Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
Jiang Shuaijun; Meng Weifeng; Wan Yongqing; Qin Weihua; Liu Xiaoqing; Lan Yanhua
(School of Environment and Safety Engineering, North University of China, Taiyuan 030051, China)
Abstract: Cyclotriphosphazene derivatives can effectively improve the flame retardancy and fire safety of epoxy resins (EPs)via their influence on the pyrolysis process. In this work, the effects of hexa(5-methyl-2-pyridinoxyl)cyclotriphosphazene(HMPOP) incorporation on the initial pyrolysis of an EP at 500–3500 K were studied using the ReaxFF method. The pyrolysis fragments, initial reaction pathways, and main products were identified for the EP and EP/HMPOP composites.The activation energies were derived by fitting the weight percentage curves for solid species during the pyrolysis reactions and the obtained values were in good agreement with experimental data. The initial EP pyrolysis reactions included four major decomposition modes, which primarily involved the cleavage of C-O and C-N bonds. The main pyrolysis products were H2O, CO, C2H4, and CH2O. HMPOP bonded with the oxygen-containing fragments to form larger molecular fragments and reduced the amounts of C0–C4 products, especially that of the harmful gas CH2O. Thus, HMPOP promoted the formation of carbon clusters and reduced the generation of combustible gases, ultimately decreasing the capacity for fire propagation.
Key words: epoxy; cyclotriphosphazene; ReaxFF; pyrolysis; flame retardancy
1 Introduction
Epoxy resins (EPs) feature good thermal stability,chemical stability, dimensional stability, mechanical properties, adhesion, formability, and thermal insulation characteristics. However, despite these benefits, the flammability of EPs has severely restricted their broader application in areas requiring high fire resistance, such as electronics[1], aviation[2], composite materials[3], coatings[4],and other fields[5]. In an effort to address this problem,various flame retardants suitable for incorporation in EPs have been developed.
Cyclotriphosphazenes are promising non-conjugated phosphorus and nitrogen hybrid compounds with potential applications as flame retardants because of their advantages of being free of halogens, having a high flame-retardant efficiency, producing less smoke during combustion compared with conventional alternatives,and generating no toxic or corrosive gases[6]. For example, Liu et al.[7]prepared cyclotriphosphazene flameretardant EP with excellent flame retardancy and good thermal stability. The cyclotriphosphazene moieties promoted the formation of an intumescent phosphorusrich carbonaceous char layer on the EP surface that provided shielding from heat and oxygen diffusion.Furthermore, Yang et al.[8]synthesized a polyphosphazene flame retardant containing active amine groups, which significantly reduced the generation of small molecules after introduction into EP composites and improved their fire resistance, thus suppressing smoke generation and release during combustion.
Analysis of the combustion and pyrolysis processes plays a vital role in the safety and advanced applications of materials[9–11]. Ning et al.[12]utilized an aminothiazolebased cyclotriphosphazene derivative as a reactive flame retardant for EPs. Characterization of the gaseous products by thermogravimetric analysis–Fourier-transform infrared spectroscopy and pyrolysis–gas chromatography–mass spectrometry revealed the presence of –CH2–, C=N, and C=C groups as well as NH3. Liang et al.[13]synthesized bisphenol-A-bridged penta(phenoxy)cyclotriphosphazene (A-BP) and used it to prepare flame-retardant EP/A-BP samples. The main groups detected in the reaction products were P–O–Ph, P–N, O–P–O, P–O–P, C–O, P–O, C=O, and P=O. Miao et al.[14]investigated the pyrolysis properties of EPs prepared using tri(o-phenylenediamino)cyclotriphosphazene as a flame retardant. The gaseous pyrolysis products included H2O, phenol, CO2, carbonyl compounds, aromatic ethers,hydrocarbons, and aromatic species. In order to detect the radicals and macromolecular fragments, especially those present in only low concentrations, molecular dynamics(MD) simulations incorporating the reaction force field(ReaxFF) were used to predict the radicals, intermediates,final products, and chemical reactions in the polymer pyrolysis process, which could provide a theoretical supplement to experimental studies[15].
ReaxFF employs a bond-order formalism to describe both reactive and non-reactive interactions between atoms. It has been used to accurately describe a diverse range of materials including those involving both covalent and electrostatic interactions[16], such as hydrocarbon compounds[17], ammonia borane[18], highenergy substances[19], metal oxide and transition-metal catalysts[20], and lithium–sulfur batteries[21]. Furthermore,ReaxFF has also been applied to achieve remarkable results in the field of polymer pyrolysis. For example,Zhao et al.[22]used the technique to explain the pyrolysis mechanism of polycarbonate and describe the mechanism of main-chain breakage and the formation of the main products based on the simulated trajectories, where the obtained findings were consistent with the experimental results. Diao et al.[23]utilized ReaxFF to examine the thermal decomposition characteristics of EPs, which revealed that decomposition was initiated by cleavage of the ether linkages. The main small-molecule products included H2O, CO, and H2. Zhang et al.[24]investigated the decomposition process of EPs cured by anhydride using ReaxFF. The production of CH2O was found to be dependent on the decomposition of the epoxide functional groups, while H2O was generated by free radical collision and dehydration. Li et al.[25]reported that the first pyrolysis stage of crosslinked EPs was initiated by the cleavage of the crosslinking bonds and ether bonds,followed by polycondensation and carbonization in the second stage. These studies demonstrate that ReaxFF is a reliable method for predicting the product or intermediate distributions and reaction processes of polymers.
In the present work, the pyrolysis process of EP composites at high temperatures was investigated by establishing realistic models of crosslinked EPs in the absence and presence of the flame retardant hexa(5-methyl-2-pyridinoxyl)cyclotriphosphazene (HMPOP).ReaxFF was used to study the initial reaction pathways,intermediate reactions, and main product distributions of the flame-retardant EP at the molecular level. The generated products were classified according to the number of carbon atoms, and the solid species (C40+)were compared with the experimental findings. The obtained results allowed the pyrolysis mechanism of the EP and the flame retardancy of the EP/HMPOP composite to be explained at the atomic level. This work is expected to prove valuable for future studies analyzing flame retardation mechanisms and the screening of new flameretardant materials.
2 Simulation Methods
Models of the crosslinked EP and flame-retardant EP/HMPOP composites were established. The commonly used bisphenol A diglycidyl ether (DGEBA) was selected as the monomer, and 3,3'-diaminodiphenyl sulfone (DDS) was used as the crosslinking agent. The molecular structures of DGEBA, DDS, and HMPOP are shown in Figure 1.

Figure 1 Molecular structures of DGEBA, DDS, and HMPOP
In this EP system, a three-dimensional network structure is formed by crosslinking reactions between the –NH2groups of DDS and the epoxide groups of DGEBA, as illustrated in Figure 2. Because each DDS molecule contains four active hydrogen atoms, the molar ratio of DGEBA to DDS was 2:1.

Figure 2 Crosslinking reactions of EPs
The crosslinking process of the EP was simulated according to the algorithm described in a previous work[26]. To facilitate the crosslinking procedure, three assumptions were made: (1) the primary and secondary amine hydrogens had the same reactivity, (2) etherification reactions were negligible, and (3) the reactions were diffusion controlled. On the basis of these assumptions, the following simulation procedures were employed:
Step 1: For DGEBA/DDS, an amorphous cell containing 30 DGEBA molecules and 15 DDS molecules was constructed based on the self-avoiding random walk method[27], as shown in path (a) of Figure 3. For DGEBA/DDS/HMPOP,five HMPOP molecules were added into the amorphous structure of DGEBA/DDS, as shown in path (b) of Figure 3. The initial density of DGEBA/DDS and DGEBA/DDS/HMPOP were set to 0.5 g/cm3. Annealing via an iterative procedure was then performed, beginning with an MD calculation first with the isothermal-isobaric (NPT)ensemble and then with the canonical (NVT) ensemble at a temperature of 600 K and a pressure of 0.1 MPa for 500 ps.In the subsequent iterations, the temperature was decreased by 50 K and the process was repeated until the specified temperature was 300 K.

Figure 3 Construction of the DGEBA/DDS and DGEBA/DDS/HMPOP models
Step 2: The two models were subjected to energy minimization for 10000 steps, followed by MD simulations with the NPT ensemble for 500 ps to compress and optimize the systems. The pressure and temperature were 0.1 MPa and 453 K, respectively. The time step was 1.0 fs, and the initial velocity was set to be random. Next, MD simulations with the NVT ensemble were performed for 500 ps at 300 K[28].
Step 3: The oxygen atoms in the epoxide groups of DGEBA and the nitrogen atoms of DDS were set as the reaction sites. When the distance between a pair of these reaction sites was within the reaction cutoff distance range of 3.0–10 Å, harmonic restraint was imposed on them to make them close to each other (Figure 4(a)). The DDS structure was simplified for the purposes of clarity. Then the C–O bonds in the epoxy ring broke (Figure 4(b)) to form C–N bonds (Figure 4(c)). The hydrogen atoms were adjusted and balanced (Figure 4(d)). The atom types were recalculated (Figure 4(e)) and the crosslinking tags were reidentified (Figure 4(f)), so that they did not participate in the subsequent site reactions. Finally, the crosslinked models were optimized (Figure 4(g)). The detailed crosslinking process is illustrated in Figure 4.

Figure 4 Crosslinking process for the EP and EP/HMPOP composites
Step 4: Another MD cycle with the NVT and NPT ensembles was performed to relieve any unfavorable interactions and compress the models after the formation of new bonds. The system was then checked for the possible occurrence of ring catenation or spearing during bond formation. When ring catenation or spearing had occurred, the new bonds were deleted from the system.
Step 5: Steps 2, 3, and 4 were repeated until there were no reaction sites within a reaction cutoff distance range of 3.0–10 Å. Next, annealing was performed from 600 to 300 K with an interval of 50 K. Finally, models of the crosslinked EP and EP/HMPOP with a crosslinking degree of 83.33% were obtained. The final densities of the two models were 1.14 and 1.16 g/cm3, respectively,which is in good agreement with the densities of 1.1–1.2 g/cm3reported in the literature for EPs[29–31].
ReaxFF simulations of the crosslinked EP and EP/HMPOP composites were conducted to analyze their pyrolysis products. Computational power limitations restrict the time scale of such simulations (picoseconds) to far less than the actual experimental time scale (seconds). As such,researchers routinely select higher simulation temperatures than those used experimentally to accelerate the reaction[32-33]. In this work, the simulations were performed for 50 ps with the NVT ensemble at temperatures ranging from 500 to 3500 K at 500 K intervals to better observe the chemical reactions on the simulation time scale. All of the simulations in this work were performed using the Reax package in the LAMMPS software.
The Berendsen thermostat with a damping constant of 10 fs was used for temperature control. Intermediates and products formed during the simulations were analyzed with a bond order cutoff of 0.3 to identify molecules.The two models were calculated through the reactive forcefield parameters from reference[34].
3 Results and Discussion
3.1 Analysis of pyrolysis kinetics
The major intermediates and final products obtained from the pyrolysis process were broadly classified as small gases(C0–C4), light liquid products (C5–C13), heavy liquid products(C14–C40), and solid species (C40+)[35–37]. Figure 5 presents the weight percentages of these products over the temperature range of 500–3500 K. Taking C40+ as an example, its weight percentage (WC40+) was obtained by Eq. (1):

Figure 5 Product distribution for EP and EP/HMPOP at various temperatures
whereidenotes a C40+ species,Wiis the molecular weight of speciesi,niis the number of molecules of speciesiat timet, andWis the total molecular weight for the EP or EP/HMPOP system.
The weight percentage of C0–C4products increased with increasing temperature for both systems, whereas the C40+ species displayed the opposite trend. Meanwhile, the weight percentages of C5–C13and C14–C40products were significantly lower for EP/HMPOP than for EP, whereas the weight percentage of C40+ species at temperatures exceeding 2000 K was markedly higher. At the highest temperatures of 3000 and 3500 K, more C40+ species remained than at 2500 K.
Figure 6 shows the temperature dependences of the weight percentages of C40+ species for EP and EP/HMPOP along with the corresponding fitting curves.With the addition of HMPOP, the weight percentages of C40+ species at 3500 K was markedly higher for the EP/HMPOP composite (37.66%) than for EP (7.85%).The weight percentage fitting curve for C40+ species was similar to the TG curve[38], and the final char yields of EP/HMPOP and EP in the TG curve were 31.46%and 7.60%, respectively. Thus, HMPOP increased the weight percentage of residual solid species in both the simulations and experiments. These results were in accordance with previous reports of flame-retardant EPs,where the cyclotriphosphazene decomposed prematurely to form a compact char layer that protected the matrix from fire[39]. Greater char formation limits the production of combustible carbon-containing gases and decreases the exothermicity owing to pyrolysis reactions, as well as reducing the thermal conductivity at the surface of the burning material[40].

Figure 6 Temperature dependences of the weight percentages of C40+ species for EP and EP/HMPOP
Li et al.[41]simulated the pyrolysis and combustion processes ofn-eicosane at 2000–3500 K and fitted the number evolution curves ofn-eicosane using the Arrhenius equation. Lu et al.[42]also studied the pyrolysis kinetics of polyimide by fitting the number of polymer molecules at 2800–3800 K. The obtained activation energies were in good agreement with experimental results. In this work, the weight percentage of C40+species at various temperatures (2000–3500 K) was used to analyze the kinetic parameters of the pyrolysis reaction,as shown in Figure 7. The pyrolysis performances of EP and EP/HMPOP were found to be similar, with the weight percentage of C40+ species decreasing more rapidly with increasing temperature. Thus, a higher temperature accelerated the decomposition of both EP and EP/HMPOP and more small fragments were generated. However, the curves for EP decreased more quickly than those for EP/HMPOP. Thus, the addition of HMPOP inhibited the pyrolysis of the EP.

Figure 7 Time dependences of the weight percentages of C40+ species for EP and EP/HMPOP at various temperatures
The simulation data were fitted into a kinetic model and the reaction rate coefficients were obtained. The pyrolysis activation energies (Ea) were determined using the Arrhenius equation. It was assumed that the decomposition reactions conformed to a first-order decay exponential model, as expressed in Eq. (2):
whereW0is the initial weight percentage of C40+ species in the EP or EP/HMPOP system,t0is the start time of decomposition,kis the effective reaction rate coefficient,andW(t) is the weight percentage of C40+ species at timet.After the exponential fitting, the initial pyrolysis reaction ratekcan be obtained.
Table 1 lists the reaction rate coefficientsk1andk2calculated for the EP and EP/HMPOP composites,respectively, over the temperature range of 2000–3500 K. Both reaction rate coefficients increased significantly with increasing temperature, indicating that a higher temperature shortened the time required for EP decomposition. Moreover, the coefficients were consistently higher for EP than for EP/HMPOP,demonstrating that the addition of the flame retardant HMPOP was beneficial for reducing the rate of EP decomposition.

Table 1 Pyrolysis reaction rate coefficients for EP and EP/HMPOP at various temperatures.
The activation energy is another important temperaturedependent parameter for analyzing reactions. In this case,the activation energy reflects the difficulty of the pyrolysis reaction[43]. The activation energy can be conveniently evaluated using the Arrhenius equation. Taking the logarithm of both sides of the Arrhenius equation affords Eq. (3):
whereTis the temperature,Ais the pre-exponential factor,Eais the activation energy, andRis the ideal gas constant. Therefore, the pre-exponential factorAand activation energyEaof the pyrolysis reaction can be obtained by linearly fitting the reaction ratekof the target model according to the relationship between lnkand 1000/T. The obtained fitting lines and parameters are shown in Figure 8.

Figure 8 Logarithmic fitting lines of reaction rate to temperature
From Figure 8, theEafor EP was 141.72 kJ/mol, which is in good agreement with the previously reported experimental values of 102.0–160.3 kJ/mol[44,45]. TheEafor EP/HMPOP was lower at 116.40 kJ/mol. The corresponding linear correlation coefficients were 0.98 and 0.99, respectively. Thus, the flame retardant HMPOP significantly decreased theEarequired for the thermal decomposition of the EP, thereby making HMPOP/EP easier to ignite. This lower energy requirement was ascribed to the initial decomposition of some of the HMPOP to form the char layer that blocked oxygen and heat. Thus, HMPOP reduced theEaof the EP decomposition reaction and accelerated the initial decomposition. Previous studies[6,8]have also reported that most phosphorus-containing flame retardants impart excellent flame retardancy to the EP thermoset at the expense of the initial decomposition temperature. These results demonstrated that the ReaxFF MD method was suitable for examining the decomposition process of the EP and its composites.
3.2 Analysis of pyrolysis fragments
The bond-breaking behavior and number of intermediate products were expected to govern the thermal pyrolysis reactions of the EP and EP/HMPOP composites, as well as the rate of subsequent reactions. The overall evolution of new species (including both intermediates and final products) for the EP and EP/HMPOP composites is plotted in Figure 9. The total number of species increased rapidly at the beginning of the simulations and then gradually stabilized. It was clear that the variety of pyrolysis products was enriched upon increasing the simulation temperature. The number of pyrolysis products was small in the temperature range of 500–1500 K and greatly increased in the range of 2000–3500 K. These results indicate that the temperature exerted a marked influence on the pyrolysis products. The curves for EP/HMPOP displayed more pronounced fluctuations than those for EP, especially in the later stages of the reaction.This led us to the conclusion that more bonding and decomposition processes occurred after the addition of HMPOP.

Figure 9 Evolution of total species number for EP and EP/HMPOP at various temperatures
The evolution of small-molecule gaseous (C0–C4) products and solid (C40+) species during the pyrolysis reaction was further analyzed, as shown in Figure 10. From Figures 10(a) and 10(b), the weight percentage of C0–C4products increased with increasing temperature. After a short period, the weight percentage of C0–C4products did not further increase for the temperature range of 500–1500 K, which was primarily because the simulation time was not sufficiently long to achieve complete pyrolysis. At temperatures of 2000 K and above, the formation rate of small-molecule gaseous products was higher, and the weight percentage reached a state of relative equilibrium.EP and EP/HMPOP displayed similar trends, although the addition of HMPOP led to a lower weight percentage of C0–C4products at a given temperature. Accordingly,the generation rate of C0–C4products was relatively slow for EP/HMPOP. Because most C0–C4gases are readily flammable, the addition of HMPOP mitigated heat generation and the further decomposition of the EP.
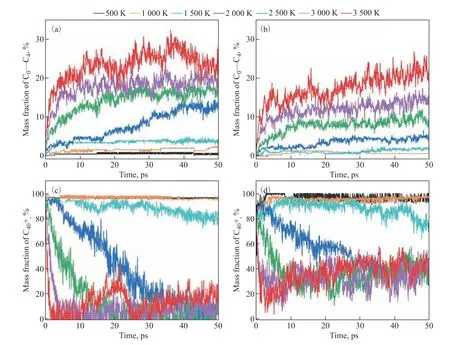
Figure 10 Evolution of weight percentage of C0-C4 products for (a) EP and (b) EP/HMPOP and weight percentage of C40+species for (c) EP and (d) EP/HMPOP
Figures 10(c) and 10(d) show the variation of the weight percentage of C40+ species during the pyrolysis process. In stark contrast to the results observed for C0–C4products, the weight percentage of C40+ species initially decreased. The pyrolysis rate of the C40+ species increased with increasing temperature from 2000 to 3500 K, and the weight percentage of C40+ species eventually reached a state of relative equilibrium. For EP at 3500 K, the curve for the C40+ species drastically fluctuated,indicating that some of the char residues formed from smaller fragments broke down again. Overall, 50 ps was sufficient for analyzing the pyrolysis of the EP and EP/HMPOP composites at 2000–3500 K. From 500 to 1500 K, however, the weight percentage of C40+ species remained almost unchanged, indicating that the extent of pyrolysis was minimal and a large number of residual solid molecules were still present. In contrast to EP,at higher temperatures, the final weight percentage of C40+ species in EP/HMPOP rapidly decreased and then gradually increased. These results confirmed that HMPOP played a positive role in the simulated pyrolysis process and increased the amount of carbon residue. This would be expected to hamper the spread of flames, thus reducing the risk of fire.
To more directly examine the variation of the number of C40+ species during the reaction process, the average number of carbon atoms in the C40+ species derived from the EP and EP/HMPOP composites was calculated at temperatures of 2000 and 3000 K, as presented in Figure 11. The average number of carbon atoms initially decreased rapidly for both systems. Notably, the curves for EP gradually flattened out over the course of the simulations. By contrast, the curves for EP/HMPOP reached minimum values after the initial decline then displayed an upward rebound as the simulations proceeded.

Figure 11 Average number of carbon atoms in the C40+species for EP and EP/HMPOP (the shaded regions indicate the deviation of the statistical results)
In particular, fragments containing more than 400 carbon atoms were generated in the later stage of the simulations,indicating the formation of large carbon clusters. Moreover,increasing the temperature accelerated the pyrolysis rate for both EP and EP/HMPOP, although the curve for the former appeared less affected by the temperature change.The higher temperature of 3000 K was also conducive to the formation of larger char fragments in the EP/HMPOP system. Yang et al.[8]also reported that polyphosphonitrile compounds accelerated the decomposition of EPs at lower temperatures but formed a stable carbon layer that acted as a good flame retardant.
3.3 Pyrolysis processes of EP and EP/HMPOP
3.3.1 Pyrolysis process of EP
The EP model comprised a three-dimensional intersecting network structure. Representative segment models with major functional groups were extracted to describe the pyrolysis process of EP, as depicted in Figure 12. Four major decomposition modes were observed during the initial pyrolysis reactions of the EP.

Figure 12 Major pyrolytic initiation reactions observed for the EP
The oxygen bridging bonds (C–O) and nitrogen bridging bonds (C–N) were easily broken (P1, P2, and P3)because of their lower bond energies[46]. The C–O bonds in Figure 12 are connected to different groups (labeled with ①, ②, ③,and ④), such that they possess different bond dissociation energies. According to Hückel’s rule,fragments with aromatic structures exhibit superior thermal stability to those without aromatic structures,necessitating a higher activation energy to cleave the C–O bond. Thus, the C–O bonds in the ② and ③ position were more difficult to break, favoring cleavage of the C–O bonds in the ① and ④ position during EP decomposition(P2 and P3). Moreover, CH3· radicals generated by the P4 pathway accounted for a small proportion of the observed products. The resulting small-molecule radicals would continue to participate in the reaction and trigger the subsequent pyrolysis reactions of the EP chain.
In the following chain reaction, the formation of some important radicals and final products was observed,as shown in Figure 13. Some irrelevant products were eliminated to avoid overlap the target groups and species.Atoms colored gray, white, red, blue, yellow, and pink correspond to carbon, hydrogen, oxygen, nitrogen, sulfur,and phosphorus, respectively. In the pyrolysis reaction, the evolution of the typical functional groups in Figure 13(a)(corresponding to the representative unbroken structure in Figure 12) was monitored from snapshots taken at various simulation times. The methyl group of EP was generated through the P4 of Figure 12 at 1.10 ps (Figure 13(b)), and some H· reacted with the OH· to produce water at 1.20 ps(Figure 13(c)). SO2molecules were also appeared at 1.28 ps (Figure 13(d)). Additionally, at 1.75–1.82 ps, ethylene and formaldehyde were observed in Figure 13(e), and then CO molecules were created by the decomposition of formyl groups at 1.82–4.65 ps (Figure 13(f, g)). The C–N bond broke through P1 pathway to generate –NH2group in the subsequent reaction (Figure 13(h)). The specific formation mechanism is described in Section 3.4.2.
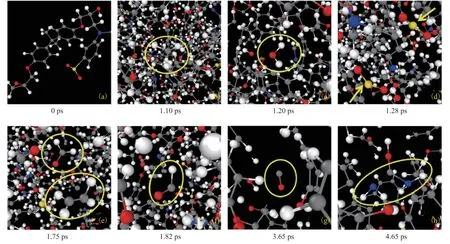
Figure 13 Formation of important products observed in the EP crosslinked network
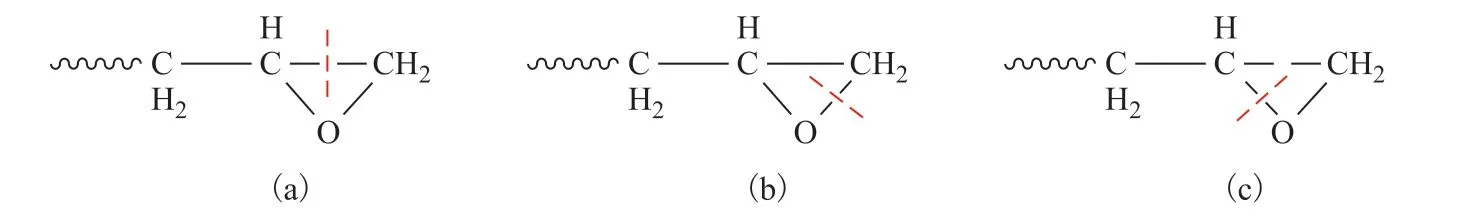
Figure 14 Schematic illustrations of epoxide ring-opening modes

Figure 15 Snapshots showing the simulated trajectories for the three epoxide ring-opening modes
Furthermore, three ring-opening modes were observed for the epoxide group during the reaction process, as shown in Figures 14 and 15. The first of these was cleavage of the C–C bond (Figures 14(a), 15(a), and 15(b)), which was mainly attributable to the difference in electronegativity between carbon and oxygen atoms.The electrophilicity of epoxide carbon atoms makes them susceptible to reactions with nucleophiles. In addition,the two C–O bonds were usually broken by the attack of H· (Figures 14(b), 14(c), and 15(c)-15(f)), which is a common ring-opening mode for epoxide groups.
3.3.2 Pyrolysis process of EP/HMPOP
For a comprehensive analysis of the influence of HMPOP on the flame retardancy of EPs, it was crucial to study the pyrolysis behavior of EP/HMPOP. Thus,the entire HMPOP molecules were labeled to track their decomposition process in the simulated EP/HMPOP system. The typical products observed during the pyrolysis process are shown in Figure 16. The 5-methyl-2-pyridinoxyl moieties of the cyclotriphosphazene ring were first lost by cleavage of the P-O bonds. Furthermore,a ring-opening reaction occurred in the pyridine moieties because of the lower energy of the C–N bond, resulting in the generation of more stable –NH or –NH2groups(Figure 16(a)). The cleaved pyridine rings then reacted with H· in the pyrolysis process to form OH·. This captured some of H· generating from the pyrolysis of EP to inhibit the pyrolysis reaction (Figure 16(b)). The cyclotriphosphazene ring remained stable for some time,but it eventually opened with cleavage of the N–P bonds.The P· gradually contacted oxygen-containing fragments,which mainly originated from the aromatic ether linkages in EP. Eventually, these oxygen fragments bonded with phosphorus to form a PO· fragment structure, such as PO2·, PO3·, and PO4·, et al. These then reacted with other intermediates to generate larger fragments PxOyR(where R denotes an organic component) (Figure 16(c)),which is the main reason why cyclotriphosphazenebased flame retardants promote carbon char formation.In a previous work[13], combustion fragments containing P–O–Ph, P–N, O–P–O, P–O–P, C–O, P–O, C=O, and P=O bonds were detected in a flame-retardant EP based on a phosphonitrile derivative. Cheng et al.[47]also reported that the heating of EP composites incorporating phosphorus compounds led to the generation of phosphorus-containing acids that facilitated the formation of char residuals.

Figure 16 Main product fragments of HMPOP pyrolysis
3.4 Analysis of main products
3.4.1Effects of temperature and flame retardant on the EP
The pyrolysis product with the longest reaction lifetime and highest production was defined as the main product of the reaction. At various pyrolysis temperatures, the model system gradually decomposed from macromolecular fragments to smaller fragments. Among them, H2O,CO, formaldehyde (CH2O), and ethylene (C2H4) were the main products with the highest production at different temperatures, which has also been observed experimentally[46]. The weight percentages of these four main products with respect to temperature are plotted in Figure 17.

Figure 17 The main products distribution at various temperatures for (a) EP and (b) EP/HMPOP
The weight percentages of H2O, CO, and C2H4showed an increasing trend with temperature, while the amount of CH2O reached maximum at 2000 K for EP. This was mainly because the high temperature was more conducive to the formation of CO rather than CH2O for EP. The amount of CH2O consistently increased for EP/HMPOP. The generation and consumption pathways of these products are described in Section 3.4.2. CH2O and C2H4were formed at relatively low temperatures by the decomposition of the acrolein (C3H4O) or vinyl methyl ether radical (C3H5O·), which was in accordance with a previous work[23]. After the addition of HMPOP,the main products formed were similar, although the weight percentage of CH2O at a given temperature was significantly lower, which is beneficial in terms of minimizing harmful gas emissions. The production of C2H4was significantly increased at 3000 K, and unsaturated hydrocarbon bonds are an important precursor for the further generation of polycyclic aromatic hydrocarbons (a key component of soot)[48].
The time evolution of each of the key combustible gases (CH2O, CO, and C2H4) for EP and EP/HMPOP is presented in Figure 18. Higher temperatures were found to promote the generation of these components. The weight percentages of C2H4and CH2O were lower for EP/HMPOP than for EP. Almost no C2H4was generated below 2500 K for EP/HMPOP, whereas this product was observed at 2000 K for EP. In addition, CH2O started to be generated at 1500 K for EP/HMPOP, while it was produced at 1000 K for EP. The weight percentage of CO was also slightly elevated for EP/HMPOP. High temperatures promoted the conversion of CH2O to CO.Overall, HMPOP reduced the production of the key combustible gases and increased the temperature required for their generation.

Figure 18 Time evolution of (a) C2H4, (b) CH2O, and (c) CO for EP and (d) C2H4, (e) CH2O, and (f) CO for EP/HMPOP
3.4.2 Generation pathways of main products
Following the analysis of the weight percentages of the main products, we explored their formation mechanisms at the atomic scale. The formation pathways of the main products are depicted in Figure 19. The main smallmolecule gases in the EP decomposition products were CH2O, C2H4, H2O, and CO. CH2O and C2H4were primarily derived from C3fragments produced by the pyrolysis of the EP. As previously stated, the pyrolysis of the EP generated acrolein (C3H4O) or vinyl methyl ether radical (C3H5O·) intermediate product, which continued to decompose into ethylene and CH2O (Figure 19(a)). The removal of hydroxy groups and the collision of hydroxy groups with hydrogen radicals were the main pathways to form water (Figure 19(b)).

Figure 19 Main formation mechanisms for the major products (a) CH2O, C2H4, (b) H2O, (c) CO, and (d) CH4
By tracing the oxygen atoms in the CO molecules, we found that the CO generation pathway undoubtedly involved decarbonylation reactions of the carbonyl structure. Part of the CO also generated from the hydrocarbon containing the hydroxy groups and then aldehyde compound was produced by oxidation reaction.Finally, CO was generated by a decarbonylation reaction (Figure 19(c)). CH4was also identified in the main products obtained at 3000 K, indicating that the generation of CH4required higher energy. Most of the CH4molecules in the products were directly derived from a demethylation reaction. On the other hand, CH3·captured H· to generate CH4(Figure 19(d)).
3.5 Mechanism of flame retardation by HMPOP
On the basis of the simulation results and analysis,the flame retardation mechanism of EP/HMPOP was inferred as depicted in Figure 20. In the initial stage of the reaction, HMPOP reduced the activation energy of the EP and caused the premature decomposition of EP/HMPOP. The flame retardant also captured various radicals such as H· at high temperatures to inhibit further decomposition. The cleavage of the N–P bonds in the phosphazene ring also enabled the resulting P· groups to combine with oxygen-containing aromatic fragments to form PO· structures. These structures were further converted into phosphate derivatives (PxOyR), which facilitated the combination of small-molecule fragments into carbon clusters and ultimately larger thermally stable char structures. These all inhibited the transfer of heat and combustible volatile gaseous products to a certain extent and contributed to improving the thermal stability of EP/HMPOP. Meanwhile, the generation of the harmful gas CH2O was significantly reduced.
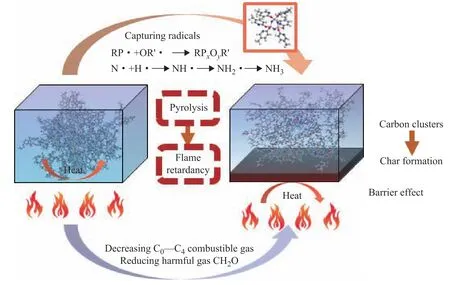
Figure 20 Flame retardation mechanism of EP/HMPOP
4 Conclusion
The pyrolysis processes of EP and EP/HMPOP composites were examined at various temperatures (500–3500 K)using the ReaxFF MD method, with consideration of the initial reaction pathways, main products, and pyrolysis and flame retardation mechanisms. The flame retardant HMPOP accelerated the initial pyrolysis of the EP and increased the weight percentage of carbon residues.The activation energies for EP and EP/HMPOP were 141.72 and 116.40 kJ/mol, respectively, indicating that the pyrolysis of EP/HMPOP was more likely to occur at relatively low temperatures, which is consistent with previously reported results. The decomposition of the EP was initiated by the breaking of bridging bonds containing nitrogen or oxygen. During the pyrolysis process, the EP released small-molecule gases such as H2O, CO, CH2O,and C2H4. For the EP/HMPOP composite, the product composition was similar to that observed for EP, although the weight distribution of C0–C4products, especially CH2O, clearly decreased, thus reducing the production of these combustible gases. Interestingly, simulation snapshots showed that the HMPOP molecules were not involved in the initial decomposition of the EP. However,phosphorus- and nitrogen-containing radicals derived from HMPOP captured some free radicals such as H· and oxygen-containing fragments during decomposition to restrict the further degradation of the EP.
Acknowledgments:This work was supported by the National Natural Science Foundation of China (51901209).
- 中国炼油与石油化工的其它文章
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations

