Degradation of differently processed Mg-based implants leads to distinct foreign body reactions (FBRs) through dissimilar signaling pathways
Xoson Lu ,Guoqn Cn ,Xonxon Zon ,Tnn Wn ,Xoon H ,Wpn Yun ,Pnpn Zn,Yn Lu,Donmn Co,Su Cn,Kn-c Mnb,Zny Jn,Tsuyos Furusm,Dmon Knt,Yn Cn,∗,Guoyn N,,Mnyon Go,∗,H L,,∗
a Cancer Research Institute,First People’s Hospital of Foshan,Foshan,Guangdong 528000,China
b Department of Rheumatology,First People’s Hospital of Foshan,Foshan,Guangdong 528000,China
cThe Second Clinical Medical College of Jinan University,Shenzhen People’s Hospital,Shenzhen,Guangdong 510632,China
d Genecology Research Centre,University of the Sunshine Coast,Maroochydore BC,QLD 4558,Australia
e Department of Medical Imaging,First People’s Hospital of Foshan,Foshan,Guangdong 528000,China
fDepartment of Surgery,First People’s Hospital of Foshan,Foshan,Guangdong 528000,China
g Department of Mechanical Engineering,Graduate School of Science and Engineering,Tokyo Metropolitan University,1-1 Minami-osawa,Hachioji,Tokyo 192-0397,Japan
h School of Mechanical,Materials,Mechatronic and Biomedical Engineering,Faculty of Engineering and Information Sciences,University of Wollongong,Northfields Ave,Wollongong NSW 2522,Australia
i Institute of Industrial Science,Department of Mechanical and Biofunctional Systems,the University of Tokyo,4-6-1,Komaba,Meguro,Tokyo 153-8505,Japan
j School of Science,Technology and Engineering,University of the Sunshine Coast,Maroochydore BC,QLD 4558,Australia
Abstract Mg alloys have mechanical properties compatible with human bones.However,their rapid degradation and associated foreign body reactions in vivo significantly limit their application for human implants.In this study,three differently processed Mg alloys,pure Mg (PM),cold extruded Mg alloy AZ31 (CE AZ31),and fully annealed AZ31 Mg alloy (FA AZ31) were comparatively investigated for their potential as implants using a rat model.All three implanted Mg alloys do not show any impact on hepato-and renal function,nor any signs of observable changes to vital organs.Proteomics analysis of tissues directly contacting the implants 2.5 months post implantation revealed that FA AZ31 activates very few inflammation and immune associated signaling pathways;while the CE AZ31 and PM produce more significant inflammatory responses as confirmed by cytokine array analyses.Further,FA AZ31 activated pathways for cell organization and development that may improve the recovery of injured tissues.Structurally,EBSD analysis reveals that the FA AZ31 alloy has a higher ratio of first-order pyramidal orientated (10–11){10–1–2} grain texture with a value of 0.25,while PM and CE AZ31 alloys have lower ratios of first-order pyramidal orientated texture with the values of 0.16 and 0.17,respectively.This is associated with recovery and recrystallisation during annealing which promotes grain texture which exhibits enhanced degradation behaviours and induces a more limited immune response in vivo.In conclusion,the FA AZ31 demonstrated better biocompatibility and corrosion resistance and is a promising candidate for metal-based degradable implants which warrants further investigation.
Keywords: Toxicity test;Proteomics analysis;Signaling pathway;Biocompatibility and corrosion resistance;First-order pyramidal slip system;Recovery and recrystallization;Foreign body reactions (FBRs).
1.Introduction
As the lifespan of humans is increasing,aging populations present a challenge to healthcare systems worldwide.Fracture is an increasingly common public health issue among the aged due to accidents and osteoporosis [1].Currently,50% of all women and about 20%of men aged over 50 years suffer from fracture at least once [2–5].Traditional bone internal fixation materials such as titanium and stainless-steel alloys have substantially different elastic moduli and mechanical properties compared to that of human bone tissue [6,7].This causes“stress shielding” of the bone surrounding an implant,which can lead to degradation of the bone and potentially failure of the implant.Additionally,the release of metal ions increases the local pH,which can result in infection or inflammatory reactions [8,9].On the other hand,polymer implant materials have limited potential as bone substitute materials due to their inferior mechanical properties [10].Additionally,whether applied as either permanent or temporary implants,the above-mentioned materials often necessitate multiple operations which causes unpleasant hospital experiences and economic burden to patients [3].Thus,materials with optimal mechanical and biocompatible properties for internal fixation,repair,and replacement of bone tissue are in high demand.
The desired mechanical and corrosion properties for orthopedic applications include mechanical properties comparable to human cortical bone with yield strengths of at least 130–180 MPa [11] and an elastic modulus close to 10–30 GPa[12],strong osseointegration potential,high corrosion resistance and excellent wear resistance.Most importantly,biomaterials for implants should elicit no or only very limited adverse reactions with the surrounding tissues when placed into service,which means they must possess high biocompatibility.As the lightest metal on earth,magnesium (Mg) has a density and modulus very close to human bones [13–15],which thus effectively reduces stress shielding compared to traditional metallic implant materials.In addition,Mg and its alloys have high specific strength and appropriate stiffness to address the mechanical requirements for hard tissue implant materials [16].Mg,as an essential element for human health is vital to many physiological processes.An adult’s daily intake of Mg2+can reach 240–420 mg,which is more than 50 times that of Fe3+(8–18 mg) and Zn2+(8–11 mg) [17].About two of thirds Mg in the body is stored in the bones and muscles which is around 30 g in total [18–20].It participates in all metabolic processes including in protein synthesis,activation of different enzymes in the body,regulation of the activities of the central nervous system and muscles,functions of the intestinal,stomach and other organs.It also antagonizes calcium [21] and functions as a signal transmitter[22].However,rapid degradation rates in physiological environments limits the clinical application of Mg alloys as bone grafts or fixation materials.Therefore,substantial research has been conducted to improve the anticorrosion behaviors of Mg alloys using various techniques,including alloying [3,23–27],processing [3,28–32],and application of protective coatings[3,33–37].
Periprosthetic infection (PPI) is the other main issue that adversely affects the usage of metal-based implant materials.The implant surface is likely to accumulate bacteria,resulting in bacterial colonization and even forming a bacterial biofilm [3,34,38,and 39].To address this issue,clinical anti-PPI treatments must be conducted,including the use of antimicrobial treatments,surgical interventions,implant removal and replacement,all involving post-operative recovery during which patients are invariably subject to unpleasant physical and mental experiences as well as unexpected expenses [40,41].Recent research has shown that pure Mg has anti-bacterial activity bothinvitroandinvivo[15],though its rapid degradation can increase the local pH to abnormal levels resulting in adverse physiological effects including alkalosis,local inflammatory reactions,and even cell death[34].Therefore,there is a need to develop Mg alloy materials with relatively slow degradation rates and enhanced antibacterial properties.Recently,we have developed a method to immobilize an anti-microbial peptide onto fully annealed AZ31,which showed improved corrosion resistance and longlasting (up to 120 hrs) bacterial resistanceinvitro[3].It was postulated that the fully annealed microstructure may provide an optimized substrate for the peptide immobilized coating [3].
The relevance of Mg alloy microstructure (e.g.,texture,grain size etc.) with respect to the biocompatibility,bacterial resistance,and corrosion resistanceinvivoremains unclear.In this study,we fabricated implants using differently processed Mg materials (pure Mg,cold extruded AZ31 and annealed AZ31 alloys) and investigated their behaviorsinvitroandinvivo,including their microstructures,influence on the local microenvironment once implanted and the modulation of inflammation signaling pathways.We seek to further reveal the correlation between the microstructure of Mg alloys and their properties,which sets the foundation for the optimization of Mg alloy design and better selection of coating materials for Mg-based implants.
2.Materials and methods
2.1. Mg alloys and specimen preparation
Three different Mg alloys were used in this study,including pure Mg (PM),cold extruded Mg AZ31 alloy (CE AZ31)and a fully annealed Mg AZ31 alloy (FA AZ31).All three Mg alloys were obtained as rods with a diameter of 5.0 mm.The FA AZ31 samples were subjected to a full recrystallisation annealing heat treatment.They were heated to 330–350◦C in an inert gas atmosphere (Argon),kept for 3–5 hrs,and furnace cooled.Specimens were manufactured as small pins with the sizeφ0.5 × 2 mm (Fig.1A,Scale 20:1).Before theinvitroandinvivoexperiments,the alloy samples were polished with 400 grit silicon carbide paper for 1–3 min to remove the original oxide layer and then polished by 800–2400 grit silicon carbide paper for 2–5 min to improve the sample surface qualities and obtain homogeneous roughness.After each step of polishing and grinding,all the specimens were rotated 90°to ensure that the following grinding and polishing removed the scratches generated in the previous step.Finally,all the samples were ultrasonically cleaned in 70% ethanol at room temperature for 5 min.
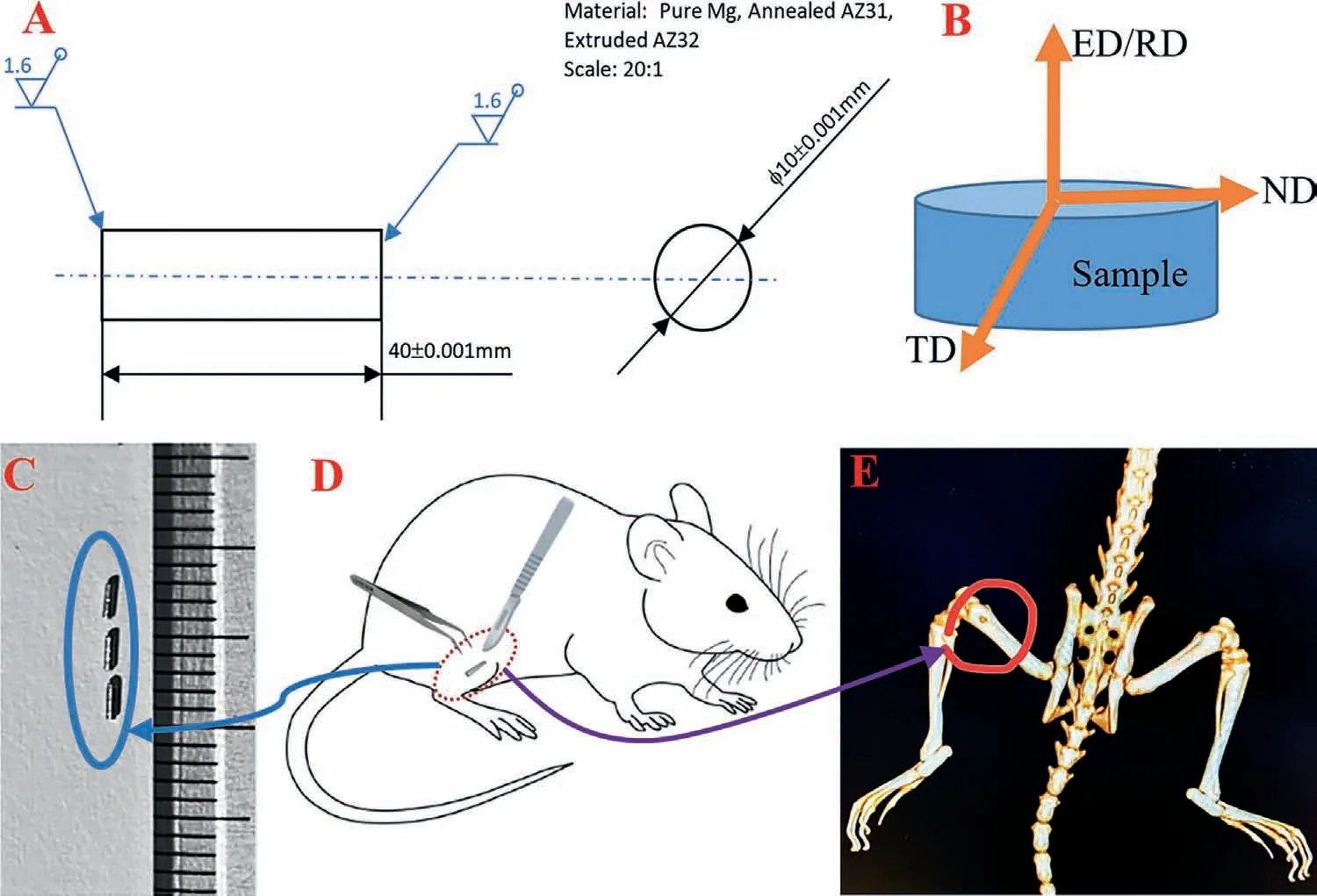
Fig.1.Mg’s EBSD test and implantation: (A) specimen size,(B) EBSD mapping position,(C) Mg pin,(D) SD rat,(E) CT image of Mg implantation in SD rat.
2.2. EBSD mapping of Mg and alloy specimens
The EBSD mapping of the Mg alloy microstructures were conducted on a JOEL JSM-7100F field emission gun scanning electron microscope (JOEL JSM-7100F FEG SEM) at the Institute of Industrial Sciences of the University of Tokyo with the following parameters.The scanning parameters for EBSD were an accelerating voltage of 15 kV and scanning step size of 0.5 μm.Other parameters and the EBSD sample preparation method is detailed in ref.[42].Fig.1B showed the EBSD mapping position and orientation.Analyses of the EBSD mapping results were conducted using orientation imaging microscopy (OIM) V7.0.
2.3. Micro hardness tests
The micro hardness tests were performed on the rods with a diameter of 5.0 mm and length of 1 m.All the rods were cut into thin plate with a thickness of 2 mm and roughness of 800.The micro hardness tests were conducted on an HMV-G micro-Vickers hardness tester with a load(HV0.01 98.07 mN)at the Institute of Industrial Sciences (IIS) of the University of Tokyo.
2.4. In vitro corrosion tests
Theinvitrocorrosion tests of the specimen were performed by a newly developedinvitrocorrosion test device,which was described in our previous study [3].Two properties,including pH of the mixture and weight change of the Mg specimen,were measured.
2.4.1.pHmeasurement
The corrosion of Mg alloys is usually accompanied with rising pH values [43].During theinvitrocorrosion test,Dulbecco’s Modified Eagle Medium (DMEM) culture media (Carlsbad,CA,USA) was used as the corrosion solution,which contains 4 mM L-glutamine,4500 mg/L glucose,1 mM sodium pyruvate,and 1500 mg/L sodium bicarbonate.The pH value was tested at different time points (0,24,48,72,96,120 h) using a Cyberscan pH510 Bench Meter.Prior to measuring the pH values,the meter was calibrated,and the detector was cleaned with pure water after each test.
2.4.2.Weightchange
Weight changes were measured at the designated time points during immersion [3].The samples were removed from their tubes,washed with deionized water and dried in a biosafety fume hood at room temperature.They were weighed using an electronic balance(Shimadzu electronic balance AUW220D) and returned to the respective tubes.Variations in sample weight were recorded and analyzed accordingly.
2.4.3.H2evolution
During the immersion,H2evolutions of different Mg samples were measured by a developed testing device,which includes a small flask and rubber tube and scaled chambers as shown in Fig.S1.50 mL Dulbecco’s Modified Eagle Medium(DMEM) culture media was employed as corrosion solution.
2.5. In vivo tests
2.5.1.Rats
Six to eight weeks old Sprague Dawley (SD) rats were purchased from the Animal Resource centre of Guangdong Province and kept at the Animal Facility of the Foshan First People’s Hospital,Guangdong,China.Experiments were approved by and performed in compliance with the guidelines of Animal Experimentation Ethics Committee (Ethics Approval Number: FAHGPU20160316).All rats were kept in Specific pathogen free (SPF) conditions on a 12-hr light/dark cycle at 22 °C with humidity of 75%.One rat was kept in each cage,provided with sterilised standard mouse food and water.Rats were sacrificed by CO2inhalation at the end of each experiment,confirmed by the cessation of breath and heart function[44].
2.5.2.Metalimplantsinratfemur
The ultrasonically cleaned specimens were further exposed to UV radiation for 30 mins on each side for sterilization[3,45].The implantation was conducted in the animal house of Foshan First People’s Hospital.Twelve 8-week-old male SPF SD Rats were weighed at 266.646 ± 43 g.Rats were randomly divided into four groups,including control (no implants),pure Mg (PM),cold extruded AZ31 (CE AZ31) and fully annealing AZ31 (FA AZ31) groups (Fig.1C).Rats were anesthetised by intraperitoneal (i.p.) injection of 1% sodium pentobarbital solution with a dose of 40 mg/kg.A sterile blade was used to cut about 1 cm perpendicular to the femoral shaft,then the subcutaneous tissue and muscle were separated until the femoral condyle of the rats was exposed.A grinding drill was used to drill a hole located at the lateral condyle of the rats’femur perpendicular to the longitudinal axis of the femur(Figs.1D and 1E).The control group was not embedded with any implant after drilling.The incision was sutured layer by layer with 4–0 absorbable sutures.
2.5.3.Degradationandbiocompatibilityofimplants
After 9 days’ implantation,the rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital at the dose of 40 mg/kg.Peripheral blood samples were collected by eye bleeding and then investigated for serum electrolyte,liver and kidney function.The rats were sacrificed after the peripheral blood was taken.The implants were removed from the femoral condyle,cleaned,dried and disinfected.After the attached soft tissue was completely removed,the implants were photographed to evaluate the degree of degradation.The organ tissues (including heart,liver,spleen,lung,kidney,brain,and testis) were collected and fixed with 10% formalin for hematoxylin-eosin (HE) staining.
2.5.4.SEM-EDStest
The Mg alloy specimens were removed from the SD rats after 9 days of implantation,then cleaned ultrasonically with 70% ethanol and rinsed in distilled water for 3–5 min.SEM-EDS analysis was conducted on Zeiss Sigma 300 Field Emission Electron Gun (FEG)-Scanning Electron Microscopy(SEM) with the following parameters: EHT is 3.00 KV,WD is 5.4 and Mag is 100×.
2.5.5.Magneticresonanceimaging
All three-dimensional computerized tomography (3D CT)and magnetic resonance imaging (MRI) were undertaken in the First People’s Hospital of Foshan.For the 3D CT,a clinical 64 slices CT system (Model: GE Discovery 64 role (GE Healthcare,Waukehsa,USA) was used,and for the MRI,a clinical 3.0T MR system (Model: GE Discovery 750 w (GE Healthcare,Waukehsa,USA) with a 4-channel receive and small animal RF coil (Model: WK602,SN: 1236,Magtron,Jiangyi,China) were used.This coil was set to the headimaging mode.All 3D CT and MR imaging were performed while rats were under an anesthetic state [46].The detailed methods for the CT and MRI are provided in Supplementary Methods.
2.5.6.Elementanalysisofimplantedbonetissues
Bone tissues from the metal implanted areas were collected and analysed using the Agilent ICP-MS 7700 (Agilent,Santa Clara,USA) in Foshan First People’s Hospital.Parameters used for the ICP-MS analysis are detailed in Table S1.Four metal elements were analyzed,including Mg,Ca,Al and P.The concentration of each element was calculated as follows:
where,mis the mass of the sample used for the analysis which was measured using an analytical balance.V0is the volume after the sample is digested.fis the dilution factor.C0is the concentration of the test solution element,C1is the element concentration of the original solution of the sample digestion solution,which is calculated by Eq.(2).Cxis the final test result of the measured element.The final test result of the measured element is expressed as a percentage,w,calculated by Eq.(3).
2.5.7.Serumtest
The blood electrolyte was measured at the Department of Pathology,Foshan First People’s Hospital using a VITROS 4600 Chemistry System (Johnson &Johnson,Raritan,USA).In addition,liver and kidney functions were evaluated at the Department of Pathology,Foshan First People’s Hospital using ADVIA 2400 Chemistry System (SIEMENS,Germany)
2.5.8.CytokineELISA
The cytokine ELISA kits for rat sera TNFα,IL-10,MCP-1 and IL-1βwere purchased (R&D system,Minneapolis,USA)and performed following the protocol provided by the manufacturer.
2.5.9.Cytokinearray
The detailed protocol for cytokine array was described elsewhere [47].Briefly,tissues adjunct to implants were sampled before implants were collected from the rats,and cytokine antibody arrays were processed using the Proteome Profiler Rat XL Cytokine Array Kit (Cat.No.ARY030;R&D Systems,Minneapolis,MN,USA).After preparation,the concentration of proteins was adjusted to 500 μg/mL.Cytokines and chemokines in these blots were detected digitally using a C-digit machine (LI-COR Biosciences,Cambridge,UK).Digital copies were quantified using Image J software (version 1.53,National Institutes of Health,Bethesda,MD,USA)[48].
2.5.10.MTTarray
Cell proliferation was determined with an MTT assay(Sigma,St.Louis,MO,USA) following the manufacturer’s instructions as described previously [49,50].Briefly,5 × 103TC-1 or HeLa cells were cultured in flat-bottomed 96-well plates.Approximately 0–15 μg of different peptides or gels were added to the cells and cultured overnight at 37 °C with 5% CO2.Each treatment was performed in triplicate.Then 10 μL of MTT stock solution were added to the wells and incubated for an additional 4 h.Finally,150 μL DMSO was added to stop the reaction.Results were analyzed using an ELISA plate reader (Multiskan GO,Thermo Fisher Scientific) at 570 nm according to the manufacturer’s protocol.The survival percentage (S%) was calculated using the following equation:S%=(Treated-Control)/(Untreated-Control) × 100%.
2.5.11.Quantitativeproteomiccomparisonoftissues collectedfromcontrolandimplantgroups
2.5.11.1.Proteinsamplepreparation.Samples were homogenised in SDT buffer(4%SDS,100 mM Tris–HCl,1 mM DTT,pH7.6),and 200 μg of proteins for each sample were subjected to trypsin digestion according to the filter-aided sample preparation (FASP) procedure described elsewhere[51,52].The protein suspensions were digested with trypsin(Promega) overnight at 37 °C,and the resulting peptides were desalted on C18 Cartridges (EmporeTMSPE Cartridges C18,bed I.D.7 mm,volume 3 mL,Sigma),and lyophilized by vacuum centrifugation for TMT10plex labeling.A total of 100 μg peptide mixture of each sample was labeled using TMT reagent according to the manufacturer’s instructions(Thermo Scientific).Labelled peptides were fractionated by SCX chromatography using an AKTA Purifier system (GE Healthcare).The collected fractions were desalted on C18 Cartridges,lyophilized and resuspended for LC-MS/MS analysis (see Supplementary Methods for more details).
2.5.11.2.nanoLCtandemQ-Exactivems/msanalyses.The peptide samples were analysed using a Q Exactive mass spectrometer coupled to Easy nLC (Thermo Scientific).The peptides were loaded onto a reverse phase trap column (Thermo Scientific Acclaim PepMap100,100 μm×2 cm,nanoViper C18) connected to the C18-reversed phase analytical column(Thermo Scientific Easy Column,10 cm long,75 μm inner diameter,3 μm resin) in buffer A (0.1% Formic acid) and separated with a linear gradient of buffer B (84% acetonitrile and 0.1% Formic acid) at a flow rate of 300 nL/min.The mass spectrometer was operated in positive ion mode.MS data was acquired using a data-dependent top10 method dynamically selecting the most abundant precursor ions from the survey scan (300–1800m/z) for HCD fragmentation (see Supplementary Methods for more details).
2.5.11.3.Proteinidentificationandquantitation.
The MS/MS data was searched against Ensembl_Rattus_29,107_20,200,311 (76,417 sequences,downloaded on Dec 12,2014) database for protein identification using Mascot2.2 (Matrix Science,London,UK) and Proteome Discoverer1.4 software (Thermo Fisher Scientific,Waltham,MA,USA) with the following search settings:enzyme trypsin;two missed cleavage sites;precursor mass tolerance 20 ppm;fragment mass tolerance 0.1 Da;fixed modifications: Carbamidomethyl (C),TMT 10plex (N-term),TMT 10plex (K);variable modifications: oxidation (M),TMT 10plex (Y).The results of the search were further submitted to generate the final report using a cut-off of 1%FDR on peptide levels and only unique peptides were used for protein quantitation.All peptide ratios were normalized by the median protein ratio,and the median protein ratio was 1 after the normalization.The protein showing a fold change ≥1.2 compared to the control group and theP-value<0.05 were considered significantly regulated.
2.5.11.4.Geneontology,domainandKEGGpathwayanalysis.The protein sequences of the selected differentially expressed proteins were locally searched using the NCBI BLAST+client software Version.2.2.28,and gene ontology (GO) terms were mapped,and sequences were annotated using the software program OMICSBOX (https://www.biobam.com/omicsbox/).Protein sequences are searched using the InterProScan software embedded in OMICSBOX to identify protein domain signatures from the InterPro member database Pfam.The proteins were blasted against the online Kyoto Encyclopedia of Genes and Genomes(KEGG)database(http://geneontology.org/) to retrieve their KEGG orthology identifications and were subsequently mapped to pathways in KEGG.Enrichment analysis were applied based on the Fisher’exact test,considering the whole quantified proteins as background dataset.Benjamini-Hochberg correction for multiple testing was further applied to adjust derivedP-values.Only functional categories and pathways withP-value <0.05 were considered as significant.
2.5.11.5.Protein-proteininteractionanalysis.The protein–protein interaction (PPI) information of the studied proteins was retrieved from IntAct molecular interaction database[53] (http://www.ebi.ac.uk/intact/) by their gene symbols or STRING software (http://string-db.org/).The results were downloaded and visualized using Cytoscape software (http://www.cytoscape.org/,version 3.2.1).The statistical analysis of the PPI was performed using the Network Analyzer [54]in Cytoscape.
3.Results
3.1. Characterization of microstructures,micro-hardness,and in vitro degradation of Mg alloys
The original microstructure and texture of the three differently processed Mg alloys obtained by EBSD mapping are shown in Figs.2A-2C.The average grain sizes are 22 μm for the PM,18.2 μm for the CE AZ31 and 15.9 μm for the FA AZ31 alloy.From the EBSD results,we observed that compared to PM and CE AZ31 samples,the FA AZ31 sample had the highest fraction (0.25) of pyramidal slip systems with soft orientation (The Schmid factor is 0.5) as shown in Fig.2F[55,56].However,PM and CE AZ31 samples have lower ratios of first-order pyramidal slip systems of 0.16 and 0.17,respectively.This means that for FA AZ31 pyramidal slip texture dominates,while other slip systems including basal slip(0001){11–20},prismatic slip (10–10){1–210},and second order pyramidal slip (11–22){-1–123} systems do not have high ratios of soft orientation (0.5) as shown in Figs.2D,2E and 2G.The pole figure {10–10} shows that CE AZ31 has the highest intensity of texture along the extrusion direction as shown Fig.2I,while PM and FA AZ31 have lower intensity of {10–10} texture along the extrusion direction as shown in Figs.2H and 2J.
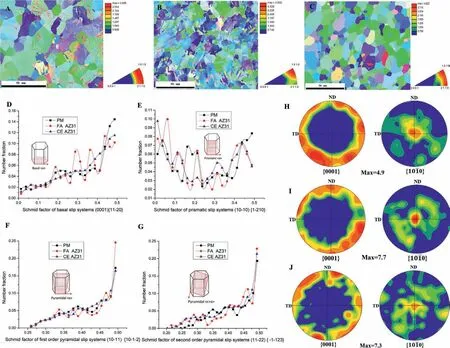
Fig.2.Microstructures and EBSD analysis of three Mg materials.The microstructure and texture of annealed PM (A),CE AZ31 (B) and FA AZ31 (C);(D)Basal slip system (0001){11–20};(E) Prismatic slip systems (10–10){1–210};(F) First-order Pyramidal slips systems (10–11){10–1–2};(G) Second-order pyramidal slip systems (11–22){-1–123};Pole figures {0001} and {10–10} of PM (H),CE AZ31 (I) and FA AZ31 (J).
The micro-hardness analysis revealed that the CE AZ31 group had the highest microhardness both in the center (79.0 HV) and edge regions (84.1 HV),followed by the FA AZ31 group with microhardness values of 67.1 HV and 65.9 HV at the edge and center regions,respectively (Fig.3A).The PM specimen had the lowest microhardness for both the center and edge regions,which were 42.8 HV and 40.1 HV,respectively.

Fig.3.The in vitro analysis of micro-hardness,the change of pH and weight.(A) Vickers Micro Hardness of PM,CE AZ31 and FA AZ31;(B) pH values in a Dulbecco’s Modified Eagle Medium (DMEM) solution;(C) hydrogen evolution;(D) Hela MTT assay for different Mg samples.
On immersion,the solutions containing the three specimens had similar pH values,around 7.3,which increased after immersion (Fig.3B).The initial rate of corrosion into Mg2+and OH-ions was rapid as reflected by the pH values increasing sharply within the first 24 hr,subsequently reduced to a steady rate at longer periods.The PM group maintained a higher reaction rate than the AZ31 alloy specimens.Over 120 hr of immersion,the pH values of the PM specimen increased significantly from 7.3 to 8.86,while both the FA AZ31 group and CE AZ31 group showed a slightly increase from 7.3 to 8.71 and 8.41 respectively (Fig.3B).After 120 h immersion,both FA AZ31 and CE AZ31 groups showed an intact surface without any obvious corrosion products (white magnesium hydroxide),while PM group has obvious corroded surface with white magnesium hydroxide (Fig.3B).Moreover,hydrogen evolution showed that CE AZ31 has the highest hydrogen evolution rate among three groups (Fig.3C).After 120 hr immersion,its hydrogen evolution reached 280 μL.While FA AZ31 and PM group had the lower hydrogen with the values of 119.9 μL and 104.7 μL respectively.PM group take place oxidation quickly and surface cover with oxidation layer which result in low volume hydrogen evolution.Normally weight loss test will last for longer period(3–6 months).While current weight loss test only lasted 120 h (Fig.S2),the relevant results could not address corrosion issues accurately.A longer period weight-loss test (3 to 6 months) is being planned for further investigation.Considering results of pH values and hydrogen evolution,the FA AZ31 specimen presented the best corrosion resistance.
The cytotoxicity of the FA AZ31 implant was assessed on the proliferation of HeLa cells using the MTT assay(Fig.3D).It can be seen that the viability of HeLa cells in the FA AZ31 group was of similar magnitude compared to the control group.The PM significantly inhibited the growth of HeLa cells (P-value=0.0096),indicating a high cytotoxicity,while the CE AZ31 showed an insignificant antiproliferative activity.
3.2. In vivo anti-corrosion properties
3.2.1.MRIandCTanalysis
We first investigated the MRI signal intensity between the four intervention methods compared with the corresponding healthy side of the animal in each case.At the implant sites,the average signal intensities in decreasing order were obtained from the PM,followed by FA AZ31 and then the CE AZ31 alloy,while there were no significant differences for the healthy sides of the animals (Table 1 and Fig.S3).This may indicate that differing magnesium alloys and different processing lead to different biological effects influencing the relative rates of degradation.The CT results were comparatively displayed in Fig.S4,which showed a similar tendency.
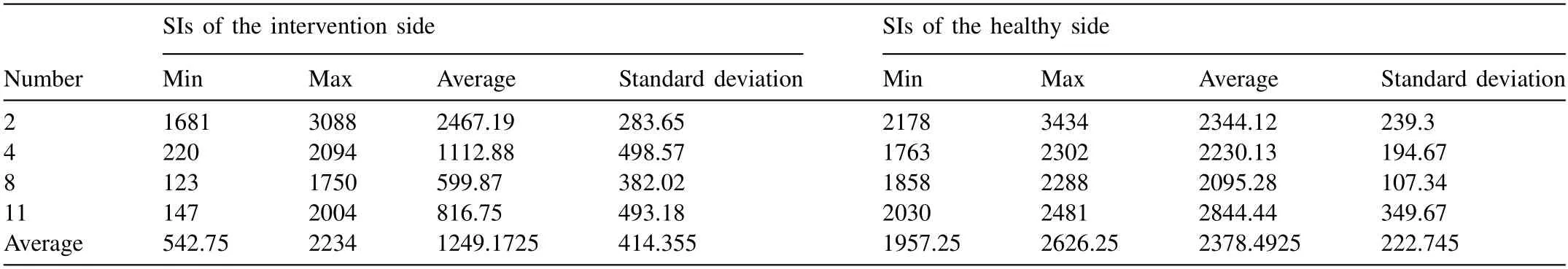
Table 1MRI signal intensity (SI) comparisons between the four intervention methods and that of the healthy side of the animals.
3.2.2.SEM-EDXanalysis9dayspostimplantation
After implantation for 9 days,the size of the PM samples was significantly smaller than the other Mg alloy samples,while no obvious difference was observed between the two AZ31 Mg alloy groups(Figs.4A,4B,4C and 4D).SEM map ping showed that the FA AZ31 and CE AZ31 maintain an almost intact surface with evidence of slight surface corrosion.Conversely,severe corrosion took place in the PM group with the surface entirely corroded away in some samples (Figs.4E and 4F).Since corrosion products of Mg and its alloys include magnesium hydroxide and hydrogen gas,only magnesium hydroxide is likely deposited on the Mg-based samples.The EDX analyses revealed that the FA AZ31 group was the most stable with fewer oxides detected on the specimen surface,while the CE AZ31 and PM group both had higher levels of oxides,as shown in Fig.4G.Overall,the FA AZ31 has higherinvivocorrosion resistance which was in accordance withinvitroobservations of corrosion performance.
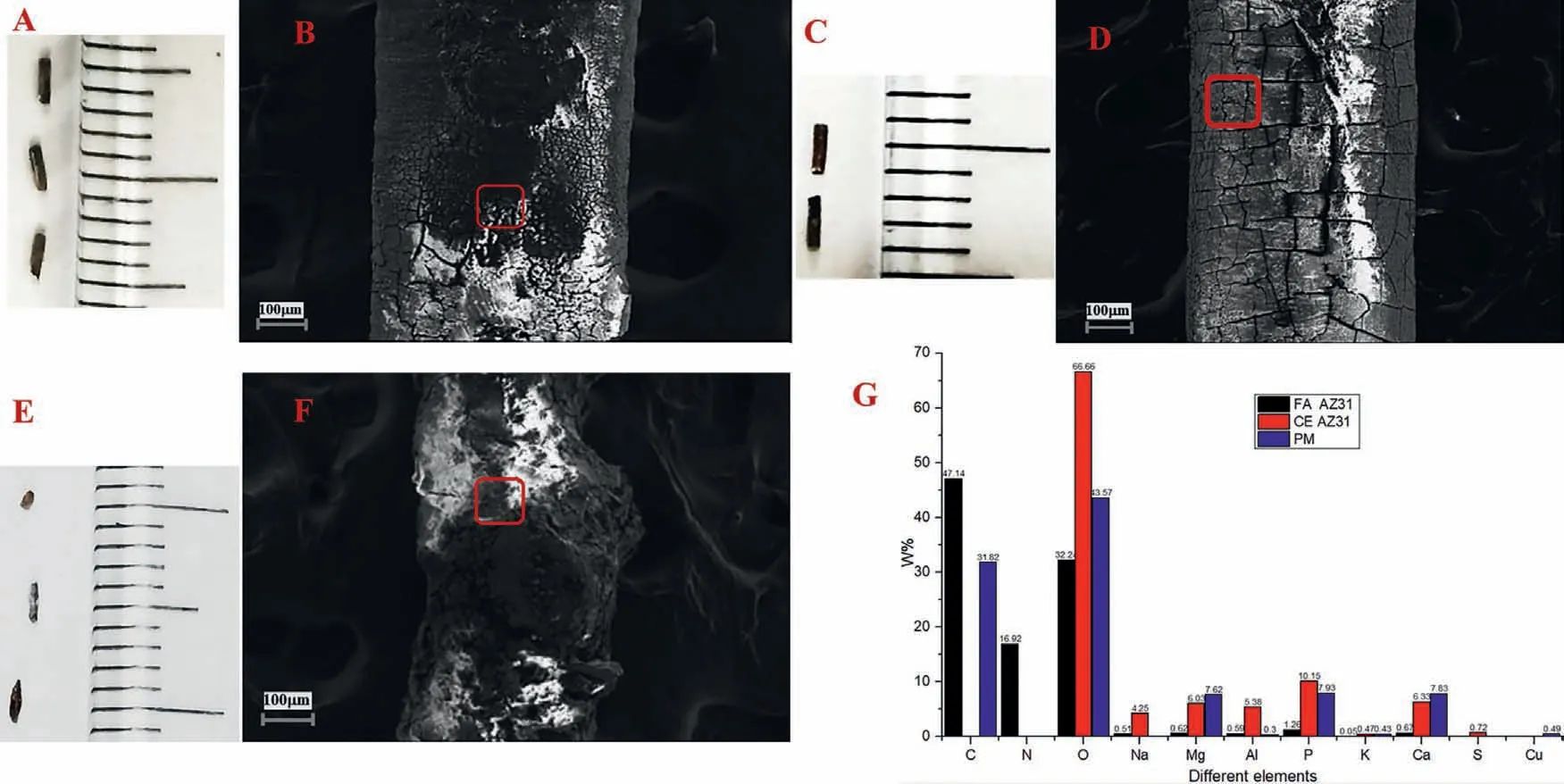
Fig.4.SEM-EDX analyses of the three specimens of each Mg material nine days post the in vivo implantation.(A) FA AZ31 specimen,(B) SEM image of FA AZ31 specimen,(C) CE AZ31 specimen,(D) SEM image of CE AZ31 specimen,(E) PM specimen,(F) SEM image of PM specimen,and (G) the analyses of EDX results of three specimens.

Fig.5.HE stained sections of liver,spleen and lung of four groups of rats (×200): (A) liver,(B) spleen,(C) lung.
3.3. In vivo compatibility
3.3.1.Elementalanalysisrevealslowestofthebonetissues isolatedfromimplantsurrounds
Results from elemental analyses of bone tissues surrounding the implantation site and from the healthy side of the animals for each experimental group after 9 days into the implantation were compared (Table 2,S1 and Fig.S5).The FA AZ31 group showed the lowest Mg content (3850 mg/kg)at the site of implantation,while the PM group displayed the highest Mg content of 4153 mg/kg (Table 2) which accords with theinvitroobservation that the PM group exhibited greater corrosion.The control group and CE AZ31 group had similar contents of Mg which were 3949 and 3936 mg/kg,respectively.The contents of Ca and P were also lowest in the FA AZ31 group at 186,209 and 3850 mg/kg,respectively.The PM group had the highest contents of Ca(200,972 mg/kg)and P (99,447 mg/kg) among all groups,followed by the control group with 197,822 mg/kg of Ca and 97,324 mg/kg of P.Thus,it was evident that the FA AZ31 alloy had the lowest degradation,followed by the CE AZ31,while the PM showed the highest degradation.As expected,the right-side bone tissues did not show any trend in terms of elemental contents(Fig.S5).

Table 2The analysis of the elements in the bone tissues with different implants by the ICP-MS and the electrolytes.
3.3.2.Analysisofelectrolytelevels,andliverandkidney functions
We investigated electrolytes as well as liver and kidney function of the different groups nine days after implantation.The FA AZ31 group had the lowest concentration of serum Mg2+(0.95 mmol/L) while the CE AZ31 group had the highest concentration of Mg2+(1.12 mmol/L),fol lowed by the control group (0.99 mmol/L) and then the PM group (0.97 mmol/L) (Table 2).In terms of serum Ca2+level,the PM group showed the highest concentration (2.73 mmol/L),while the FA AZ31 and the CE AZ31 groups had similar concentrations (2.62 and 2.61 mmol/L,respectively) (Table 2).The control group had the lowest concentration of 2.59 mmol/L.As shown in Table 2,the CE AZ31 group had the highest concentration of serum P(5.35 mmol/L),followed by the control group (4.07 mmol/L)and the FA AZ31 group (4.05 mmol/L).The PM group presented the lowest concentration of serum P (3.49 mmol/L).
Interestingly,the CE AZ31group had the highest alanine transaminase (ALT) (80 IU/L) and aspartate transaminase(AST) enzyme levels (368 IU/L) whereas the implantation of FA AZ31 resulted in the lowest ALT and second lowest AST levels (Table 2).The PM group presented the lowest concentration of AST (138 IU/L).The PM and CE AZ31 groups had similar levels of creatinine (CRE) which were the highest among the four groups(Table 2).The FA AZ31 group showed a similar CRE level (about 25 μmol/L) to the control group.These results suggest that FA AZ31 had more limited influence on hepato-and renal function compared to PM and CE AZ31.
3.3.3.HEstainingofimportantorgans
Important organs were collected from the rats sacrificed nine days post the implantation,and HE stained to observe for organ damage potentially caused by the implanted metals.The central vein,liver lobule and portal area of the liver among different groups were normal,and the liver cells showed no obvious necrosis (Figs.5 A1-A4).There were no obvious abnormal cells in the red and white pulp of the spleen(Figs.5 B1-B4).The lung parenchyma and interstitial cells were normal (Figs.5 C1-C4),and the cardiomyocytes of the four groups showed no obvious cell necrosis under the cardio scope (Figs.6 A1-A4).In addition,the brain tissue structure of the four groups was normal with no obvious necrotic areas (Figs.S6 B1-B4) and the structure of the renal cortex and renal medulla was normal.The renal corpuscles and tubules were also normal (Figs.S7 A1-A4).There was no obvious necrosis of seminiferous tubules,Sertoli cells and Leydig cells in the testes of the four groups (Figs.S7 B1-B4).The results indicate that the implantation of Mg specimens did not lead to damage of important organs.
3.3.4.FAAZ31inducedlessinflammatoryresponsesby proteomicsanalysis
TMT 10plex labeling quantitative proteomic analysis identified a total of 3679 proteins in all samples (Table S2),based on which the proteins differentially expressed (FC ≥1.2,P-value <0.05) between the different implant groups samples were derived (Table S3).In comparison to the control,the CE AZ31 group showed the highest number of significantly upregulated (140) and downregulated (118) proteins,whereas FA AZ31 induced the least number of proteins with significant regulation (Fig.6A).The profiles of proteins regulated with significance was compared,the CE AZ31 and PM groups showed higher overlaps than with the FA AZ31 group(Fig.6B).Among the 11 upregulated proteins in the FA AZ31 group,the abundance of Rarres2,Pdcd2l and Aif1 were previously shown to positively correlated with inflammation(Fig.6C).
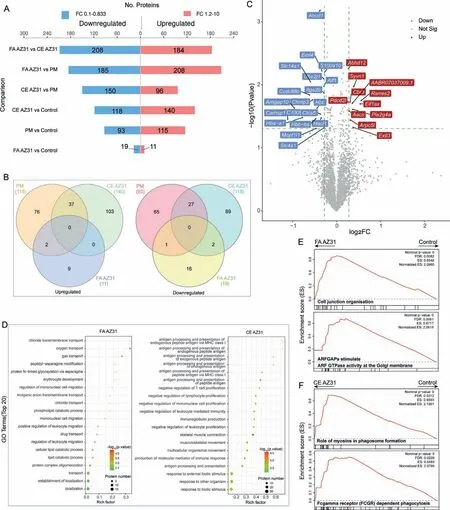
Fig.6.Quantitative proteomics analysis of soft tissues collected from the implantation sites 9 days post the operation.(A) Comparison of the numbers of significantly regulated proteins between four groups,including the control,PM,CE AZ31 and FA AZ31 groups.(B) Venn diagrams show the overlaps of differentially expressed proteins in PM,CE AZ31 and FA AZ31 groups.(C) Volcano diagram shows the significantly regulated proteins in the FA AZ31 group with respect to the control.(D) The top 20 biological processes enriched by the significantly regulated proteins identified in the FA AZ31,CE AZ31 groups relative to the control,respectively.The top 2 Reactome pathways enriched by the proteins quantified in the FA AZ31 (E) and the CE AZ31 (F) groups in comparison to the control by GSEA analysis.See Table S4 for full enrichment analysis.
The top 20 biological processes enriched by the significantly regulated proteins in the three different implant groups with respect to the control group were compared (Fig.6D,S8A and Table S4).The FA AZ31 group was enriched with several cellular transport processes,as well as migration processes related to mononuclear cells and leukocytes.For the CE AZ31 group,a few antigen-processing and presentation processes were enriched,so were several other immune response associated processes,such as negative regulation of T cell proliferation,negative regulation of leukocyte mediated immunity and immunoglobulin production.The enrichment of immune response relevant processes was also largely observed in the PM group (Fig.S8A).The GSEA based on the contents of all proteins in each implant group was performed with respect to the control group.The FA AZ31 did not show the enrichment of any pathway directly relevant to an immune response,but exhibited the activation of cell development,including ‘cell junction organisation’ (FDR=0.0082) and ‘ARFGAPs stimulate ARF GTPase activity at the Golgi membrane’(FDR=0.0061) (Fig.6E).The CE AZ31 group showed remarkable elevation for ‘myosins in phagosome formation’ (FDR=0.0212) and ‘FCGR dependent phagocytosis’(FDR=0.0226),whereas the PM group was highly enriched in ‘innate immune system’ (FDR=0.0054) and ‘exocytosis of secretory granule membrane proteins’ (FDR=0.005)(Fig.6E and Fig.S8B).
3.3.5.FAAZ31stimulatedlowerinflammatorycytokineand chemokinelevels
Based on the proteomic analysis,we thus examined cytokine and chemokine levels from the same tissues isolated using cytokine array [48].Compared with the PM group,unsurprisingly,the matrix metalloprotein 9 (Mmp9),IL-1α,IL-1βwere barely detected in the FA AZ31 group (Figs.7A-7C).The levels of CCL3 and CXCL2 were much lower in the FA AZ31 group (Fig.7C).Only CCL21 was higher in the FA AZ31 group,while the adiponectin,galectin-1 and Igfbp3 levels were similar between the two groups.
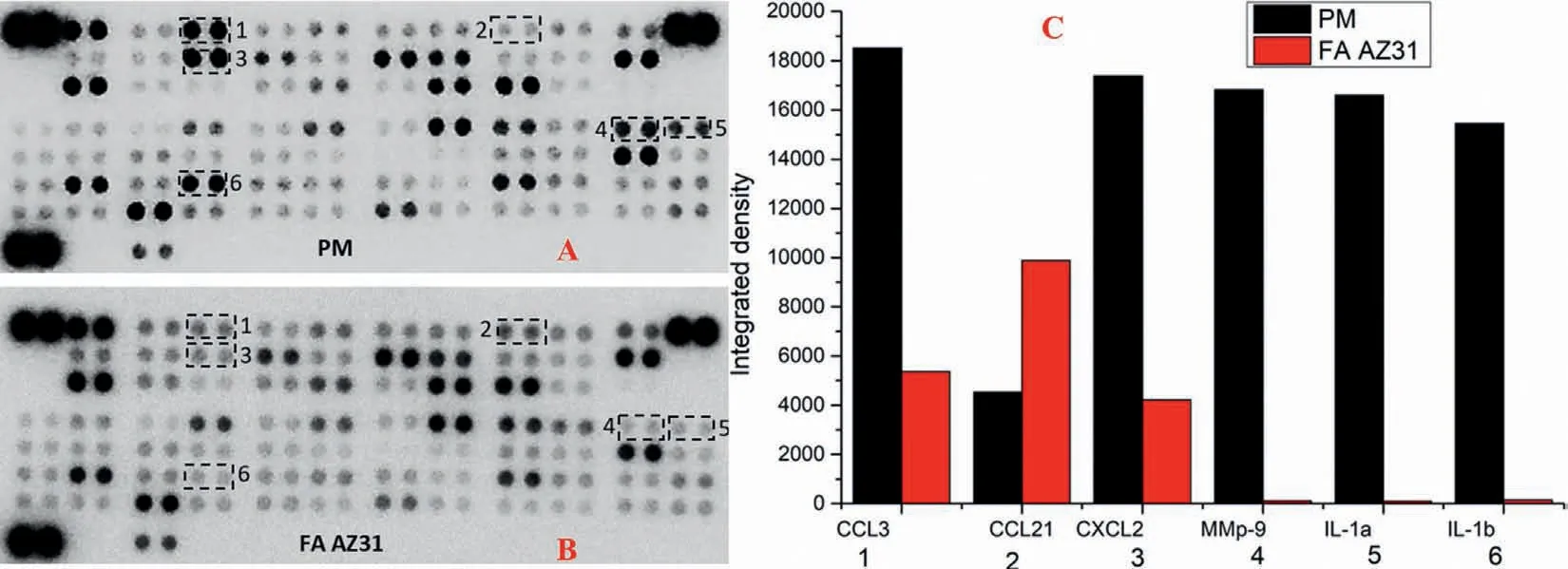
Fig.7.The comparison of cytokine and chemokine levels in the PM and FA AZ31 groups.The images show the cytokine array results of the PM (A) and FA AZ31 (B) groups.(C) Quantitative analysis of protein expression between the two groups.The spots were coded as in (A) and (B).
Therefore,FA AZ31 not only has the similar mechanical properties to those of human bone,but also has high corrosion resistance and high biocompatibilities,including lower cytotoxicity,no observable influences on important organs,the induction of low-level inflammatory responses and lower inflammatory cytokine and chemokine levels.
4.Discussion
4.1. Alloying elements led to distinct in vitro and in vivo corrosion behaviors
The results consistently showed that CE AZ31 and FA AZ31 groups had substantially superior corrosion resistance and mechanical properties compared with the PM,which may be attributed to alloying with elements that improve the microstructure and mechanical properties.Common Mg alloying elements include Al,Zn,Mn,Ca,Li,Sr,Sc,and rare earth,to alter different properties [57,58].In this study,the AZ31 Mg alloy contains around 3% Al and 1% Zn [59].Al modifies the mechanical properties and anticorrosion behaviors of Mg alloys [60–63] through significant grain refinement and solid solution strengthening effects.When the mass ratio of Al is between 1% and 5%,it helps to generate smaller,more equiaxed grains [58,64,65].The Al dissolves in the Mg solution or precipitates as a secondary Mg17Al12phase in the form of a continuous network along grain boundaries [66] or via lamellar growth [67].The yield strength for as-cast Mg alloys mainly depends on grain size and dendrite arm spacing[68].Thus,the finer grain sizes of CE AZ31 (18.18 μm) and FA AZ31 (15.85 μm) may contribute to their higher hardness and strength relative to PM (22 μm).
Moreover,the different electrode potentials of theα-phase Mg matrix (hexagonal central pack) and Mg17Al12(β-phase)may also contribute to different corrosion resistance.When corrosion occurs via galvanic reaction,the Mg17Al12phase acts as the cathode with respect to theα-phase of the Mg matrix,which accelerates the corrosion of the Mg alloy [65].However,Mg17Al12acts as a barrier to reduce corrosion due to due to its inertness [65,66,69-73].In addition,passivation layers also play an important role in the corrosion of Mg alloys.In this study,the PM has a single-phase (α-Mg) structure and a weak passivation layer of magnesium oxide layer,while both CE AZ31 and FA AZ31 contain dual phases (α-Mg andβ-Mg) with a strong passivation layer including zinc oxide,aluminum oxide and magnesium oxide,which hinders corrosion.
Zn,not only enhances solid solution strengthening of magnesium alloys leading to increased strength,but also promotes activation of non-basal slip in Mg alloys at room temperature and improves their plastic processability [73].However,when Zn content exceeds 2.5%,corrosion of Mg alloys is accelerated due to the high-volume fraction of Mg–Zn phases that act as the cathode accelerating corrosion of theα-Mg matrix in surrounding regions [65].In this study,the AZ31 alloy contains 1 wt% Zn which is more influential in enhancing mechanical properties than adversely impacting corrosion resistance.It has been reported that for Mg-Al-Zn alloys in general,secondary phases (such as Mg-Zn intermetallic compounds) and grain refinement are the main factors impacting mechanical properties and corrosion behaviors[74–78].Hence,CE AZ31 and FA AZ31 have superior mechanical properties (micro-hardness) and corrosion resistance(pH value and weight change) relative to PM.In addition,CE AZ31 had the highest Vickers micro-hardness due to increases in the dislocation and grain boundary densities caused by work hardening and dynamic recrystallization during processing.
4.2. First-order pyramidal orientated (10–11){10–1–2}texture in FA AZ31 led to the better in vitro and in vivo behaviors
Processing plays an important role in achieving optimal mechanical and anticorrosive properties.Typically,Mg alloys have low strength and poor plasticity as only the basal (0001){11–20} slip system is active at room temperature.Basal slip has the lowest critical resolved shear stress (CRSS),around two orders of magnitude lower than non-basal slip,leading to low strength,while only two independent slip systems provide limited plasticity as it does not meet Taylor’s five-slip-system requirement for ductile polycrystal deformation [79].However,with elevated temperature processing other slip systems including prismatic (10–10){1–210} and pyramidal slip (firstorder (10–11){10–1–2} and second-order (11–22){-1–123})systems become active.High-temperature annealing which activates these systems and enables extensive cross-slip facilitates recovery processes involving nucleation and growth of new refined grains by grain boundary migration [80–82].Therefore,the annealing heat treatment leads to recrystallization of the original microstructure and texture,relieves defects,and stresses,and generates new refined equiaxed grains with a homogeneous microstructure which may contribute to the betterinvitroandinvivodegradation behaviors observed for FA AZ31.
In this study,the annealing temperature for FA AZ31 was between 330 and 350 °C and the proportion of Al was 3 wt.% so that the microstructure included a high fraction ofα-Mg phase with only limited amounts of theβ-Mg phase(Mg17Al12) [83,84].The high annealing temperature activated first-order pyramidal slip (10–11){10–1–2} and cross-slip enabling recovery and recrystallisation to occur.The equiaxed microstructure promotes homogenous mechanical properties and improves the plasticity of FA AZ31 alloy.The CRSS for first-order pyramidal slip of around 68 MPa is significantly higher than that of basal slip at 25 MPa,so that compared to basal slip texture,this first-order texture was more stable due to its high strength.Therefore,the degree of first-order pyramidal texture(10–11){10–1–2}may contribute to the differentinvitroandinvivocorrosion behaviors among the three groups.
There is limited research focused on the role of grain orientation on the corrosion behaviors of Mg alloys and the influence of different grain orientations (texture) remains unclear.Generally,the surface energy associated with a close-packed crystalline surface is the lowest so that for Mg alloys the basal or close packed {0001} plane has the lowest surface energy.Theoretically,the corrosion rate of the basal {0001}plane is 18 ∼20 times lower than those of the {1010} and{1120} planes [57].Songetal.[85] found that the surface of hot rolled AZ31 alloy had a strong preference toward a{0001} grain texture with lower surface energy and higher corrosion resistance compared with the {1010} and {1120}planes.Xin et al.[86] discovered that rolled AZ31 had a low{0001} texture intensity,but high intensities of {1010} and{1120} textures accompanied by a substantial increase in the degradation rate in 3.5% NaCl solution.Zhaoetal.[87] reported that the TD–ND planes of rolled AZ31 dominated by prismatic {10–10},second pyramidal {11–20} and first-order pyramidal {10–11} plane orientations showed a higher corrosion resistance compared with those of the RD–TD planes consisting mainly of {0001} oriented grains.This was observed in our FA AZ31 alloy,which had dominant first-order pyramidal{10–11}oriented grains,exhibiting the highest corrosion resistance for bothinvitro(Figs.3A and 3B) andin vivotests (Figs.4A and 5A).Conversely,due to the influence of cold work [88],the CE AZ31 with accumulated dislocation networks and increased grain boundaries density had an increased surface energy which may promote higher rates of corrosion compared to FA AZ31.
Moreover,the FA AZ31 samples also showed a greater structural stability when implanted into the physiological environment.The controversy on the role of surface structure and texture on the degradation response remains.Hagihara et al.attributed the different corrosion behavior of different grain orientations to the high atomic-packing densities[89],while others believed that the generated twin boundaries might act as physical barriers to corrosion attack [90].Our study strongly suggests a positive correlation between the first-order pyramidal dominant texture of the FA AZ31 and its superiorinvitroandinvivobehaviors.It should be noted that this observation is currently limited to the behavior of fully annealed AZ31 and further studies are required to determine if it applies more widely to other magnesium alloys.
4.3. FA AZ31 showed better biocompatibility
Normally,Mg based implants form a degradation layer,where the implant reacts with the local environment [91].The degradation layer is affected by both the implant substrate chemistry and structure,and the neighboring fluid environment.After the implantation,Mg was released from the degradation layers of the implants and into the surrounding bone and tissue (Figs.8).The SEM-EDX evaluation of the degradation layer found that the FA AZ31 group had the lowest weight ratio of Mg,likely due to its superiorinvivoanticorrosion behavior(Fig.8C).In the physiological environment,hydroxyapatite (Ca1n(PO4)6(OH)2) is one of the main degradation products of Mg implants [92–99],while the weight ratios of Ca and P were the lowest in the degradation layer of the FA AZ31 group (Figs.8B to 8D),which indicates a relatively lower concentration of OH-was derived from the corrosion.Besides,the comparison of HE stained tissues demonstrated that implantation of the Mg alloy specimens into SD rat femurs did not show any obvious influence on the organs in agreement with other studies [100–103].The levels of ALT,AST and CRE of the FA AZ31 group was the closest to the control.The SEM and CT imaging also confirmed that the FA AZ31 group showed the most intact surface morphology,indicating that the degradation was slower compared to the implants,which was supported by the MRI results showing that FA AZ31 had the highest SI value.Thus,the FA AZ31 exhibited excellent biocompatibility in all aspects.
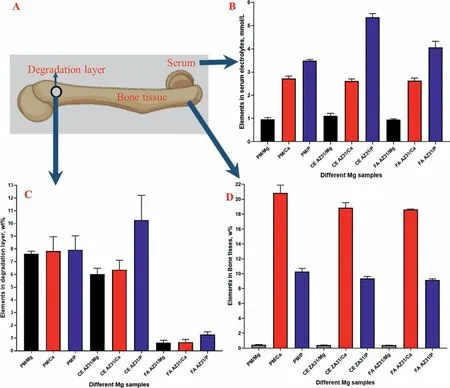
Fig.8.Element analysis of Mg,Ca and P in the three groups: (A) schematic shows the locations for tissue collection and where the elements were analyzed.The contents (wt.%) of Mg,Ca and P elements of the three groups in degradation layer (B),bone tissues (C) and serum electrolytes (D).
4.4. Degradation of FA AZ31 led to fewer inflammatory and immune responses,but triggered distinct signaling pathways related to recovery
Foreign body reactions (FBRs) are a series of acute sterile innate immune inflammatory reactions,which overlap with tissue vascularization and remodeling,and ultimately fibrotic tissue encapsulates the implants to prevent further interaction with the host tissue [104–106].All biomaterial implants can induce FBRs;however,these reactions may have significant difference in terms of the severity and clinical manifestations[107].Three stages have been proposed for FBRs occurringinvivo: i) proteins adsorption onto the biomaterial surface,ii) acute inflammation in the FBR cascade,and iii) chronic inflammation resulting in reduced biomaterial function(Fig.9) [108–110].Due to the relatively short 9-dayinvivotests,our results mainly apply to the understanding of protein adsorption onto the biomaterial surface and acute inflammation stages in the FBR cascade.

Fig.9.The three-stage model of foreign body reactions (FBRs) in response to biomaterials [108].(A) Proteins adsorption onto the biomaterial surface.(B)Acute inflammation in the FBR cascade.(C) Chronic inflammation in the FBR results in reduced biomaterial function.(D) Activation of the inflammasome results in the secretion of chemokines.
The quantitative proteomic analysis found that the FA AZ31 group showed the least number of proteins significantly regulated with respect to the control,which may be attributed to its better corrosion resistance and biocompatibility.Several most significantly upregulated proteins in the FA AZ31 group relate to inflammation regulation,includingRarres2,Syvn1,Extl3,Pla2g4a,Cbr3,andPdcd2l.Rarres2recruits immune cells expressing the orphan G protein-coupled receptor chemokine-like receptor 1 to sites of tissue injury,playing an important role in the immune response including on inflammation [111].Upregulation ofSyvn1by the proinflammatory cytokines IL-1βand TNF-αis implicated in the overgrowth of synovial cells [112],while the overexpression ofExtl3enhances TNF-αinduced NF-κB activity[113].Pla2g4awas identified as an important regulator of central effectors of inflammation and joint destruction,includingMmp3,Il8,Cox2,andPge2[114],and was found to be upregulated in inflamed macrophage RAW 264.7 cells[115].The upregulation ofCbr3under inflammatory conditions was observed [116].The overexpression ofPdcd2lwas found to effectively attenuate the release of TNF-αin Daudi cells exposed to LPS,suggesting its anti-inflammatory effects[117].Thus,a physiological environment with both pro-and anti-inflammatory effects was formed post the implantation of FA AZ31,yet the extent of the effects was limited since the FC values were relatively low (Supplementary Table S3).Notably,most of the biological processes enriched by the FA AZ31 implantation were associated with cell proliferation and development,while no immune response relevant process was enriched,further indicating better biocompatibility of the FA AZ31.The enrichment of the transport of oxygen and chloride probably related to the elevated levels of cations such as Ca2+,Mg2+and Zn2+.The GSEA identified cell junction organization as the most enriched pathway in the FA AZ31 group,which suggested that cell growth was more active in the cell-extracellular matrix.This meant late stage-1 FBRs took place at the site of the FA AZ31 implants (Fig.9A),where the implants adsorbed host serum immunomodulatory proteins that can enhance cell adhesion and may contribute to recovery of the “injured” tissues [118–120].
In terms of the PM and CE AZ31 groups,most of the top 20 enriched biological processes were related to immune responses.The high presence of the antigen processing and presentation in the CE AZ31 group was potentially due to the high concentration of phosphorus detected in both the degradation layer and serum.In physiological environments,phosphorus normally takes the form of phosphate ions,the level of which significantly influences many vital biological pathways including phosphorylation of proteins and metabolites.The aberrantly upregulated phosphorylation was shown to induce more antigen processing and presentation signaling [121].The Reactome pathways highly enriched in the PM group,including ‘scavenging of heme from plasma’ and‘binding and uptake of ligands by scavenger receptors’,indicated more active phagocytosis,which is the characteristic of stage-2 FBR cascade [122–127].Additionally,the enrichment of ‘exocytosis of secretory granule membrane proteins’implicated a neutrophil-mediated inflammatory response,and the neutrophils established contact with activated vascular epithelium at the implantation site [128].This was also consistent with stage-2 FBR of the recruitment and activation of neutrophils (Fig.9B) rapidly localized to the implantation site upon release of chemoattractants by activated platelets[129,130] and endothelial cells [131–133].The Reactome pathways relevant to phagosome were the most enriched in the CE AZ31 group,indicating the stage-2 FBRs.Moreover,the activation of vascular endothelial growth factor (VEGF)relevant pathways was suggested as signature of the stage-3 FBRs (Fig.9C),while the ‘signaling by VEGF’ was significant (P<0.05) only in the PM and CE AZ31 groups.The chemokine signaling KEGG pathway were enriched in the PM group.Thus,it was evident both stage-2 and 3 FBRs in these two groups,which is distinct to the observation for the FA AZ31 group.The cellular mechanisms underlying the long-lasting stage-1 FBRs of the FA AZ31 group warrants further investigation.
Although the three samples did not show significant differences forinvitrodegradation rates,there were more obvious differences observed for theinvivotesting with the PM samples showing substantially greater degradation after 9 days of implantation,while FA AZ31 exhibited the highestinvivocorrosion resistance.In the structural assessments of the alloys,substantial differences were detected in the texture components (for example first-order pyramidal texture was substantially greater in the FA AZ31) and there were differences in grain sizes as well as chemical variation for the pure Mg and AZ31 alloy samples which may result in different local interactions with large biomolecules (especially proteins) in the physiological environment,further contribute to the different immune responses observed after implantation.In addition,the biological coordination between biomolecules and common transition metal ions (e.g.,Fe,Zn,Cu,and Ni)have been found to relate to essential physiological functions,such as catalysis,gas transport,electron transfer,metal scavenging,and signal transduction [134].These factors might lead to the significantly different immune behaviors in the FA AZ31 group observed in this study.
More work will be required to further evaluate the potential of the FA AZ31 as a medical implant,such as the effects of different Mg alloys on degradation processesin vivo,the comparison of long-term implantation (i.e.,the differences in stage-3 FBRs),the analysis of bacterial growth at the implantation sites,and hemolysis tests.In addition,the post-implantation mechanical properties should also be studied.
5.Conclusions
In this study,we showed that a fully annealed AZ31 Mg alloy as a degradable biomaterial presents superior biocompatibility compared to cold rolled AZ31 Mg alloy and pure Mg bothinvitroandinvivo.The annealed AZ31 exhibited greater corrosion resistance.The EBSD analysis demonstrated that higher first-order pyramidal slip (10–11){10–1–2} surface texture of the annealed AZ31 alloy might play a key role.Unlike the cold rolled AZ31 and pure Mg alloys,the annealed AZ31 implants did not elicit any obvious inflammatory responses,yet it significantly activated pathways associated with cell organization and development,which may assist recovery and healing of injured tissues.The cold rolled AZ31 alloy showed a high enrichment of innate immune system and exocytosis of secretory granule membrane proteins.Overall,fully annealed AZ31 Mg alloy presented excellentin vitroandinvivobehaviors and might be an ideal candidate for biodegradable implant material.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The last corresponding author thanks Japanese JSPS committee and the University of Tokyo for providing financially support of the research of Mg treatment and microstructure analysis,Mr.Bernhard Black,Mr.Ross Barrett and Mr.Hugh Allan from the University of the Sunshine Coast for samples machining and preparation.We thank Doctor Yajun Ye,Doctor Guowei Liu and Doctor Weixiong Tang from the Department of Radiology of the First People’s Hospital of Foshan for CT and X ray imaging of experimental rats.
Funding sources
This study was supported in part by JSPS research grant(No.P16718),Natural Science Foundation of Guangdong Province (No.2020A1515010855),National Science Foundation of China (31971355) and Genecology MCR Seed Funding of University of the Sunshine Coast.Deng Feng Project of Foshan First People’s Hospital (2019A008).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.03.017.
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Ameliorating the re/dehydrogenation behaviour of MgH2 by zinc titanate addition
- Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2Y (in wt.%) alloy
- PEO coating on Mg-Ag alloy: The incorporation and release of Ag species
- Underlying mechanisms of variation in yield asymmetry and strain hardening behavior of extruded pure Mg with Gd addition
- Edge crack damage analysis of AZ31 magnesium alloy hot-rolled plate improved by vertical roll pre-rolling
- High sintering and dielectric performance: The improved (Mg,Zn)3B2O6 ceramics with the help of the DFT calculation
