C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
Li Xiuping; Wei Yuanyuan; Liu Xiaoyi; Zhao Rongxiang
(1. School of Petrochemical Engineering, Liaoning Petrochemical University, Fushun 113001, China 2. Daqing Petrochemical Research Center of PetroChina, Daqing 163714, China)
Abstract: A C9H10O2:0.5ZnCl2/SG catalyst was synthesized using a one-step sol-gel method with silica gel (SG) as the carrier and C9H10O2:0.5ZnCl2 deep eutectic solvent (DES) as active component. The structure of the supported catalyst was characterized by FT-IR, XRD, SEM, and N2 adsorption-desorption, and the DES was found to have successfully permeated the SG through its pores. The removal of dibenzothiophene (DBT) in model diesel was studied using C9H10O2:0.5ZnCl2/SG as a catalyst and H2O2 as an oxidant. The influence of loading dose of DES, reaction temperature, catalyst dosage, O/S molar ratio, and sulfide type on the desulfurization rate was investigated. The removal rates of DBT, 4,6-dimethyldibenzothiophene(4, 6-DMDBT), and benzothiophene (BT) under optimal reaction conditions were 99.4%, 96%, and 78.2%, respectively.C9H10O2:0.5ZnCl2/SG catalyst could be recycled five times with a little decrease of oxidative desulfurization activity, and the adsorption-oxidation desulfurization mechanism was examined.
Key words: C9H10O2:0.5ZnCl2/SG; sol-gel; oxidative desulfurization; dibenzothiophene; DFT calculation
1 Introduction
SOxis produced during the burning of sulfur compounds in fossil fuels, causing many environmental and industrial problems, such as acid rain and smog. Many countries, therefore, have introduced laws to limit the sulfur content in such fuels[1], meaning that clear motivation exists to develop ultra-clean fuel. According to European and American regulations, the sulfur content of fuel must not exceed 10 µg/g[2]. At present, the most common desulfurization method in industry is the hydrodesulfurization (HDS) method. However, HDS,which removes aromatic sulfur compounds, requires high pressure and temperature and is expensive[3-4]. Oxidative desulfurization (ODS) is a new method of desulfurization and has been researched extensively due to its mild reaction conditions and impressive desulfurization performance. In ODS, thiophene compounds are readily oxidized to sulfone compounds under catalytic oxidation conditions, and then extracted from the oil phase. ODS,therefore, makes up for the shortcomings of HDS and can even replace HDS in industrial applications[5-7].
DESs have gained in popularity as inexpensive and biofriendly alternatives to ionic liquids. It combines two or three components through hydrogen bond interactions.DESs have physical and chemical properties similar to those of traditional ionic liquids (ILS), such as low toxicity and strong biodegradability[8]. In recent years,DESs have been widely used in catalysis[9], extraction[10],separation[11], synthesis[12], and electrochemistry[13]. DESs can also be used as an extractant in the desulfurization of fuel oil[14]. FeCl3-based DESs were synthesized, as an extractant, by Gano et al.[15], and the removal rates of dibenzothiophene (DBT) and benzothiophene (BT)were 64% and 44%, respectively. Li et al.[16]synthesized carboxylic-acid-based DESs for extractive desulfurization.The desulfurization rates of DBT and BT were 80.47%and 72%, respectively. Various researchers have applied DESs in ODS. Liu et al.[17]synthesized [EMIM]DEP/2C3H4O4DESs for ODS and showed experimentally that the removal rate of DBT was 98.2%. ChCl/p-TsOH DESs were synthesized by Dai et al.[18]to remove sulfur from model oil, and the removal rate of DBT was as high as 89%. However, in most cases, the DES used is a viscous liquid, which is only recycled with difficulty after the reaction. In order to solve this problem, our group has loaded DESs as active components on a suitable carrier.It not only improves the activity of the catalyst, but also solves the environmental problem of catalyst recycling.
In recent years, supported ionic liquid catalysts have been widely used in ODS, for example [Omim][HSO4]/Silica Gel[19], NMP·FeCl3/γ-Al2O3[20], IL-3DOMSiO2[21], IL/G-h-BN[22], [pmim]FeCl4-SBA-15[23], [C4mim]3PW12O40/SiO2[24], [Bmim]FeCl4/Silica Gel[25], [Omim]FeCl4/Silica Gel[26], and [PrSO3HMIm]HSO4@MIL-100(Fe)[27]. There is no doubt that these ionic liquid-supported catalysts exhibit high catalytic activity. However, their preparation is complex. Compared with ionic liquids, deep eutectic solvents have significant advantages in terms of ease of preparation and cost of production.
ILs or DESs can be loaded onto carriers with a large specific surface area by simple impregnation, grafting,polymerization, sol-gel, and encapsulation methods[28].Among these, the sol-gel method has attracted considerable attention due to its ease of preparation, low cost, and less loss of active components of the prepared catalyst. Active components play an important role in regulating the structure of silica gel[29]. The sol-gel method is beneficial to improve the catalytic performance of the supported catalyst[30].
Our group has studied the preparation of C9H10O2:XZnCl2DESs and their oxidative desulfurization performance[31].During desulfurization, the DESs plays a different role in the process of oxidative desulfurization, such as a catalyst and complexation extractant. In addition, the double acidity (Bronsted acidity and Lewis acidity) of DESs is conducive to oxidation. However, there are some problems,such as the high viscosity, difficult recycle of catalysts. In this study, a C9H10O2:0.5ZnCl2/SG catalyst was synthesized using the sol-gel method, with the C9H10O2:0.5ZnCl2DES as the active component and silica gel as the carrier. The removal rate of aromatic sulfide in model oil was studied using the C9H10O2:0.5ZnCl2/SG as a catalyst and adsorbent and H2O2as an oxidant. The optimal reaction conditions for ODS were investigated.Under optimal reaction conditions, a reaction kinetic analysis of the removal of different sulfides was carried out. The optimal structure of the interaction between different sulfides and DES was calculated by using the density functional theory. Finally, the recycling performance of the catalyst and the reaction mechanism were investigated.
2 Experimental
2.1 Materials
Benzothiophene (98%), tetraethyl orthosilicate (97%),dibenzothiophene (98%), 4,6-dimethyldibenzothiophene(98%), and 3-phenylpropionic acid (98%) were purchased from Aladdin Reagent Co., Ltd. Zinc chloride (98%),n-octane (98%), anhydrous ethanol (98%), and HCl (36%)were purchased from Sinopharm Chemical Reagent Co.,Ltd. H2O2(30%) was purchased from Liaoning Quanrui Reagent Co., Ltd.
2.2 Synthesis of C9H10O2:0.5ZnCl2/SG
C9H10O2:0.5ZnCl2DES was prepared in line with Ref.[31]. C9H10O2:0.5ZnCl2/SG catalysts were synthesized as follows (scheme 1). C9H10O2:0.5ZnCl2(0.17g) was placed in a 50 mL beaker along with ethanol (7 mL)and tetraethyl orthosilicate (10 mL) and the contents stirred at 60oC. After stirring for 10 min, concentrated hydrochloride acid (4 mL, 36%–38%) and distilled water(7.0 mL) were added. After 4 hours, the mixture began to gradually coagulate, finally producing a transparent colloidal material. The obtained solid material was dried at 130 °C for 4 h. The immobilized catalysts were denoted 6%-C9H10O2:0.5ZnCl2/SG. Using a similar process,12%-C9H10O2:0.5ZnCl2/SG and 18%-C9H10O2:0.5ZnCl2/SG were prepared.
12%-C9H10O2:0.5ZnCl2/SG (im) was prepared using an impregnation method: 0.208 g of C9H10O2:0.5ZnCl2, 20 mL of absolute ethanol, and 1.525 g of silica gel were mixed and stirred for 3 h in 50 mL beaker. Then the mixture was placed in a heating sleeve to remove the liquid ethanol, leaving the 12%-C9H10O2:0.5ZnCl2/SG(im).
2.3 Desulfurization

Scheme 1 Synthesis of C9H10O2:0.5ZnCl2/SG using the solgel method
Model oil containing 500 µg/g sulfur was prepared by dissolving 1.437 g DBT in 500 mLn-octane. The tests of catalytic oxidative desulfurization were carried out in a 50 mL glass reactor under stirring and condensation conditions. The specific process was as follows: a certain amount ofn%-C9H10O2:0.5ZnCl2/SG (n= 6, 12, and 18)and model oil was added to a glass reactor. Then, 30%H2O2was added to the reactor. The mixture was stirred vigorously at a constant temperature. The upper oil phase was sampled every 20 min and its sulfur concentration determined using a WK-2D micro coulomb analyzer. The sulfur removal was calculated according to the formula (1):
whereC0is the initial concentration of sulfides, µg/g; andCtis the sulfide concentration in the oil phase at timet,µg/g.
2.4 Characterization techniques
Fourier transform infrared spectroscopy (FT-IR) was performed using an FT-IR spectrophotometer (Nicolet iS50 FT-IR, Thermo Scientific). The crystallographic structure of the catalysts was determined by X-ray powder diffraction (XRD) with a TD-3500X-ray diffractometer(Dandong Tongda Science & Technology Co., Ltd.). The surface morphology of the catalyst was determined using scanning electron microscopy (SEM) (Gemini SEM 500,ZEISS, Germany). N2adsorption-desorption isotherms were recorded using a BELSORP-mini II High Precision Surface Area and Pore Size Analyzer (Micrometrics Instrument). The Brunauer-Emmett-Teller (BET) method was used to calculate the specific surface area The pore size distribution (PSD) was obtained using the Barrett-Joyner-Halenda (BJH) model from adsorption data. The DES content was determined using inductively coupled plasma optical emission spectrometry (ICP-OES)(ICP-5000, TITAN, Co., Ltd. China).
3 Results and Discussion
3.1 Characterization
3.1.1 FT-IR and ICP analysis
Figure 1 shows FT-IR spectra of silica gel andn%-C9H10O2:0.5ZnCl2/SG (n=6, 12, 18). The absorption peaks at 793 cm-1and 1074 cm-1of the samples are attributed to the stretching vibration of the Si-O bond in the silica gel[32]. The absorption peaks around 3500 cm-1and 1623 cm-1are attributed to the vibration of the Si-OH bond[33].The absorption peak ofn%-C9H10O2:0.5ZnCl2/SG (n= 6, 12, 18) at 1713 cm-1is attributed to the bending vibration of the carbonyl group (C=O) in the carboxylic acid fromn%-C9H10O2:0.5ZnCl2; the wide and scattered peaks at 2500–3200 cm-1are attributed to the stretching vibration of the O-H group in the carboxylic acid[31]; the absorption peak at 1467 cm-1is attributed to the C=C bond in the benzene ring of the phenylpropionic acid in the DESs; and the vibration at 697 cm-1is attributed to the C-H bond in the benzene ring. The DESs have a very strong absorption peak at 3200–3600 cm-1, which is attributed to the O-H bond of phenylpropionic acid molecules. Usually, the broadening the characteristic peak of hydroxyl groups means the formation of hydrogen bonds between two ligands[34]. In summary, the FTIR spectrum of C9H10O2:0.5ZnCl2/SG possesses the characteristic peaks of silica gel and C9H10O2:0.5ZnCl2.Therefore, the DESs travel into the pores of the silica gel. The DES concentration in the catalyst was measured using ICP-OES, the results of which are shown in Table 1. The experimental results for the DES concentration are basically consistent with the theoretical results.

Table 1 Concentration of DES in the catalyst
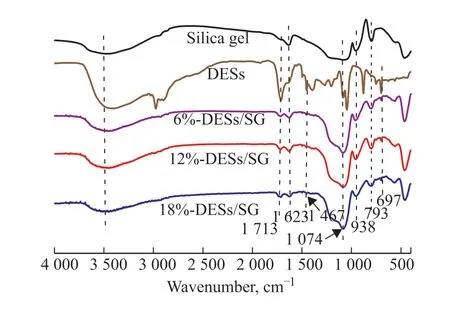
Figure 1 FT-IR spectra of silica gel and n%-C9H10O2:0.5ZnCl2/SG (n=6, 12, 18)
3.1.2 XRD analysis
Figure 2 shows the XRD spectrum of silica gel andn%-C9H10O2:0.5ZnCl2/SG (n=6, 12, 18). All curves indicate a relatively strong peak at around 2θ= 20°–30° due,probably, to the non-crystalline nature of silica[35].After immobilization of DES with different loadings on the SG, This characteristic peak at around 2θ= 20°–30° due was still observed. Therefore, the SG structure remained intact during sol-gel synthesis. In addition, an increase in DES loading from 6% to 18% decreased the intensity of the silica peak because of filling of the silica pores by the DES. In practice, The characteristic diffraction peak intensity of silica gel decreases with the increase of the amount of DES[26].
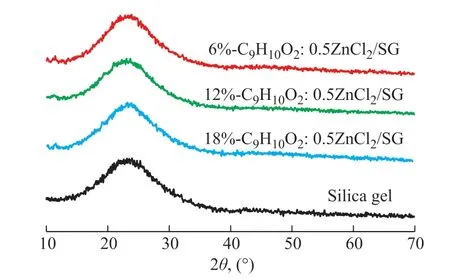
Figure 2 XRD spectra of silica gel and n%-C9H10O2:0.5ZnCl2/SG (n=6, 12, 18)
3.1.3 BET analysis
Table 2 shows the specific surface area (SBET), pore volume, and pore diameter of several samples. The SBETof silica gel decreases from 664 to 458 m2/g with increasing DES load, from 6% to 18%. With the increase of the DES dosage, the pore volume of the catalyst decreases and the pore size increases. The tendency of this variation is attributed to micropores being filled by DESs[36]. Typical N2adsorption-desorption isotherms are displayed in Figure 3(a). Several samples exhibit an obvious hysteresis ring between 0.38 and 0.75. The hysteresis ring belongs to the fourth type of adsorption isotherm curve, and there are mesopore structures in several of the samples.BJH pore size distribution plots are shown in Figure 4(b). The sample has mesoporous structure, but it also possesses a small number of micropores. Notably, the samples prepared by impregnation had the smallest surface area and smallest pore size. Compared to the impregnation process, the sol-gel method was more suitable for the preparation of DES-loaded catalysts.
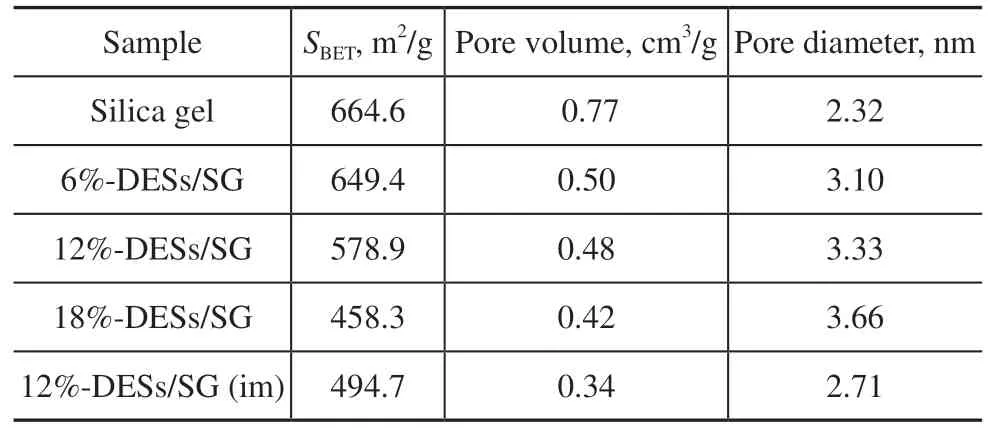
Table 2 Specific surface area and pore structure of samples

Figure 3 (a)N2 adsorption-desorption isotherms ; (b) BJH pore size distribution curves of sample

Figure 4 SEM images of (a) silica gel, (b) 12%-C9H10O2:0.5ZnCl2/SG, and (c) EDS of 12%-C9H10O2:0.5ZnCl2/SG
3.1.4 SEM and EDS analysis
The morphology of silica gel and 12%-C9H10O2:0.5ZnCl2/SG was recorded using SEM. The silica gel has an irregular block structure and a smooth appearance [Figure 4(a)]. After DES loading, the catalyst maintains this silica gel structure. Several particles are gathered on the surface of the silica gel, making it rough. The morphology of the catalyst shows that the DESs have been successfully loaded in the silica gel. The elemental composition of the catalyst is shown in Figure 4(c), with five elements, C, O,Zn, Si, and Cl, indicating that DES has been successfully loaded onto the SG support.
3.2 Influence of different desulfurization systems on desulfurization rate
Using four different desulfurization systems, the effect of desulfurization system type on the DBT removal rate was studied, and the results are listed in Table 2.The desulfurization rate of 12%-C9H10O2:0.5FeCl3/SG is 44.4%, and the desulfurization rate of 12%-C9H10O2:0.5SnCl2/SG is 94.2%. The desulfurization performance of the 12%-C9H10O2:0.5ZnCl2/SG catalyst synthesized by the sol-gel method is the best (99.4%).This shows that the type of metal chloride used significantly influences the desulfurization rate. However,the desulfurization rate of the 12%-C9H10O2:0.5ZnCl2/SG(im) catalyst is only 53.4% because impregnation results in a smaller surface area and pore volume than the sol-gel method.
3.3 Influence of reaction conditions on desulfurization rate
The desulfurization performance of a catalyst with three different loading doses of C9H10O2:0.5ZnCl2was investigated, the results of which are shown in Figure 5(a). When the amount of C9H10O2:0.5ZnCl2loaded on SG increases from 6% to 12%, the DBT removal rate increases from 86% to 98.6%. When the loading dose of C9H10O2:0.5ZnCl2increases to 18%, the desulfurization rate is 93.4%. This is because too much active component C9H10O2:0.5ZnCl2enters the pores of the silica gel, resulting in smaller specific surface area of C9H10O2:0.5ZnCl2/SG, fewer active sites, and weaker adsorptivity. These all reduce the desulfurization activity of the catalysis[37]. We determined that the optimal loading of C9H10O2:0.5ZnCl2DESs is 12%.

Figure 5 (a) Influence of DESs loading on desulfurization rate at m(cat.)/m(oil)=0.06, O/S=6, T=70 °C; (b) Influence of reaction temperature on desulfurization rate at m(cat.)/m(oil)=0.06, O/S=6; (c) Influence of m(cat.)/m(oil) ratio on desulfurization rate at O/S=6, T=70 °C; (d) Influence of O/S molar ratio on desulfurization rate at m(cat.)/m(oil)=0.06, T=70 °C
The reaction temperature greatly influenced the reaction.Figure 5(b) shows the effect of reaction temperature on the desulfurization rate of DBT. The desulfurization rate increases from 75.6% at 50 °C to 99.4% at 70 °C in 200 min. From the perspective of chemical reaction kinetics, raising the reaction temperature speeds up the diffusion of molecules and increases the efficiency of molecular collision, thereby promoting catalytic activity. However, when the reaction temperature is 80 °C, the desulfurization rate decreases to 98%. A reaction temperature threshold exists for an optimal desulfurization rate. This is because the oxidant in this experiment was hydrogen peroxide, which decomposes at high temperature, decreasing oxidation of sulfide[38].The desulfurization rate was investigated form(cat.)/m(oil) ratios (catalyst-to-oil mass ratio) of 0.02, 0.04,0.06, and 0.08, the results of which are shown in Figure 5(c). When them(cat.)/m(oil) ratio increases from 0.02 to 0.06, the sulfur removal rate increases from 74.8% to 99.4%. This is because as the amount of catalyst increases, the number of active sites also increases[39]. When them(cat.)/m(oil) ratio is 0.08, the desulfurization rate decreases from 99.4% to 93.2%.Excessive catalysis can easily lead to agglomeration of the active component, limits catalyst contact with DBT,and affects the diffusion of reactants and products[40].In addition, too much catalysis leads to an increase in the decomposition of H2O2, causing a decrease in the desulfurization rate in ODS[36]. In summary, the bestm(cat.)/m(oil) ratio is 0.06.
The effect of the O/S ratio on the performance of oxidative desulfurization was investigated. Figure 5(d)shows that the desulfurization rate does not change much when the O/S molar ratio increases from 2 to 4. However,when O/S is increased to 6, the removal rate of sulfur rapidly increases to 99.4%. When O/S is lower than 6, the effective collision probability between the oxidant and the reactant is low, resulting in a low removal rate of DBT.With increasing H2O2amount, H2O2comes into contact and reacts more easily with DBT, significantly increasing the efficiency of ODS[41]. When the O/S ratio is 8, the desulfurization rate drops to 97.3%. This is because there are two competing reactions in the ODS system: DBT was oxidized to DBTO2by H2O2, and H2O2decomposed[42].
Excessive H2O2increases the adsorption of H2O on the surface of the catalyst[43], resulting in a reduction in the effective specific surface area of the catalyst. This, in turn, inhibits the oxidative desulfurization reaction. In summary, O/S = 6 is selected as the best oxygen-sulfur ratio.
3.4 Removal of different sulfur compounds
Fuel contains many sulfur-containing compounds;therefore, the removal of different sulfides was studied. Simulated oils with an S-content of 500 µg/g for BT, 4,6-DMDBT, and DBT, were prepared. The desulfurization rates of model oil containing different sulfides under optimal reaction conditions are shown in Figure 6(a). Desulfurization rate is listed in the order:BT < 4,6-DMDBT < DBT. According to Ref.[44], sulfur compounds with higher electron cloud densities are more easily removed. The electron cloud densities of DBT, 4,6-DMDBT, and BT are 5.758, 5.760, and 5.739,respectively[45]. BT has the smallest electron cloud density and so is the most difficult to remove. Compared to other sulfides, 4,6-DMDBT has a higher electron cloud density,it is affected by steric hindrance (it contains two methyl groups), which increases the difficulty of desulfurization.The reaction rate of ODS follows the first-order reaction kinetic equation[46]:

Figure 6 (a) Removal efficiency of different sulfides at m(cat.)/m(oil)=0.06, T=70 °C, O/S=6 ; (b) Kinetic analysis of the removal of different sulfides in ODS
wherekis the first-order reaction kinetic constant, min-1;tis the reaction time, min;C0is the initial concentration of sulfides (µg/g); andCtis the sulfide concentration (µg/g)in the oil phase at timet.
Simulation results for the first-order reaction kinetics of the 12%-C9H10O2:0.5ZnCl2/SG oxidative desulfurization system for the removal of DBT, 4,6-DMDBT, and BT are shown in Figure 6(b). The attained correlative coefficient,R2, was 0.98589, 0.97186, and 0.97712, respectively,and they have a linear correlation. The larger the kinetic constantkof the first-order reaction, the faster the reaction rate and the better the effect of oxidative desulfurization(Figure 6). From the fitted values in the figure,kBT The Gaussian09 series program[47]is used to calculate the density functional theory (DFT), this has been frequently used for electronic structure calculations. The molecular structures of DESs:DBT, DESs:4,6-DMDBT,and DESs:BT were optimized using the B3lYP function under the basis set 6-311+G(d,p). The interaction energy between the active component C9H10O2:0.5ZnCl2and DBT, 4,6-DMDBT, and BT molecules was obtained. The results are shown in Figure 7. According to the density functional theory, the lower the binding energy, the more easily the molecules react with one another. That is to say, the oxidative desulfurization reaction was easy to carry out . The DFT calculation results validate the experimental results. Figure 7 Optimized structures of DESs with (a) DBT, (b) 4,6-DMDBT, and (c) BT After the oxidation desulfurization reaction, the lower catalytic phase was separated and washed three times using carbon tetrachloride. The recovered catalyst was dried in a drying oven at 80oC for 4 hours. A recovery experiment on the 12%-C9H10O2:0.5ZnCl2/SG catalyst was carried out under optimal reaction conditions. The removal rate of DBT is 94.3% after five cycles [Figure 8(a)], indicating that the catalyst has good stability. Two things lead to a decrease in catalytic activity: the first is a loss of active components[48]; the second is silica gel pores being blocked by oxidized sulfones, which affects the adsorption of DBT due to reduced specific surface area[49]. The surface area of the recovered catalyst and the DES concentration are listed in Table 3. The surface area and the DES concentration both show a decreasing trend. The stability of the recovered catalyst is shown in Figure 8(b). The FT-IR spectrum shows that the recovered catalyst retains its major characteristic peaks,indicating that the 12%-C9H10O2:0.5ZnCl2/SG catalyst has good stability. Table 3 Influence of desulfurization system on DBT removal Table 4 DES concentration and specific surface area of 12%-DES/SG before and after reaction Figure 8 Recycling and regeneration of the 12%-C9H10O2:0.5ZnCl2/SG. (a) The Desulfurization performance of regenerated 12%-C9H10O2:0.5ZnCl2/SG at m(cat.)/m(oil)=0.06, T=70°C,O/S=6; (b) The FTIR spectrum of fresh and recovered 12%-C9H10O2:0.5ZnCl2/SG After the ODS reaction, the product was back-extracted using CCl4solution[31]and subjected to FT-IR analysis.The FT-IR spectrum in Figure 9(a) shows the obvious characteristic peaks of dibenzothiophene sulfone at 1292 cm-1and 1166 cm-1. In order to investigate the mechanism of oxidative desorption, a free-radical capture experimentwas designed.P-benzoquinone and isopropanol were added to the ODS system as free radical quenchers of[·OH] and [·O2-], respectively. The DBT model oil was desulfurized under optimal conditions. The results are shown in Figure 10(b). After adding isopropanol, the desulfurization rate was 89.2% in 200 min, while the desulfurization rate after addingp-benzoquinone is 62.8%(Figure 9(b)). The reaction simultaneously generates superoxide radicals [·O2-] and hydroxyl radicals [·OH].Moreover, superoxide radicals play a dominant role in the oxidative desulfurization process. Figure 9 (a) FT-IR spectrum of oxidation products and (b) free-radical capture experiment Figure 10 Desulfurization mechanism The extraction and oxidative desulfurization of DBT are shown in Figure 10. The interaction between the C9H10O2:0.5ZnCl2DES and DBT occurs in two ways: ππ conjugation and complexation. π-π conjugation and complexation between DBT and DESs result in DBT molecules easily adsorbing to the catalyst[50]. Superoxide and hydroxyl radicals are then generated by the zinc ions in the C9H10O2:0.5ZnCl2/SG under the action of H2O2,and DBT is oxidized to DBTO2[51]. DBTO2is more easily adsorbed on the catalyst and then removed from the oil phase than DBT. A C9H10O2:0.5ZnCl2/SG catalyst was synthesized using a sol-gel method, with C9H10O2:0.5ZnCl2DES as the active component and silica gel as the carrier. The asprepared C9H10O2:0.5ZnCl2/SG was characterized by FT-IR, XRD, SEM, and N2adsorption-desorption.Compared with catalysts prepared using the traditional impregnation method, catalysts prepared using the sol-gel method showed high oxidative desulfurization activity.Using H2O2as the oxidant, and 12%-C9H10O2:0.5ZnCl2/SG as the catalyst and adsorbent, the removal rates were 99.4% for DBT, 96% for 4,6-DMDBT, and 78.2% for BT, respectively. Moreover, the removal rate of DBT remained above 94% after the catalyst was recycled five times. Under the action of the catalyst, superoxide radicals and hydroxyl radicals with higher activity were formed from hydrogen peroxide, which promotes the oxidation of sulfides. Acknowledgements:The authors acknowledge the financial support of the Natural Science Foundation of Liaoning Province(2019-ZD-0064) and the Doctoral Fund of Liaoning Province(201501105).
3.5 Recycling performance


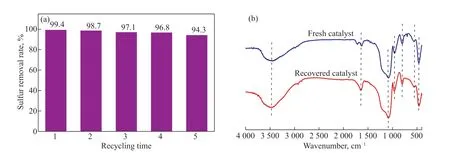
3.6 Mechanism of ODS


4 Conclusions
- 中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics

