Tenofovir alafenamide significantly increased serum lipid levels compared with entecavir therapy in chronic hepatitis B virus patients
Rui-Min Lai, Shan Lin, Miao-Miao Wang, Na Li, Jia-Hui Zhou, Xiao-Yu Lin, Tian-Bin Chen, Yue-Yong Zhu, Qi Zheng
Abstract
Key Words: Tenofovir alafenamide; Entecavir; Hepatitis B virus; Serum lipid; Metabolic factor
INTRODUCTION
Hepatitis B virus (HBV) infects approximately 240 million people worldwide, including approximately 86 million people in China[1].Chronic hepatitis B (CHB) may lead to decompensation of cirrhosis and hepatocellular carcinoma (HCC), which are the leading causes of mortality in patients with CHB[2].Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome (MetS), with a cumulative prevalence of 24% worldwide[3].In recent decades, the prevalence of NAFLD has significantly increased in China, leading to the coexistence of NAFLD and CHB.Dyslipidemia, which is characterized by high triglyceride (TG), high total cholesterol (TCHO), low high-density lipoprotein (HDL), and high low-density lipoprotein levels, is strongly associated with NAFLD and MetS[4].
Interferon and nucleoside analog therapies cannot completely eradicate HBV infection[5].Many patients require longterm anti-HBV therapy with potent oral drugs tenofovir alafenamide (TAF) and entecavir (ETV), which are recommended as first-line treatment in HBV clinical practice guidelines[6].TAF has also recently been incorporated into antiretroviral regimens for people with HIV, and we observed its impact on serum lipid levels in these individuals.A prospective cohort study showed that patients with HIV infection treated with a TAF-containing regimen had significantly worse blood lipid levels, especially those with higher LDL and TCHO[7].In a recent real-world study, switching from tenofovir disoproxil fumarate (TDF) to a TAF-containing regimen in HIV-infected patients resulted in a significant increase in serum lipid profiles[8].However, data on the effects of TAF on serum lipid levels in patients with HIV may be limited by the potentially confounding effects of concomitant antiretroviral HIV drugs.
The effect of ETV on serum lipid profiles has not yet been reported in postmarketing studies.A retrospective cohort study showed greater reductions in TCHO, LDL, and HDL levels in patients with CHB treated with TDF than in those treated with ETV[9].TAF is considered the successor of TDF; however, no studies have compared the effects of TAF and ETV on lipid profiles in HBV-treated patients.Meanwhile, there are limited data on the effects of TAF on metabolismrelated complications in real-world settings.Therefore, this retrospective cohort study aimed to characterize the effect of TAF on serum lipid levels and NAFLD risk in patients with CHB, and we compared the pretreatment and post-treatment serum lipid profile changes after initiation of either TAF or ETV anti-viral therapy.
MATERIALS AND METHODS
Eligibility/study subjects
This study included all patients with CHB older than 18 years who visited the outpatient department of the Hepatology Research Institute of the First Affiliated Hospital of Fujian Medical University between January 2020 and January 2021.Information such as patient demographics, treatment history, laboratory data, and comorbidities was extracted from the electronic medical record system.CHB was defined according to the Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2019) of China[10].Diagnosis of decompensated cirrhosis was confirmed by ultrasonography or imaging for inclusion in the study.Finally, the study included 336 participants (Figure 1) who were taking TAF or ETV and had a pretreatment serum lipid profile and repeated serum lipid assessment after initiating anti-viral therapy for 1 year.Exclusion criteria were as follows: (1) Less than 1 year of anti-viral therapy; (2) Use of other oral anti-viral drugs during the study period; (3) Use of lipid-lowering drugs; (4) Complicated with other liver disease or pregnancy; (5) Heavy alcohol intake (amounting to ethanol consumption of ≥ 40 g/day for males and ≥ 20 g/day for females).
Measurement of parameters /data collection
The collected clinical and demographic data included age, sex, body mass index (BMI), drinking and smoking habits, date of anti-viral treatment initiation, cirrhosis, and comorbidities [diabetes mellitus (DM), hypertension, and NAFLD].A fatty liver was identified using ultrasonography.Clinical laboratory information included HBV-DNA, hepatitis B surface antigen, aspartate aminotransferase, alanine aminotransferase, glomerular filtration rate, uric acid, creatinine, creatine kinase (CK), CK-MB isoenzyme, fasting serum lipid profiles (TCHO, TG, HDL, and LDL), and baseline fasting blood glucose levels.The parameters were measured by the clinical laboratory of the First Affiliated Hospital of Fujian Medical University, and data were collected before and 1 year after the initiation of anti-viral therapy.This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, China.
Statistical analysis
Statistical analyses were performed using SPSS 23.0.Normally distributed continuous variables were presented as mean ± standard deviation, which were further evaluated by Student’st-test for the different treatment groups.Categorical variables were described using frequencies and proportions, and Pearson’sχ2test was used to compare categorical variables.The pairedt-test and McNemar’s test were used to assess the differences between the levels before and after treatments in the same treatment group.We calculated the pretreatment and post-treatment differences in each lipid profile component in order to evaluate the impact of anti-viral therapy on lipid profile.
Propensity score-matched models were used to assess the effect of treatment type (TAFvsETV) on lipid profile component changes.All propensity score-matched models were adjusted for BMI, age, sex, fatty liver disease, cirrhosis, DM, hypertension, smoking, and drinking.We presented the average changes in the model coefficients.Finally, logistic regression analysis was used to estimate the odds ratio of the association between baseline factors and elevated TCHO levels.StatisticalPvalues less than 0.05 were considered significant.
RESULTS
Summary of baseline clinical and demographic data of TAF-treated and ETV-treated CHB patients
Overall, 336 CHB patients receiving anti-viral therapy (TAF,n= 214vsETV,n= 122) were included in the study.The mean age was 46.67 years, 75.60% were male, and 30.95% were cirrhotic at baseline.Patients were older in the ETV group (P= 0.001), but the two groups had a similar rate of NAFLD (TAF: 35.05%vsETV: 26.23%,P= 0.122) and BMI (TAF: 22.97vsETV: 23.78,P= 0.152).However, hypertension and cirrhosis were more prevalent in the ETV group (17.21%vs7.94%,P= 0.016 and 40.98%vs25.23%,P= 0.004, respectively).TAF and ETV had similar levels of hepatitis B surface antigen (3.07vs3.04,P= 0.787) and HBV-DNA (1.94vs1.86,P= 0.560) (Table 1) as well as no statistically significant differences in serum alanine aminotransferase and aspartate aminotransferase levels.
Comparison of serum lipid profiles before and after anti-viral therapy in TAF-treated vs ETV-treated CHB patients
TCHO, TG, LDL, and HDL values in the TAF-treated and ETV-treated individuals were comparable at baseline (prior to anti-viral medication).In the TAF-treated group, post-treatment serum lipoprotein levels were considerably higher than the pretreatment levels for TCHO (4.51 ± 0.93vs4.67 ± 0.90,P= 0.001) and TG (1.25 ± 0.67vs1.37 ± 0.81,P= 0.014), whereas HDL and LDL levels did not change in the TAF group over the duration of our study.Further, the TAF group showed significantly higher post-treatment TCHO levels compared with that in the ETV group (4.67 ± 0.90vs4.36 ± 1.05,P= 0.006).However, TAF treatment did not increase the incidence of NAFLD after 1 year of follow-up (Table 2).In the ETV cohort, there was no significant difference between the pretreatment and post-treatment serum lipoprotein levels.
TAF as an independent predictor of TCHO level change
Using propensity score-matched models for BMI, age, sex, smoking, drinking, and presence of comorbidities such as NAFLD, cirrhosis, DM, and hypertension, we assessed the impact of TAF compared to ETV on achieving an increase in the levels of the serum lipid profile (Table 3).Patients treated with TAF had a statistically significant increase in TCHO levels (P= 0.019), which was 5% higher than that in the baseline lipid profile.

Table 1 Summary of demographic and clinical characteristics at baseline of chronic hepatitis B patients on tenofovir alafenamide or entecavir therapy
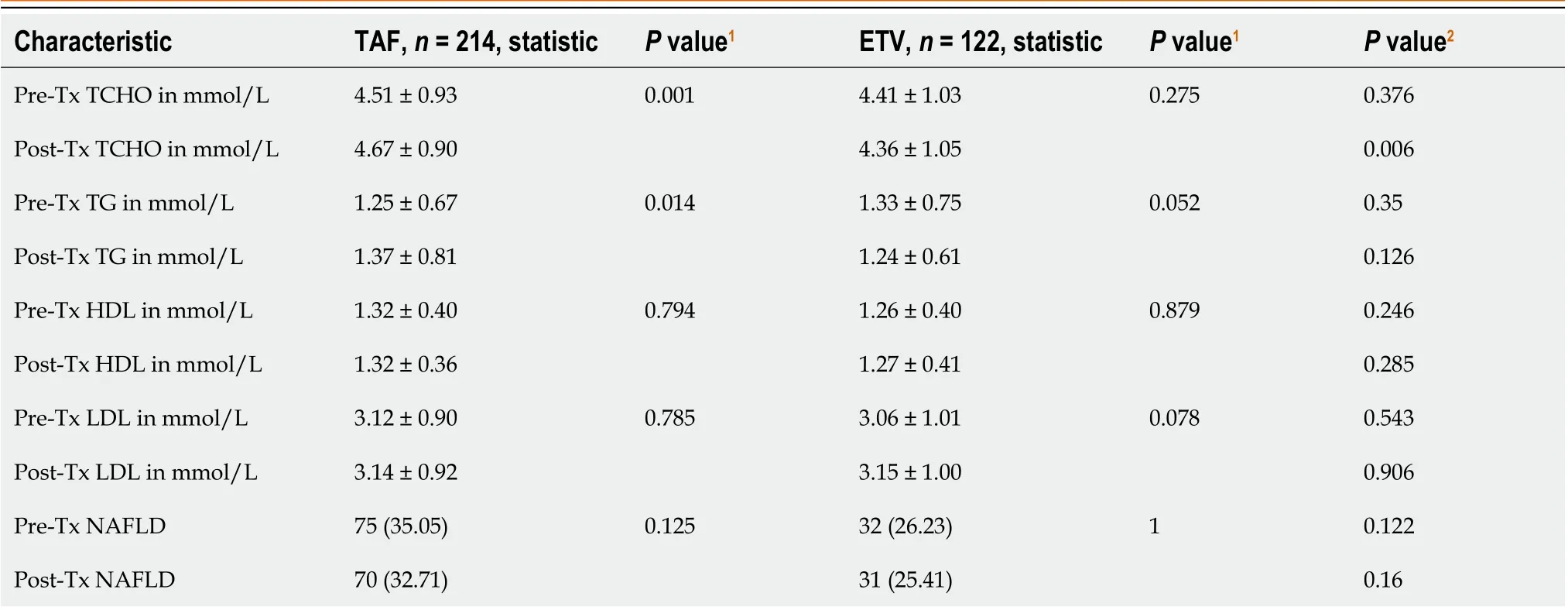
Table 2 Comparison of serum lipid profile before and after 1 year of anti-viral therapy either tenofovir alafenamide or entecavir

Table 3 Impact of tenofovir alafenamide on achieving a higher level of total cholesterol in chronic hepatitis B patients

Figure 1 Flowchart of study participants.CHB: Chronic hepatitis B; TAF: Tenofovir alafenamide; ETV: Entecavir; TCHO: Total cholesterol; HDL: High density lipoprotein; LDL: Low-density lipoprotein; TG: Triglycerides.
Risk factors were associated with elevated TCHO levels in CHB patients with 1-year TAF therapy
Logistic regression analysis was used to evaluate the risk factors associated with the worsening of TCHO levels (Figure 2).BMI, sex, hypertension, the baseline TCHO, and CK-MB levels were significantly associated with elevated TCHO levels.Furthermore, a nomogram incorporating statistically significant parameters in the logistic regression analysis was constructed, and the total points predicted the probability of elevated TCHO levels in individual patients.
DISCUSSION
In this real-life retrospective cohort study of 336 patients with CHB who received anti-viral therapy for 1 year, we compared fasting serum lipid profiles before and after initiation of either TAF or ETV treatment.In the TAF-treated cohort, we found a significant increase in the serum TCHO and TG levels compared with no difference in patients who received ETV.However, there were no concomitant significant changes in serum HDL or LDL levels in TAF-treated patients.Recent data have demonstrated that MetS increased the risk of progression of liver fibrosis independent of viral factors in patients with CHB[11,12].Elevated TCHO and TG levels as MetS risk factors were related to a high risk of cirrhotic events in patients with CHB; thus, the results of this investigation may affect the decision regarding anti-viral therapy in CHB patients with cirrhotic risk factors.
TAF has been used as first-line therapy for CHB and HIV infections.Dyslipidemia and metabolic disorders have been linked to the prolonged use of TAF to treat HIV infection.Previous studies found that the serum lipid profile increased in HIV patients switching from TDF to TAF therapy, and the effect of increased serum lipids on the risk of cardiovascular events cannot be ignored[13-15].Therefore, whether dyslipidemia due to TAF treatment in patients with CHB would lead to an increased risk of NAFLD is also a concern.Studies have reported that patients with CHB and NAFLD have better liver-related outcomes and overall mortality than those with CHB alone[16].It is increasingly recognized that metabolic factors, which are precursors of NAFLD, can also be used to evaluate the risk of HCC in patients with CHB[17,18].
In our study, patients who received TAF therapy for 1 year did not have a significantly increased risk of developing NAFLD.However, owing to the short follow-up period of our study, the effect of TAF on increasing the incidence of NAFLD still needs to be evaluated over a longer follow-up period, and more data are required to better elucidate this.

Figure 2 Nomogram plot predicted the probability of the elevated total cholesterol level for individual patients receiving tenofovir alafenamide therapy.BMI: Body mass index; CK-MB: Creatine kinase-MB isoenzyme; TCHO: Total cholesterol.
It was unclear how TAF increased lipid concentrations, but the mechanism was independent of the patient’s HIV status because a similar effect of TAF has been reported in HIV-negative patients receiving CHB therapy.Notably, a higher proportion of TAF-treated CHB patients experienced higher levels of LDL than the TDF group at the 48-wk follow-up[19,20]; however, the use of lipid-lowering agents was not described.In our study, the increased TCHO levels with TAF treatment represented a small change in the propensity score-matched model (5% higher than baseline,P= 0.019), but we did not find a significant change in the LDL profile compared to the ETV treatment group.Regular serum lipid measurements might not accurately capture the intricate alterations in lipid metabolism brought on by anti-viral medication.A recent study showed that TDF modulates lipid metabolism by upregulating hepatic CD36viaPPAR-a activation in patients with HBV infection[21].However, the mechanism by which TAF increases serum lipid profiles remains unclear, and further studies are required.
In addition, in multivariate logistic regression analysis, metabolic factors, including BMI, hypertension, baseline TCHO, and CK-MB levels, were significantly associated with elevated TCHO levels, demonstrating an important impact of metabolic factors in terms of the elevated lipid profile caused by TAF.Previous studies have reported the effects of MetS on the adverse outcomes of fibrosis in patients with CHB[22,23].Furthermore, some studies have confirmed that metabolic factors positively affected the risk of HCC in patients with HBV infection[17,24].Based on the results mentioned above, the treatment options for patients with CHB who have the aforementioned aberrant metabolic variables may need to consider the potential effects of TAF medication on blood lipid levels.
Our study had some limitations.First, not all relevant clinical data were obtained from the treatment databases, and important data elements, such as lifestyle risk factors (i.e.exercise and diet) and family history, were not fully documented.Second, this was not a multicenter study, and we adjusted for baseline factors through a propensity scorematched model.Therefore, the observed increase in the TCHO profile may be due to the effect of anti-viral treatment.Third, the effect of TAF on the incidence of NAFLD cannot be elucidated well because of the short follow-up time.However, this study provided real-world data from China regarding the relationship between TAF-treated patients with CHB and changes in serum lipid levels.Large-scale multicenter prospective studies should be conducted in the future to further evaluate the effects of CHB and anti-HBV therapies on the risk of dyslipidemia, NAFLD, cirrhosis, and HCC.
CONCLUSION
In this real-life retrospective cohort study in China, we found that TAF was significantly associated with higher TCHO levels, whereas ETV had no effect in patients with CHB treated for 1 year.Risk factors (BMI, sex, baseline TCHO and CKMB levels) were significantly associated with elevated TCHO levels.In the future, we will focus on increased NAFLD risk and not just changes in serum lipid profiles.Further studies will help elucidate the effect of TAF on long-term metabolicrelated complications in patients with CHB.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions: Lai RM and Lin S contributed equally to this work; Lai RM and Lin S conceived and designed the study; Chen TB and Zhou JH contributed to the data analysis and manuscript feedback; Li N and Wang MM collected clinical data of the patients; Lai RM and Zheng Q wrote the manuscript; All authors approved the final version of the manuscript.
Supported byNatural Science Foundation of Fujian Province, No.2021J01123300.
Institutional review board statement: This retrospective study was approved by the ethics committee at the First Affiliated Hospital of Fujian Medical University, China.
Informed consent statement: Patients were not required to give informed consent to the study as the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All the authors declare that they have no conflicts of interest related to the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding author at bei0825@163.com.
STROBE statement:The authors have read the STROBE Statement – checklist of items, and the manuscript was prepared and revised according to the STROBE Statement – checklist of items.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: China
ORCID number: Rui-Min Lai 0000-0003-2911-0273; Tian-Bin Chen 0000-0002-9136-2167; Yue-Yong Zhu 0000-0002-0746-4911; Qi Zheng 0000-0001-8006-7069.
S-Editor: Liu JH
L-Editor: Filipodia
P-Editor: Cai YX
 World Journal of Hepatology2023年8期
World Journal of Hepatology2023年8期
- World Journal of Hepatology的其它文章
- Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors
- Liver transplant in primary sclerosing cholangitis: Current trends and future directions
- Prognostic and diagnostic scoring models in acute alcohol-associated hepatitis: A review comparing the performance of different scoring systems
- Stages of care for patients with chronic hepatitis C at a hospital in southern Brazil
- Emerging therapeutic options for non-alcoholic fatty liver disease: A systematic review
