origin.
Treatment
Having hepatocellular carcinoma, decompensated cirrhosis, or other neoplasms interfere with the assessment of the patient’s profile and with the recommendation of treatment[14].Cirrhosis was shown to be a positive factor in the simple analysis, which is justified by the recent finding that having advanced cirrhosis was a necessary criterion for undertaking treatment[14].On the other hand, co-infection with HIV and institutionalized individuals proved to be risk factors for not undergoing treatment, and it should be noted that they are vulnerable groups[14].The non-white race in the simple analysis also proved to be a vulnerable group for not undergoing treatment, which reminds us of the need to consider race in the implementation of public policies[20].In the final analysis, the female sex obtained higher data in the performance of treatment; however, this data contradicts the results seen previously where women, mainly young people, tend to face barriers to engaging in any form of health care[21].Furthermore, HCV infection rates in women of childbearing potential have increased, making prenatal diagnosis a priority[22].Being connected to the HNSC was associated with a significantly higher rate of undergoing treatment, which may be related to the maintenance of followup at a specialized outpatient clinic for gastroenterology and infectology services.
SVR
Having a low level of education, only elementary school, proved to be an obstacle to collecting HCV-RNA and achieving SVR, indicating the importance of education for the perception of their health status.Counterintuitively, it is possible that having more knowledge about the natural history of the disease is associated with greater stigma of HCV infection, another barrier to be addressed in the continuous care of patients.The patients at the HNSC were mostly from the countryside and metropolitan area and had cirrhosis inferring the need and demand for specialized care.Despite these results, the training of primary care professionals is able to increase the rate of treatment of patients, with HCV cure results in the decentralization of care similar to specialty outpatient clinics[22].
CONCLUSION
Among the limitations of the study is our inability to determine the true rate of SVR due to the high number of missing follow-up HCV-PCR data.Furthermore, other missing data from the HNSC medical records and external sites, important for a thorough analysis of sociodemographic variables, are also a considerable limitation of the referred study.This study identified that the sociodemographic characteristics of patients diagnosed with HCV infection at the HNSC are similar to regional and national data.It was also possible to stratify relevant risk factors for patients failing to proceed along the treatment cascade, thereby elucidating potential targeted strategies to improve care.According to the results of the hepatitis treatment at the HNSC, the importance of specialized care was highlighted.To make hepatitis treatment more accessible to patients in the countryside and metropolitan area, teams need to be properly trained.Given that we are far from the goals defined by the WHO necessary for elimination of HCV as a public health problem, it is critical to strengthen the treatment lines and facilitate care for patients not only at the HNSC, but also throughout the South region.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
We would like to thank the Hospital Nossa Senhora do Conceição for being a supporter of the work.
FOOTNOTES
Author contributions: Vaucher MB analyzed the data and wrote the manuscript; Silva CU performed the research; Varella IRS contributed to data analysis and helped perform the research; Kim AYS contributed analytic tools and reviewed the manuscript; and Kliemann DA designed the research study and reviewed the manuscript.
Institutional review board statement: The study was reviewed and approved by the Research Ethics Committee of the Hospitalar Conceição Group (Approval No.51462421.8.0000.5530).
Informed consent statement: Informed consent was waived, subject to the patient’s commitment to confidentiality.
Conflict-of-interest statement: All the authors have no conflicts of interest to disclose.
Data sharing statement: Data can be acquired through the corresponding author.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: Brazil
ORCID number: Manoela Badinelli Vaucher 0000-0003-3876-5466; Dimas Alexandre Kliemann 0000-0003-4326-0024.
S-Editor: Chen YL
L-Editor: Wang TQ
P-Editor: Chen YL
 World Journal of Hepatology2023年8期
World Journal of Hepatology2023年8期
- World Journal of Hepatology的其它文章
- Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors
- Liver transplant in primary sclerosing cholangitis: Current trends and future directions
- Prognostic and diagnostic scoring models in acute alcohol-associated hepatitis: A review comparing the performance of different scoring systems
- Tenofovir alafenamide significantly increased serum lipid levels compared with entecavir therapy in chronic hepatitis B virus patients
- Emerging therapeutic options for non-alcoholic fatty liver disease: A systematic review
