A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
Zhou Yafen; Cheng Hong; Song Yu; Wang Qing; Zhao Wenjie;Chen Qilin; Zhou Limei; Xu Bin
(1. Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002, China; 2. Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education, Sichuan University of Science & Engineering, Zigong 643000, China)
Abstract: Polyimide (PI) is an organic polymer material with good stability and diverse sources that has attracted widespread attention in the field of photocatalysis. In this study, a series of PI photocatalysts were synthesized by a thermal polymerization approach using pyromellitic dianhydride (PMDA) and various diamine monomers (melamine (MA),4,4'-oxydianiline, and melem) as the precursors as well as different heating rates. The effects of the diamine precursor and heating rate on the structure, composition, morphology, and optical properties of the as-prepared PI materials were systematically investigated by various characterization techniques. The selective photo-oxidation of benzylamine was used as a model reaction to evaluate the photocatalytic activities of the resulting PI samples for the oxidation of amines to imines. The results revealed that the PI sample prepared using MA and PMDA as the precursors and a heating rate of 7 °C/min (MA-PI-7) exhibited the best catalytic performance, with 98% benzylamine conversion and 98% selectivity for N-benzylidene benzylamine after 4 h of irradiation. Several benzylamine derivatives and heterocyclic amines also underwent the photo-oxidation reaction over the MA-PI-7 catalyst to afford the corresponding imines with good activity. In addition, MA-PI-7 exhibited good stability over four successive photocatalytic cycles.
Key words: polyimide; photocatalyst; oxidation; amine; imine
1 Introduction
Photocatalysis is an important means of utilizing solar energy that has been extensively applied in the fields of energy conversion and environmental remediation[1].Recently, photocatalysis has also attracted widespread attention in organic synthesis owing to its mild reaction conditions and good catalyst recyclability[2]. In particular,there has been growing interest in several selective photoredox organic transformations, such as the oxidation of toluene[3], reduction of nitroaromatics[4], and oxidation of alcohols[5].
Imines are important building blocks in the production of fine chemicals, pharmaceuticals, and dyes[6-8]. Traditional synthetic route to imines via the condensation of amines and carbonyl compounds often requires Lewis acid catalysts and expensive dehydrating agents with the drawbacks of hazardous chemical waste generation and difficulties associated with the handling of unstable aldehydes[9-10], hence limiting the practical applicability of the method[9,11]. Therefore, the catalytic oxidative coupling of amines and the coupling of amines with alcohols have been considered as alternative strategies for imine synthesis with economic and environmental benefits[7,11-12].In the past decade, a number of heterogeneous catalysts based on transition metals such as supported Pd[7],Co[11], Au[13-14], Ru[15], Cu[16-17], Ni[18], and Mn[19]were successfully developed for amine oxidation. However,the thermal catalysis processes were typically conducted under high temperature and pressure, along with the utilization of precious metals and the metal contamination of the final imines, which is especially problematic during pharmaceutical production[6,20-21]. Consequently, it is crucial to develop cost-effective metal-free catalysts for the oxidation of amines under milder conditions[6,21-22].Meanwhile, from a green chemistry perspective, the photocatalytic oxidative coupling of amines to generate imines using molecular oxygen as the oxidant without the need for additives under mild conditions is an appealing approach[11,22].
In recent years, various photocatalytic materials based on graphitic carbon nitride (g-C3N4)[9,23], TiO2[8,24], AgPO4[25],CdS[23,26], Bi2MoO6[27], and BiVO4[28]have been exploited for the oxidative transformation of amines to imines.Moreover, since the pioneering application of g-C3N4as a metal-free photocatalyst for hydrogen/oxygen evolution from water by Wang et al.[29], numerous studies have focused on organic polymer photocatalysts[22,30].In particular, polyimide (PI) has recently attracted increasing attention in photocatalysis owing to its inherent advantages of facile synthesis, low cost, tunable optoelectronic properties, and good physicochemical stability[31]. Although the effects of the synthesis methods and modifications of PI materials on the performance of PI-based photocatalysts for hydrogen evolution[32],pollutant degradation[33], and H2O2synthesis[34]have been extensively investigated, the photocatalytic oxidation of amines to imines over PI-based photocatalysts has seldom been reported[35-36]. Herein, a series of PI photocatalysts were prepared by a facile calcination method using various diamine monomers and different heating rates.The photocatalytic activities of the obtained samples were evaluated by selective oxidation of amines to imines in air under UV–visible (UV–vis) light irradiation. The morphologies and physicochemical properties of the PI samples were also investigated to elucidate the origin of their varying catalytic performance. Furthermore, a possible photocatalytic mechanism is proposed.
2 Experimental
2.1 Materials
Melamine (MA, 99%), 4,4'-oxydianiline (ODA, 98%),and pyromellitic dianhydride (PMDA, 98%) were purchased from Adamas. All chemical reagents were used directly as received without further purification.
2.2 Preparation of PI
2.2.1 Synthesis of melem
Melamine (5 g) was placed in a quartz boat and heated to 425 °C at a rate of 10 °C/min in a tube furnace then maintained at this temperature for 4 h. The resulting crude product was ground, washed several times with warm water and ethanol, and dried at 80 °C for 12 h to obtain melem.
2.2.2 Synthesis of PI from various diamines
Various PI samples were synthesized by the thermal condensation approach using different diamines (MA,ODA, and melem) and PMDA as the precursors. In a typical procedure, MA (10 mmol) and PMDA (10 mmol)were mixed thoroughly in a quartz boat with a cover,which was transferred to a tube furnace and heated to 325 °C at a heating rate of 7 °C/min then calcined at this temperature for 4 h. The powder was allowed to cool to room temperature then washed several times with warm water and ethanol and dried at 80 °C overnight. The resulting PI sample is referred to as MA-PI-7. Similarly,the PI samples synthesized using ODA and melem are denoted O-PI-7 and M-PI-7, respectively.
2.2.3 Synthesis of PI at various heating rates
Various PI samples were synthesized at different heating rates using MA and PMDA as the precursors. In a typical procedure, MA (10 mmol) and PMDA (10 mmol) were mixed uniformly in a quartz boat with a cover, which was transferred to a tube furnace and heated to 325 °C at various heating rates (3, 5, 7, and 9 °C/min) then calcined at this temperature for 4 h. The powder was allowed to cool to room temperature then washed several times with warm water and ethanol and dried at 80 °C for 12 h. The resulting products are denoted MA-PI-X, whereXrepresents the heating rate used during the synthesis.
2.3 Characterization
X-ray diffraction (XRD) measurements were performed on a Rigaku Dmax/Ultima IV diffractometer with Cu Kα radiation. Scanning electron microscopy (SEM) images and elemental mappings were obtained on a GeminiSEM 500 instrument. Fourier-transform infrared (FT-IR)spectra were recorded on a Thermo Scientific Nicolet 6700 spectrometer. The spectral response ranges of the samples were analyzed by UV-vis diffuse reflectance spectroscopy (DRS) on a Shimadzu UV-3600 system using BaSO4as the reference. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo ESCALAB 250Xi spectrometer using Al Kα as the radiation source.
2.4 Photoelectrochemical measurements
All photoelectrochemical measurements were conducted on an electrochemical workstation (CHI760E,Shanghai Chenhua Apparatus Co., Ltd.) in the threeelectrode configuration with 0.1 M Na2SO4solution as the electrolyte. The working electrode was prepared by mixing the sample (20 mg) and Nafion (10 µL) in absolute ethanol (1 mL). After sonication for 0.5 h,an aliquot of the slurry (90 µL) was coated on an ITO glass substrate and allowed to dry. A Pt electrode and a saturated Ag/AgCl electrode were used as the counter electrode and reference electrode, respectively. Transient photocurrent response and electrochemical impedance spectroscopy (EIS) measurements were performed using a 300 W xenon lamp (PLS-SXE300, Beijing Perfect Light Co., Ltd.).
2.5 Photocatalytic oxidation of amines
The photocatalytic oxidation of amines was performed at room temperature under an air atmosphere. In a typical procedure, the catalyst (10 mg), amine (0.3 mmol), and acetonitrile (10 mL) were added to a reactor fitted with a reflux condenser. The resulting suspension was stirred in the dark for 30 min to achieve sufficient dispersion and an adsorption/desorption equilibrium. Next, the mixture was irradiated under a 300 W xenon lamp with continuous stirring. After the desired irradiation time, the catalyst was removed by centrifugation and the obtained supernatant was analyzed by gas chromatography with a flame ionization detector (GC-FID, Agilent 7890A) and determined by gas chromatography-mass spectrometer(GC-MS, Agilent, 5977B).
3 Results and Discussion
3.1 Characterization
The XRD patterns of the PI samples prepared from different diamine precursors are presented in Figure 1(a). It can be seen that the XRD patterns of MA-PI-7,M-PI-7, and O-PI-7 were clearly different. For MAPI-7, several characteristic peaks in the 2θrange of 10°–30° were observed, indicating a high degree of crystallinity[32]. Similar diffraction peaks were present in the XRD pattern of M-PI-7, although the crystallinity was reduced compared with MA-PI-7[37]. By contrast,no sharp diffraction peaks were detected in the XRD pattern of O-PI-7 and only two diffuse peaks appeared in the 2θrange of 10°–30°, which corresponded to typical amorphous diffraction peaks[38]. The positions and intensities of the characteristic peaks of M-PI-7 and O-PI-7 were slightly shifted compared with those of MAPI-7, implying that the structure of the diamine monomer affected the crystal structure of the resulting PI. From Figure 1(b), it can be seen that the XRD patterns of the PI samples prepared under different heating rates using MA as the diamine precursor were similar, but the peak intensities displayed obvious differences. In particular,MA-PI-7 exhibited the most intense characteristic diffraction peaks, suggesting that the heating rate during calcination affected the PI crystallinity.

Figure 1 XRD patterns of the as-prepared PI samples

Figure 2 FT-IR spectra of the as-prepared PI samples
The FT-IR spectra of the PI samples prepared from different diamine precursors and at different heating rates are presented in Figs. 2(a) and 2(b), respectively. All of the PI samples displayed the characteristic absorption bands of PI. The bands at approximately 1772, 1725,and 727 cm-1were ascribed to the symmetric stretching,asymmetric stretching, and bending vibrations of the imide carbonyl groups, respectively[33,39]. The band at 1376 cm-1was assigned to the stretching vibration of C–N-C in the five-membered imide ring[39]. In addition, the bands at 3430 and 1625 cm-1were associated with the surface adsorption of water molecules[39]. These FT-IR spectroscopy results confirmed the successful synthesis of the various PI samples using the different diamine precursors and heating rates.
The SEM images of the as-prepared PI samples are shown in Figure 3. The PI samples synthesized from melem [Figure 3(a)] and melamine [Figure 3(c)]displayed a lamellar structure, while that prepared using 4,4'-oxydianiline [Figure 3(b)] was composed of irregular particles. No significant differences were observed in the morphologies of the MA-PI-Xsamples prepared at various heating rates [Figs. 3(c–f)]. Elemental mappings of MA-PI-7 confirmed the homogeneous distributions of C, O, and N and ruled out the presence of other elements in the sample.
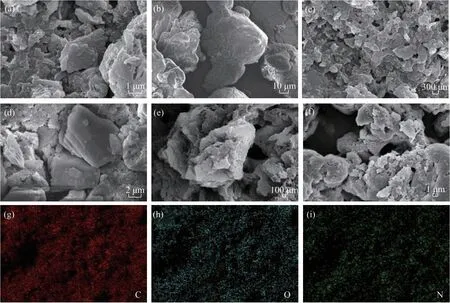
Figure 3 SEM images of the as-prepared PI samples: (a) M-PI-7, (b) O-PI-7, (c) MA-PI-7, (d) MA-PI-3, (e) MA-PI-5, and (f)MA-PI-9. (g)-(i) Elemental mappings of MA-PI-7
The light absorption properties of the as-prepared PIs were examined by UV–vis DRS. As shown in Figure 4(a),the optical absorption edges of M-PI-7, O-PI-7, and MAPI-7 were located at approximately 399, 376, and 440 nm,respectively, which indicates that the different diamine precursors used in the synthesis greatly influenced the optical absorption characteristics of the obtained PI samples. Figure 4(c) displays the UV–vis DRS spectra of the PI samples prepared at different heating rates. The absorption band edges of MA-PI-3, MA-PI-5, MA-PI-7,and MA-PI-9 were situated at approximately 430, 431,440, and 430 nm, respectively. Thus, it is clear that the heating rate had a less pronounced effect on the optical absorption properties of the PI samples than the choice of diamine precursor. The band gap energy was estimated according to the following equation:
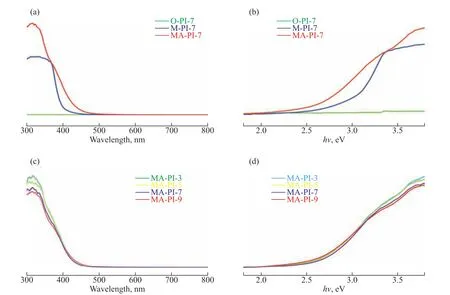
Figure 4 (a and c) UV-vis DRS spectra and (b and d) plots of (αhν)2 versus hν for the PI samples
whereα,ν,Eg,A,andhdenote the absorption coefficient,light frequency, band gap, a constant, and Planck’s constant, respectively. Herein, the value ofnis 4 because the absorption edge of the samples in the range of 200–480 nm was attributable to indirect band absorption[40].Plots of (αhν)2versushνfor the PI samples are presented in Figs. 4(b) and 4(d). The estimated band gap energies for M-PI-7, O-PI-7, and MA-PI-7 were 3.01, 3.31, and 2.60 eV, respectively[40], demonstrating a clear difference.By contrast, the band gap energies for MA-PI-3, MAPI-5, MA-PI-7, and MA-PI-9 were similar at 2.67, 2.64,2.60, and 2.65 eV, respectively. The narrower band gap for MA-PI-7 indicated that this sample should display much higher visible-light utilization efficiency than the other samples.
The chemical state and elemental composition of MAPI-7 were analyzed by XPS, as shown in Figure 5.The survey spectrum [Figure 5(a)] contained peaks corresponding to C, N, and O[41]. In the C 1s XPS spectrum [Figure 5(b)], the peak centered at 284.80 eV was attributed to surface adventitious carbon, which was used as a reference, while the peak at 287.97 eV was ascribed to carbon atoms bonded to three nitrogen atoms in the PI space lattice[42]. In the N 1s spectrum[Figure 5(c)], the peaks located at 398.07 and 399.49 eV were assigned to the C=N-C moieties of the triazine units and C–N groups connected to two C=O groups in the five-membered imide rings, respectively[43]. In the O 1s spectrum [Figure 5(d)], the peaks observed at 531.24 and 532.80 eV were ascribed to the C=O moieties in the five-membered imide rings and the C-O-C groups in the cyclic anhydride units, respectively[44].

Figure 6 (a and c) Transient photocurrent responses and (b and d) EIS Nyquist plots for the PI samples
The effectiveness of charge separation and transfer in the samples was studied by transient photocurrent response measurements using the same light source as in the photocatalytic tests. As shown in Figs. 6a and 6c, the photocurrent was generated rapidly upon turning on the light and increased quickly to a constant value. Compared with the other PI samples synthesized using the different diamine precursors and heating rates, MA-PI-7 exhibited a clearly superior light response, revealing a much higher separation efficiency of the photogenerated electron–hole pairs for MA-PI-7. EIS is an effective method for elucidating the transfer and migration processes of photogenerated electron–hole pairs, which play a key role in the photocatalytic performance[40]. In general, a smaller arc radius in the EIS Nyquist diagram suggests a higher separation efficiency of the photoexcited electrons and holes[41]. As observed in Figs. 6b and 6d, MA-PI-7 also displayed a smaller arc radius than the other PI samples,demonstrating that it possessed the best charge separation efficiency. These results are consistent with the transient photocurrent response measurements.
3.2 Photo-oxidative coupling of amines
Benzylamine was selected as a model substrate to investigate the photocatalytic performances of the asprepared PI samples for the oxidative coupling of amines to generate imines under UV–vis light irradiation and ambient air. The results are presented in Figs.7a and 7b. It can be seen that all of the PI samples exhibited high selectivity for the corresponding imine,N-benzylidene benzylamine (>98%), although they displayed clearly different activities. MA-PI-7 afforded the best performance compared with the other PI samples synthesized using different diamine precursors [Figure 7(a)] and different heating rates [Figure 7(b)], with 98%conversion of benzylamine and 98% selectivity forN-benzylidene benzylamine. In addition, as illustrated in Figs. 7(c) and 7(d), the pseudo-first-order rate constant(k) for the oxidative coupling of benzylamine over MAPI-7 was considerably higher than those observed for the other PI samples. Therefore, MA-PI-7 was selected as the optimal catalyst for a more in-depth evaluation of the photo-oxidative coupling of amines.
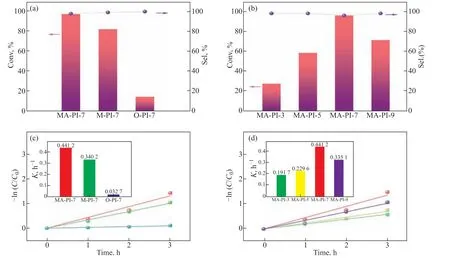
Figure 7 (a and b) Photo-oxidative coupling of benzylamine over the PI samples and (c and d) pseudo-first-order kinetics. Reaction conditions: benzylamine (0.3 mmol), catalyst (10 mg), CH3CN (10 mL), 300 W xenon lamp irradiation, 4 h, air, room temperature
A series of control experiments for the selective oxidation of amines to imines were next performed and the results are presented in Table 1. Under standard conditions,with MA-PI-7 as the catalyst and ambient air as the oxidant, the conversion of benzylamine and selectivity forN-benzylidene benzylamine both reached 98% within 4 h of irradiation (entry 1). The benzylamine conversion was negligible (<1%) when the reaction was performed in the absence of either MA-PI-7 (entry 2) or light (entry 3),demonstrating that both the photocatalyst and light were crucial for the reaction. Under argon atmosphere, even in the presence of MA-PI-7 and light, the benzylamine conversion was greatly reduced to only 14% (entry 4), confirming the key role of oxygen in the oxidative coupling of amines. It is worth emphasizing that ambient air rather than pure oxygen was used as the oxidant in this catalytic system, which avoids the energy consumption associated with the separation of oxygen from air[25].Moreover, the present oxidation reaction was conducted at ambient temperature, which corresponds to milder conditions compared with many other catalysts previously reported in the literature[25], where an elevated reaction temperature was required to obtain high conversion. To further demonstrate the simple preparation and excellent performance of the MA-PI-7 catalyst, the current results are compared with those obtained for the photo-oxidative coupling of benzylamine over several previously reported metal-free catalysts[22,30]and polymer semiconductorbased catalysts[9,23]in Table 2. The catalytic activity of MA-PI-7 was comparable to that of the CdS@g-C3N4composite (CdCN-10) and much higher than that of g-C3N4and the g-C3N4/Uio-66-NH2(CNUIO-5)nanocomposite. In addition, the MA-PI-7 catalyst was prepared by a facile thermal polymerization method.

Table 1 Control experiments for the photo-oxidative coupling of benzylamine

Table 2 Catalytic performance comparison for several catalysts in the photo-oxidative coupling of benzylamine
Encouraged by the excellent performance of the MA-PI-7 catalyst in the photo-oxidative coupling of benzylamine,we next investigated the photocatalytic oxidation of various benzylamine derivatives over MA-PI-7 under the optimized reaction conditions. The results are presented in Table 3. All of the examined substituted benzylamines were converted to their corresponding imines with high selectivity (≥99%, entries 2–6). However, the conversion rate was clearly influenced by the nature and position of the substituents. It was found that para-substituted benzylamines with a mildly electron-donating substituent(–CH3, entry 2) and an electron-withdrawing substituent(–Cl, entry 6) did not exhibit a significant difference in the rate of conversion, while substitution with a stronger electron-donating group (–OCH3, entry 5) further improved the conversion rate. In addition, benzylamines featuring methoxy substituents at different positions displayed conversions that increased in the order of ortho(47%) < meta (81%) < para (98%) (entries 3–5), which was ascribed to the decrease in steric hindrance[9,11].Furthermore, heterocyclic amines also underwent the photo-oxidation reaction to the corresponding imines with good conversion and selectivity under the same conditions (entries 7–9).
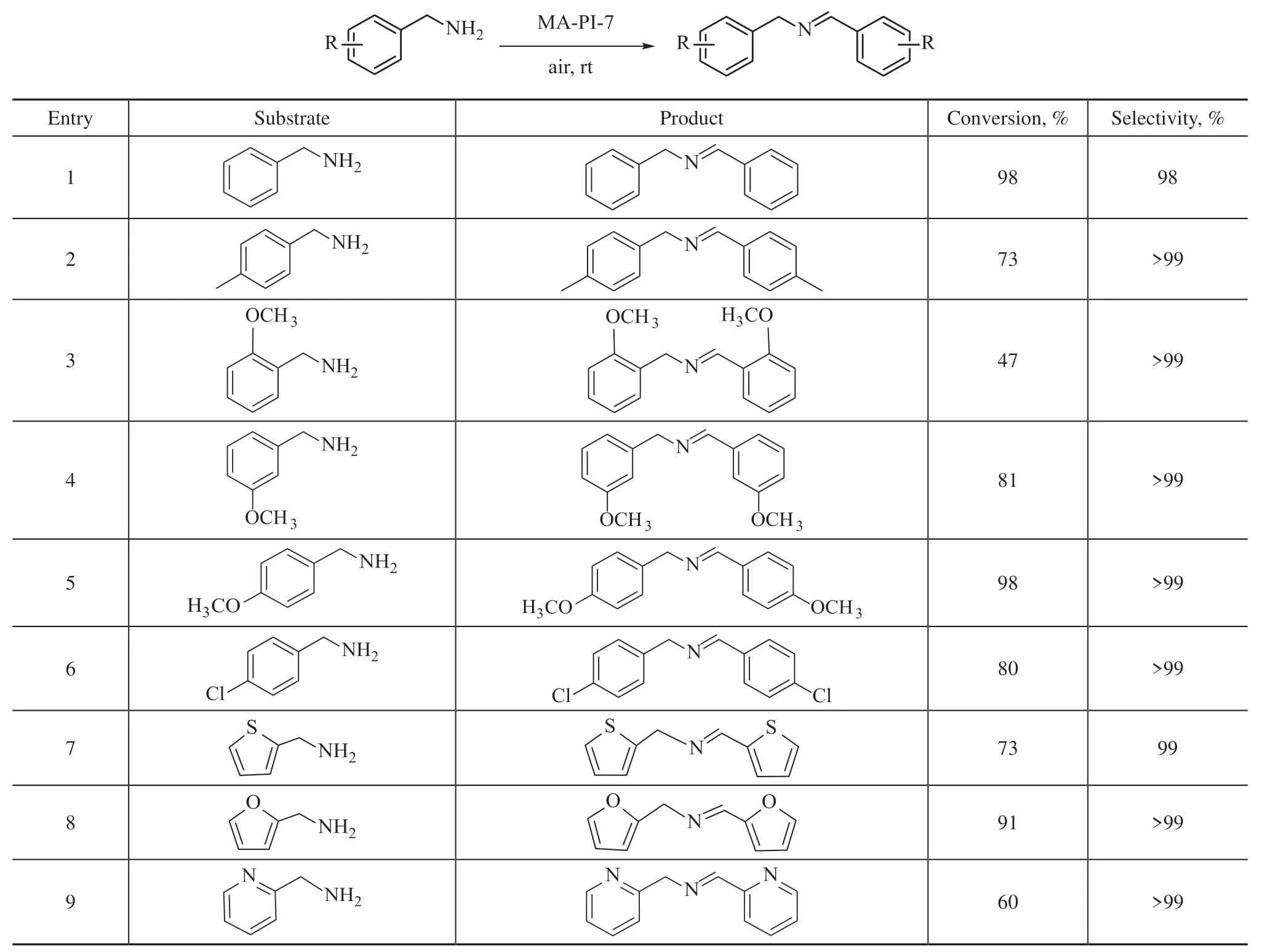
Table 3 Photo-oxidative coupling of benzylamine derivatives over MA-PI-7
Photocatalyst stability and recyclability are extremely important for practical applications. A recycling experiment was thus performed to evaluate the photocatalytic stability of MA-PI-7. After each cycle, the photocatalyst was separated from the reaction solution by simple centrifugation and washed three times each with ethanol and acetonitrile. As shown in Figure 8a,after four successive cycles, only a slight decrease in the benzylamine conversion was observed, while the selectivity forN-benzylidene benzylamine remained in excess of 98%. In addition, no obvious change was observed in the XRD pattern of MA-PI-7 before and after the recycling experiment (Figure 8b), indicating the good structural stability of the catalyst. These results suggest the excellent stability and reusability of the MA-PI-7 catalyst in this photocatalytic reaction.

Figure 8 (a) Recycling test for the photocatalytic oxidation of benzylamine over MA-PI-7 and (b) XRD spectra of MA-PI-7before and after the recycling experiment
Trapping experiments were next conducted to identify the active species involved in the photocatalytic oxidation of benzylamine over MA-PI-7. The influence of several scavengers, namely, AgNO3for electrons (e-), isopropanol(IPA) for hydroxyl radicals (·OH), ammonium oxalate (AO)for holes (h+), andp-benzoquinone (p-BQ) for superoxide radicals (·O2-), on the photocatalytic performance of MA-PI-7 was investigated and the results are shown inFigure 9. The addition of AgNO3into the reaction system brought about a drastic drop of the conversion to only 21%, revealing that photogenerated e-are the major active species in this photocatalytic process. Meanwhile,an obvious decrease in the activity was observed whenp-BQ or IPA was added, which indicated that ·O2-and ·OH radicals are also involved in the reaction.
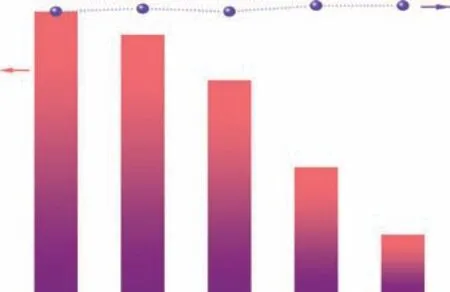
Figure 9 Trapping experiments to identify the active species in the photocatalytic oxidation of benzylamine over MA-PI-7
On the basis of the above experimental results, a plausible reaction mechanism is proposed as depicted in Scheme 1. First, MA-PI-7 is excited under the light irradiation to produce photogenerated e-and h+. Subsequently, an adsorbed O2molecule is reduced by the photogenerated eaccumulated in the conduction band of MA-PI-7 to form an ·O2-radical. Meanwhile, the benzylamine substrate is oxidized by the photogenerated h+in the valence band of MA-PI-7 to yield a nitrogen-centered radical cation[45],followed by electron transfer to afford a carbon-centered radical cation. Next, the ·O2-radical abstracts a proton from the carbon-centered radical cation to give an imine intermediate and H2O2[46]. At the same time, the produced H2O2can generate ·OH radicals, which transform the benzylamine substrate to an imine intermediate. Finally,condensation of the imine intermediate and benzylamine forms the final imine product. Alternatively, the ·OH radical can react with benzylamine to produce a small amount of water, which mediates hydrolysis of the imine intermediate to afford benzaldehyde. The condensation of benzylamine and benzaldehyde would also yield the imine product[26].

Scheme 1 Proposed mechanism for the photocatalytic oxidation of benzylamine over MA-PI-7
4 Conclusions
Three PI materials were prepared by a facile thermal condensation method using various diamines (MA, ODA,and melem) and PMDA as the precursors. An additional series of PI samples were synthesized from MA and PMDA using different heating rates. The photocatalytic activities of the resulting PI materials in the selective aerobic oxidation of amines to imines under UV–vis light irradiation were evaluated using benzylamine as a model substrate. The MA-PI-7 sample synthesized from MA and PMDA at a heating rate of 7 °C/min was found to exhibit the best photocatalytic performance. The excellent photoactivity of MA-PI-7 can be ascribed to its narrower band gap, more efficient visible-light absorption, and higher separation efficiency of the photogenerated electron–hole pairs, which were confirmed by detailed UV–vis DRS, transient photocurrent response, and EIS measurements, respectively. Furthermore, MA-PI-7 exhibited excellent stability during recycling runs. The developed PI material holds great application prospects in the field of photocatalysis on account of its low cost,environmental friendliness, and good stability.
Acknowledgments:This work was supported by the Opening Project of Key Laboratory of Green Catalysis of Higher Education Institutes of Sichuan (Grant number: LZJ2101)and the Fundamental Research Funds of China West Normal University (Grant number: 19D038).
- 中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics

