Adsorption and Adsorption-Photocatalytic Degradation of VOCs Based on Carbon Materials
Li Ying; Zhang Hongxing; Tao Bin; Zhang Jianzhong; Li Jianzhe
(State Key Laboratory of Chemical Safety, SINOPEC Research Institute of Safety Engineering Co., Ltd.,Qingdao 266104, China)
Abstract: Adsorption and the combination of adsorption and photocatalysis are prospective strategies for treating lowconcentration volatile organic compounds (VOCs). Behind the adsorption technology of VOC treatments are carbon-based materials with large surface areas and high VOC uptake. This review summarizes the research progress in carbon-based adsorbents and adsorbent-photocatalysts for VOC removal. Firstly, the VOC adsorption performances of various carbon materials, including activated carbon, activated carbon fiber, biochar, graphene and its derivatives, and carbon nanotubes,are summarized, and the adsorption mechanism of VOCs on carbon materials is analyzed. Then, the VOC adsorptionphotocatalytic properties of composites comprised of different carbon materials and photocatalysts are presented. Finally,perspectives on the adsorption and adsorption-photocatalysis of VOCs via carbon materials are proposed. This review provides an optimal reference for the research and development of adsorbents and adsorption-photocatalysts of VOCs.
Key words: volatile organic compounds; carbon-based materials; adsorption; adsorption-photocatalysis
1 Introduction
Volatile organic compounds (VOCs) include alkanes,olefin, aromatic hydrocarbons, and oxygen-containing compounds such as alcohols, aldehydes, and ketones.These organic compounds have boiling points of 323–533 K and saturated vapor pressures of more than 0.133322 kPa at room temperature[1]. Industrial VOC emissions come primarily from petroleum refining, solvent production, fossil fuel use, coal combustion, and other processes[2]. VOCs are vital precursors of ozone (O3),photochemical smog, etc., severely harming human health and ecological environments[3]. Therefore, highly efficient VOC emission control technology has emerged. Several VOC removal technologies have been developed and are divided into destructive and nondestructive categories.Nondestructive technology includes adsorption,absorption, membrane separation, and condensation,which recover VOCs by changing the temperature and pressure conditions. Destructive technology contains photocatalytic oxidation, ozone catalytic oxidation,thermal-catalytic oxidation, plasma catalysis, and biofiltration, decomposing VOCs into CO2, H2O, lowchain hydrocarbons, and other less toxic compounds.Adsorption is one of the most promising methods for medium- and low-concentration VOC treatment due to its simple operation, low energy consumption, and high recovery efficiency. Adsorbents are the key to guaranteeing the adsorption capacity and technical feasibility of adsorption technology. Carbon-based materials have been widely used for VOC removal thanks to their abundant raw materials, low cost, high adsorption capacity, and selectivity[4-5]. The hybrid treatment of VOCs via adsorption and photocatalysis is more efficient and attractive than individual treatments because of the environmental protection, low energy consumption, and high removal efficiency of low-concentration VOCs[6].In this integrated technology, carbon-based materials can concentrate VOC gases from the environment around the active sites of photocatalysts, and the photocatalytic process subsequently oxidizes VOCs into CO2and H2O. This process regenerates the adsorbents and realizes the efficient adsorption and degradation of VOCs by improving the adsorption performance of the photocatalysts while further reducing the regeneration energy costs of the adsorption process[7].
This article summarizes recent applications of carbonbased materials in the adsorption and adsorptionphotocatalytic removal of VOCs. In this work, VOC adsorption and adsorption-photocatalytic performance of various porous carbon-based materials, such as activated carbon, activated carbon fiber, biochar, graphene, and carbon nanotubes, are comprehensively summarized.The VOC treatment mechanisms of carbon materials via adsorption and adsorption-photocatalysis process are briefly discussed. Finally, prospects and challenges on adsorption and adsorption-photocatalytic degradation of VOCs based on carbon materials are proposed. This work provides important guidance for further research on the adsorption and adsorption-photodegradation of VOCs.
2 VOC Adsorption via Carbon Materials
Different carbon materials, such as activated carbon (AC),activated carbon fiber (ACF), biochar, graphene, and carbon nanotubes, have varied VOC adsorption properties.The physiochemical and VOC adsorption properties of different carbon materials are summarized in Table 1.
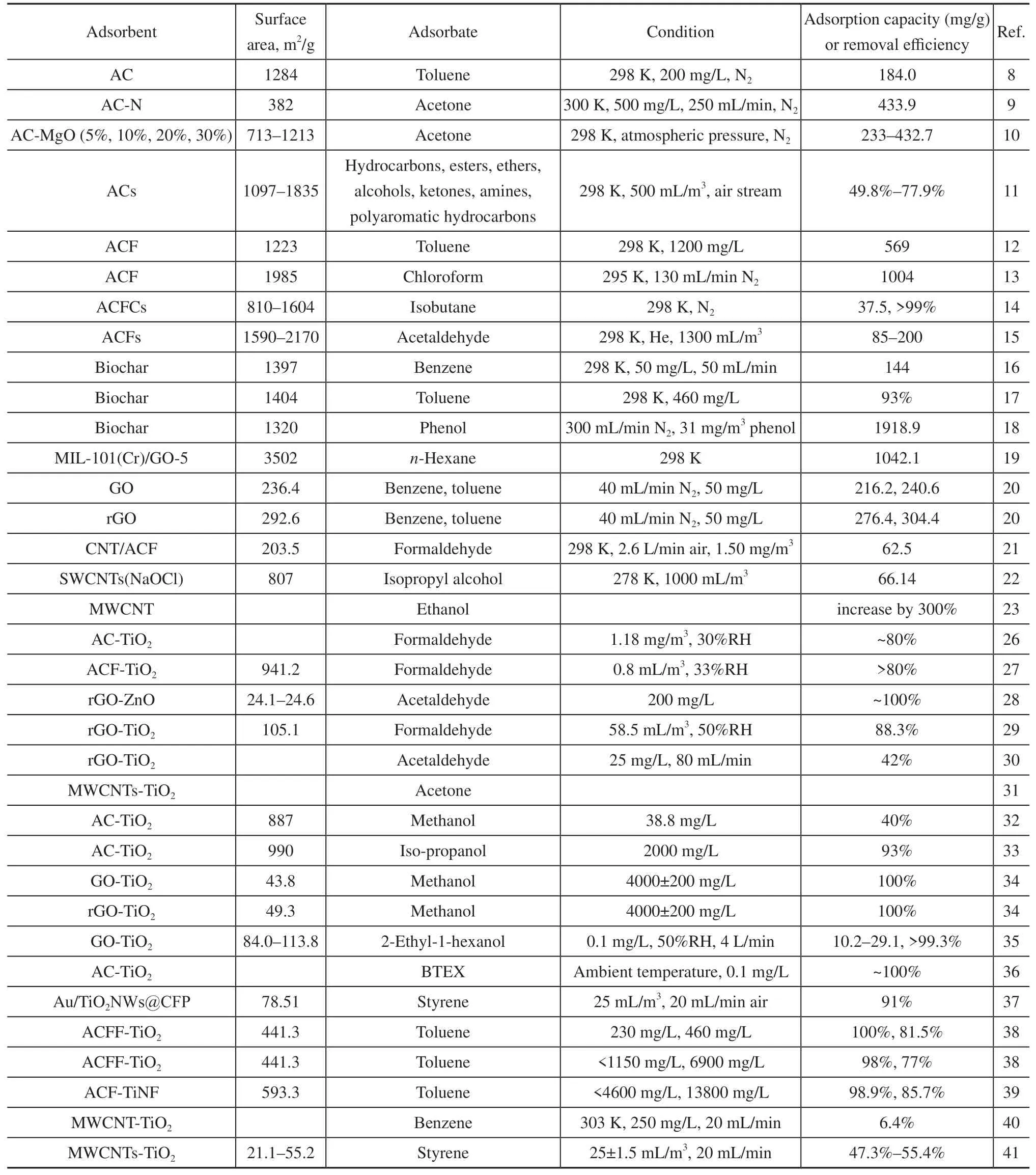
Table 1 Summary of different carbon-based material properties for VOC adsorption and degradation
2.1 Carbon materials
2.1.1 Activated carbon
ACs have large specific surface areas, high porosities, and excellent adsorption capabilities among VOCs, which are commonly prepared by processing low-cost carbonaceous material precursors into powders, pellets, granules, or spheres. For example, Yang et al. prepared five kinds of AC materials based on coal, wood, and coconut shell as raw materials and investigated their toluene adsorption behaviors[8]. The AC prepared from wood exhibited the greatest surface area and highest pore volume, resulting in the maximum toluene adsorption.
Appropriate chemical modifications from activated carbon enhance the adsorption capacity of polar VOCs because of the strong interaction forces between surface functional groups and polar VOCs. Yu et al. prepared a series of ACs with different surface modifications for acetone adsorption and evaluated the impact of pore structure and functional groups on the acetone adsorption capacities[9].AC modified by HNO3(AC-N) with a surface area of 382 m2/g had the greatest acetone adsorption of 433.9 mg/g due to the introduced microporous structures and rich carboxyl functional groups. Similarly, Zhou et al. synthesized five ACs decorated with MgO through evaporation-induced self-assembly and pyrolysis treatments while studying the application of acetone capture[10]. Among them, the AC-MgO-10% material did not exhibit the optimal surface area and microporosity.However, the maximal adsorption of acetone was up to 432.7 mg/g due to the strong adsorption affinity between oxygen functional groups and polar acetone.
Activated carbon with a large specific surface area and VOC adsorption capacities often contains developed microporous structures. However, too many micropores cause heel formation from irreversible adsorption,including chemical adsorption, which reduces the service life and regeneration cost. Adsorbents with appropriate mesopores are conducive to VOC adsorption without forming a heel, leading to a longer adsorbent service lifetime. For instance, Lashaki et al. evaluated the influence of heel formation on the lifetime and regeneration cost of five AC beads available in the market with different pore size distributions and microporosities[11]. The results showed that the high energy adsorption sites had a relatively high proportion in their highly microporous adsorbent structure, in which most adsorbed VOCs cannot be desorbed and converted into the heel. Therefore, ACs with appropriate mesoporosity proportion generally exhibit a high adsorption capacity and good adsorption/regeneration cycle, making them ideal materials for VOC adsorption.
2.1.2 Activated carbon fiber
Due to the thin fiber shape and short and straight micropores, ACFs can overcome the hardship of high pressure drops and inhibit mass transfer limitations during adsorption. This provides quicker adsorption kinetics and mass transfer rates than ACs. ACFs usually have fewer surface oxygen groups, and original ACFs tend to capture nonpolar VOCs instead of their polar counterparts.For example, Lin et al. investigated the toluene adsorption properties of three ACFs at different tolueneconcentrations and temperatures[12]. The best toluene adsorption was 569 mg/g at 298 K and 1200 mg/L. To achieve toluene adsorption at high concentrations, ACFs should have surface oxygen groups and high mesopore volumes.
Yue et al. fabricated ACF webs via melt blowing with an isotropic pitch, stabilization in air, carbonization in nitrogen, and activation in CO2/H2O mixtures for polar VOC adsorption (Figure 1)[13]. The ACFs with a surface area of 1985 m2/g showed a maximal chloroform adsorption of 1004 mg/g due to the strong adsorption affinity. Similarly, activated-carbon fiber cloths (ACFCs)prepared by Liu et al. exhibited unique structures with a micropore surface area of 810–1604 m2/g and pore width of 0.61–0.69 nm[14]. The ACFC-15 exhibited a high capture efficiency for isobutane (>99%) and adsorption of 37.5 mg/g under 5%RH–80%RH.
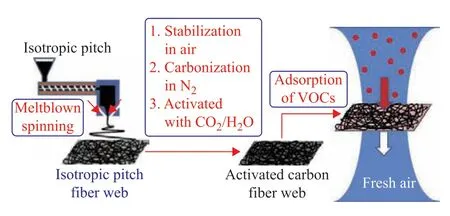
Figure 1 Diagram of ACF preparation and VOCadsorption. Reprinted with permission[13]. Copyright 2017 Elsevier B. V.
Due to the hydrophobic characteristics of ACFs, nonpolar or weak polar VOCs are more readily adsorbed by ACFs. Introducing oxygen-containing functional groups can improve the affinity between the ACF surface and polar VOCs. For instance, Baur et al. prepared ACF adsorbents via impregnation with nanoprecursors of different metal oxides (CaO, MgO, ZnO, La2O3, and Al2O3) for acetaldehyde adsorption[15]. They showed a ten-fold increase in acetaldehyde adsorption capacities(85–200 mg/g) relative to pristine ACFs. The adsorption capacities of modified ACFs increased with the basicity and proportion of metal oxides. The ACF-La2O3(20%)showed a maximum CH3CHO adsorption of 200 mg/g.Although the microporosity of ACFs is better than that of ACs in terms of VOC adsorption, its high fiber precursors and processing costs limit their industrial application. In addition, the high proportion of microporous structures in ACFs is not conducive to desorbing adsorbed VOCs,resulting in a short service life.
2.1.3 Biochar
Biochar is a potential substitute for ACs by virtue of its rich feedstock, low cost, and moderate preparation conditions. Despite the poor pore structure and adsorption performance of the original biochar, physical and chemical modifications can greatly improve the VOC adsorption capacities. In one study, Khan et al. developed gasification biochar from mixed feedstock using KOH activation and investigated the benzene adsorption performance[16]. This modified biochar had a specific surface area of 1397 m2/g, much higher than that of pristine biochar (228 m2/g), leading to a greater benzene adsorption of 144 mg/g. Similarly, Tham et al. prepared durian shell-activated biochar by H3PO4impregnation at various concentrations and studied the adsorption performance of toluene[17]. The activated biochar fabricated by impregnating 30% H3PO4showed the greatest surface area of 1404 m2/g and maximal removal efficiency of 93%. In addition to the increased surface area, introducing oxygen functional groups via H3PO4improved the biochar chemisorption.
In another study, Shen et al. synthesized porous biochar through pyrolysis of pelletized rice husk and KOH activation assisted by ball-milling[18]. They investigated the adsorption of the semi-volatile organic compound phenol, a primary gas in the pyrolysis process of biomass.This modified biochar had a surface area of 1320 m2/g and a relatively high phenol adsorption in the vapor phase(Figure 2). Due to the high boiling point of phenol (455 K), natural volatilization had difficulty occurring under environmental conditions. Therefore, thermal desorption is a feasible method to reproduce waste biochar. Although biochar has the advantages of abundant raw materials,high adsorption efficiency, inexpensive, and low energy costs compared with ACs, their drawbacks include inflammability, hydroscopicity, and easy obstruction.The complex interactions between VOC molecules and surface functional groups in biochar require further study.

Figure 2 Adsorption capacities of phenol on different biochars. Reprinted with permission[18]. Copyright 2019 Elsevier B. V.
2.1.4 Graphene
Graphene (Gr) and its two typical derivatives of graphene oxide (GO) and reduced graphene oxide (rGO) have ultrahigh specific surface areas and high mechanical strengths with potential in VOC treatment. Sun et al.solvothermally prepared a MIL-101(Cr)@GO composite and investigated itsn-hexane adsorption properties[19].The composite exhibited a highn-hexane adsorption of 1042.1 mg/g, far more than pristine MIL-101, traditional ACs, and zeolites (Figure 3). The large increase in the adsorption capacity ofn-hexane is ascribed to the high surface area of the MIL-101@GO composite and increased surface dispersive forces. The desorption efficiency calculated from five adsorption-desorption cycles experimentally reached 96.78%, proving that the MIL-101@GO composite exhibits good reusability when adsorbingn-hexane.

Figure 3 (a) Isotherms and (b) adsorption-desorption curve of n-hexane on MIL-101@GO. Reprinted with permission[19].Copyright 2014 Elsevier B. V.
In another study, Yu et al. synthesized GO and rGO through an improved Hummer’s method and investigated the benzene and toluene adsorption properties[20]. The adsorption of benzene and toluene on rGO were 276.4 and 304.4 mg/g, respectively, higher than those of GO (216.2 and 240.6 mg/g) because of the stronger hydrophobic characteristics, additional vicious sites, and lower oxygen content. The adsorbed benzene on rGO was easily desorbed out via heating treatment at 423 K,demonstrating good regenerability and recyclability.
High adsorption capacities for VOCs and good regenerability of Gr and its derivatives make them potential VOC adsorbents. However, the complex synthesis process and significant aggregation of Gr and its derivatives provide great challenges in their industrial application. Therefore, shortening the production cycle and reducing particle aggregation are the subsequent research foci of their preparation and application.
2.1.5 Carbon Nanotubes
Carbon nanotubes (CNTs) are commonly prepared into cylinders by rolling a graphene sheet. Typical types include single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). CNTs exhibit large surface areas, good hydrophobicity, and strong heat stability, demonstrating advantages in removing VOCs.The functionalization design of CNTs dramatically alters their adsorption characteristics. Yang et al. fabricated a CNT/ACF composite by directly growing CNTs on the original ACF using chemical vapor deposition and evaluated its formaldehyde adsorption behaviors[21].The adsorption was 62.49 mg/g, with a higher initial formaldehyde removal efficiency and a lower pressure resistance of 50%. The CNT surfaces were smooth and hydrophobic, producing strong interactions with VOC molecules as a promising adsorbent.
Hsu et al. prepared a functional SWCNT via NaOCl oxidation for polar isopropyl alcohol adsorption[22]. The adsorption capacity on functionalized SWCNTs reduced with the relative humidity and temperature, proving the hydrophobicity of the adsorbent and exothermic properties of the adsorption process. The adsorption capacity of isopropyl alcohol was 66.14 mg/g under a 95%RH. In addition, these SWCNTs showed good reusability of isopropyl alcohol adsorption during fifteen adsorption-desorption cycles, showing good potential as an efficient isopropyl alcohol adsorber. Hussainetal.prepared modified MWCNTs through carboxylation and nitration functionalization on their surface for polar VOC adsorption[23]. The derived MWCNTs exhibited high adsorption capacities of polar VOCs such as ethanol and relatively low adsorption of aromatic compounds and nonpolar VOCs. However, there was fast desorption kinetics for both polar and nonpolar VOCs. The penetration adsorption capacity of ethanol on modified MWCNTs improved by 300%, while that ofn-hexane dropped by 75%. Although CNTs have significant advantages in VOC adsorption, aggregation limits their wide application. Surface functionalization is an effective solution for CNT dispersion to avoid aggregation.
2.2 Adsorption mechanism
The adsorption mechanism of VOCs on porous carbon materials usually exists in three forms: physical,chemical, and competitive. Physical adsorption depends primarily on the specific surface area and pore structure of porous materials. However, the large specific surface area and well-developed pore structure (especially microporous structures) positively impact physical adsorption. The surface functional groups of porous materials are responsible for the chemical adsorption of VOCs. The reaction sites on the surface of the adsorbent come from defects, which exist in the form of unsaturated atoms at the substrate edges. Among the common surface functional groups, oxygen- and nitrogen-containing groups are considered the most important species for chemical adsorption[24]. Both the physical and chemical properties control the adsorption capacity of porous materials. The polarity, boiling point, and molecular weight of VOCs influence competitive adsorption.Highly polar VOCs exhibit a stronger adsorption affinity than adsorbents with polar surfaces. VOCs with high boiling points and relatively heavy molecular weights preferentially occupy adsorption sites during competitive adsorption processes[25]. In addition, the adsorption between water molecules and VOCs is strong in some practical industrial processes.
3 VOC Adsorption-Photocatalysis Using Carbonbased Nanocomposites
The recombination of carbon-based materials and photocatalyst nanoparticles effectively adsorbs and decomposes VOCs because various carbon materials enhance the adsorption and photocatalytic efficiency.The physiochemical properties and VOC degradation performance of different carbon-photocatalytic composite materials are summarized in Table 1.
3.1 Aldehydes and ketones
The synergistic effects between carbon-based materials and photocatalysts can improve the adsorptionphotocatalysis performance of VOCs. The intensive coupling interactions are enhanced by appropriately increasing the pore size and introducing more functional groups on the surfaces of carbon-based materials. In one study, Lu et al. fabricated AC-TiO2composites by directly immobilizing TiO2nanoparticles on the AC filter surface for the photocatalytic removal of formaldehyde under a relative humidity of 30%[26]. The degradation efficiency of formaldehyde from this AC-TiO2composite was up to ~80%. Liu et al. prepared ACF-TiO2by directly impregnating a sol-gel powder of TiO2on ACF felt and demonstrated that the number of hydroxyl groups on the surface of ACF-TiO2and formaldehyde adsorption increased after acid treatment[27]. The ACFTiO2composite with a surface area of 941.2 m2/g had a degradation efficiency of >80% under a relative humidity of 33% and 0.8 mL/m3formaldehyde.
Chenet al.used the hydrothermal method to obtain rGOZnO composites and investigated the photocatalytic properties of gaseous acetaldehyde[28]. In contrast with single ZnO, rGO-ZnO composites with 1.0 and 3.0%rGO loadings had surface areas of 84.0–113.8 m2/g and outstanding photodegradation activity (~100%)of CH3CHO, which is attributed to the promoted carrier utilization of ZnO when using an rGO support.Similarly, Yu et al. hydrothermally synthesized rGOTiO2with tetrabutyl titanate and GO as raw materials for the photodegradation of low-concentration HCHO[29].The as-synthesized rGO-TiO2composites showed excellent HCHO photodegradation capacities from indoor air. The rGO-TiO2delivered excellent moistureresistance behaviors under a relative humidity of 50%and maintained a high HCHO photodegradation capacity(84.6%) after five adsorption-photocatalysis cycles. In another study, Lin et al. solvothermally prepared rGOTiO2nanocomposites for the photocatalytic removal of CH3CHO[30]. The photodegradation activities of CH3CHO on 0.5 % (rGO)-TiO2photocatalysts were 42%,much higher than commercial titania (Degussa P25)(Figure 4). The 0.5 % (rGO)-TiO2showed continuable photodegradation performance for more than 160 min,while P25 lost its photocatalytic activity only after 25 min. These results indicate that rGO-TiO2is a promising VOC photocatalyst and provides a theoretical basis for directional photocatalyst designs with VOC removal.

Figure 4 Photocatalytic degradation of (a) o-xylene and(b) acetaldehyde on different samples. Reprinted with permission[30]. Copyright 2018 Elsevier B. V.
In addition to aldehydes, ketones can also be degraded catalytically by carbon-photocatalytic composite materials. Yu et al. hydrothermally prepared MWCNTs-TiO2nano-photocatalysts with 0–0.5% CNT loadings and titanium sulfate as the titanium source[31]. The chemicallybonded MWCNTs-TiO2photocatalysts with <0.1%MWCNT loadings displayed significantly enhanced acetone photodegradation efficiency when compared with pure TiO2and P25 photocatalysts. The 0.1% (MWCNT)-TiO2naono-photocatalysts showed the best photocatalytic performance.
3.2 Alcohols
Fixing nano-photocatalysts on the surface of carbon materials enhances the photocatalytic performance and adsorption efficiency, showing great potential in the degradation removal of VOCs. Tao et al. prepared AC-TiO2composite photocatalyst through a combined impregnation method and microwave-assisted heating for methanol removal from moist air[32]. The prepared ACTiO2material with a surface area of 887 m2/g showed a methanol removal efficiency of 40%. Horikoshi et al.used a titanium oxysulphate precursor to prepare TiO2particles supported on AC particulates[33]. They subjected the aqueous dispersion to microwave heating (MW) and oil bath heating to remove isopropanol. The degradation efficiency of the MW-produced AC-TiO2particulates was up to 93%, nearly six times more photoactive in VOC degradation than that oil-bath preparation. This method for AC-TiO2composite can be used to prepare other carbon-photocatalytic composites with high degradation efficiencies.
Roso et al. studied the correlation between graphene, GO,and rGO structures and their adsorption-photodegradation capacities[34]. The neat TiO2showed a 78% methanol abatement, and the Gr-TiO2showed an 80% conversion.The rGO-TiO2nanocomposites showed the best elimination efficiency of methanol (~100%) due to their deeper touch with photocatalysts and lower mass-transfer limitations. Chun et al. investigated the relationship between the calcination temperature and photocatalytic performance of 2-ethyl-1-hexanol (2E1H) of GOTiO2composites[35]. The GO-TiO2composites heated at 400 °C showed longer penetrations and saturation adsorption times with greater adsorption for 2E1H. The photocatalytic efficiency of GO-TiO2composites did not decrease significantly after five adsorption-photocatalysis cycles, while the average 2E1H mineralization efficiency and photocatalytic efficiency were 55.1% and 99.3%,respectively.
3.3 BTEXs
BTEXs (shorted for benzene, toluene, ethylbenzene and xylene) cause significant harm to human bodies, so controlling outdoor and indoor BTEXs is vital. Jo et al.prepared an Acs-photocatalytic oxidation hybrid system to control the BTEX content (0.1 mg/L) in indoor air at ambient temperatures[36]. Compared to the AC, the composite presented a higher removal efficiency (~100%),and there was no appreciable impact from the inlet concentration or relative humidity on the degradation efficiency of composites. The results showed a relatively high removal efficiency of >70% under a low loading of ≤5000 ng. Therefore, the AC-photocatalytic composite degrades indoor low-concentration BTEX, pushing the VOC concentration to meet the indoor air quality standard range.
Shi et al. fabricated Au/TiO2NWs@CFP ternary composites by growing TiO2nanowires modified with Au nanoparticles onto porous carbon fiber paper (CFP)by combining wet coating, hydrothermal growth, and photoreduction[37]. The synergistic action of these three compositions enhanced the absorption intensity of visible light, reduced the recombination of charges triggered by light and holes, and improved the adsorption capacity of organic pollutants. The prepared Au/TiO2NWs@CFP had a surface area of 78.5 m2/g and exhibited a high styrene photocatalytic activity (91% within 3 h) with a decomposition rate of ~90% after four recycles at a concentration of 25 mL/m3, suggesting high photocatalytic activity and regeneration for styrene degradation.
The adsorption and photocatalysis of high-concentration VOCs is still an enormous challenge. Li et al. produced porous ACFF-TiO2composites by decorating TiO2nanoparticles on activated carbon fiber felts (ACFF)[38].They realized excellent adsorption-photocatalysis of toluene at high concentrations from the synergy between ACFF and TiO2(Figure 5). When the toluene concentrations were 230 and 460 mg/L, the photodegradation efficiencies of ACFF-TiO2were 100%and 81.5%, respectively. The toluene removal efficiencies of this ACFF-TiO2composite reached ~98% and ~77%at concentrations of <1150 and 6900 mg/L, respectively.Tian et al. first prepared ACF-TiO2nanofiber (ACFTiNF) composites by depositing TiNFs within ACF felts[39]. The toluene adsorption efficiencies of the ACFTiNF composite were 98.9% and 85.7% at concentrations of <4600 and 13800 mg/L, respectively. These works provide general methods for preparing efficient photocatalysts with VOC removal. Therefore, these ACFTiO2photocatalysts with outstanding adsorption and photodegradation performance showed great potential in applying high-concentration VOC removal.

Figure 5 Reaction mechanism of ACF-TiO2 photocatalytic degradation of VOCs. Reprinted with permission[38].Copyright 2017 Elsevier B. V.
The hollow structure of CNTs is conducive to improving the stability of photocatalysts and increasing the residence time of VOCs in material pores, enhancing the photodegradation activity of VOCs. Therefore, Xu et al. fabricated CNT-TiO2nano-photocatalysts via impregnation for benzene degradation[40]. The CNTTiO2composites showed an improved photodegradation capacity of benzene over commercial P25. Moreover, the 95% (MWCNT)-TiO2photocatalyst showed an average conversion ratio of 6.4% and average mineralization ratio of 64.6%. No obvious inactivation of photocatalytic activity for benzene was observed for 95% (MWCNT)-TiO2photocatalysts. An et al. hydrothermally synthesized MWCNTs-TiO₂ photocatalysts with titanium tetrafluoride and CNTs to degrade gaseous styrene[41]. The MWCNTs-TiO2spherical photocatalysts exhibited better stability and greater styrene photocatalytic activity. The photodegradation ability of styrene on 7.2% (MWCNTs)-TiO2increased stably in the first 90 min and gradually balanced over 120 min. A degradation efficiency of 47.3%was achieved within 180 min, 1.76 times that of the single TiO2. The 18.9% (MWCNTs)-TiO2photocatalysts exhibited an increased degradation efficiency of 55.4%.
4 Conclusions
This article reviewed the adsorption properties of VOCs with various carbon-based materials and the adsorptionphotocatalytic performances of carbon-photocatalysts.
The adsorption performance of VOCs is affected primarily by the pore structures and surface chemistry properties of carbon-based materials. The large surface area and high micropore volume of carbon materials favor the physical adsorption of VOCs. Using organic polymercoated adsorbents effectively reduces the competitive adsorption with water vapor in the environment. To date,some carbon materials with good pore structures, such as biomass, have poor stability. While materials such as graphene and carbon nanotubes are expensive, limiting their industrial application. Future research will consider carbon-based adsorbents for VOC adsorption treatments that are low cost, highly stable, high adsorption capacity and selectivity, and good hydrophobicity.
Carbon materials with different physical and chemical properties have important effects on the stability of nano-photocatalysts, synergistic effects between carbon materials and photocatalysts, and VOC adsorption.The composite system has uneven photocatalyst distributions and easily detaches from the carbon material skeleton. Therefore, it is important to select suitable carbon material supports for photocatalysts. Advanced combination technologies that benefit further studies are presented as follows. (1) The synthesis of carbonbased photocatalysts with stable, highly adsorptive,and photocatalytic activity is required, especially for applications with inexpensive carbon materials like biochar. (2) In the adsorption-photocatalysis process,the pore structure of carbon materials affects the VOC diffusion, photoadsorption, intermediate species removal,etc. Thus, the relationship between the pore structure of carbon materials, adsorption, VOC degradation capacity,and the regeneration performance of composites requires further investigation.
- 中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics

