Laser-assisted synthesis of Au NPs on MgO/chitosan: Applications in electrochemical hydrogen storage
Bk Jleh ,Aid Mordi ,Mht Eslmipnh ,Sdegh Khzlpour ,Hniyeh Thzii ,Seid Azizin ,Mnoj B.Gwnde
a Department of Physics,Bu-Ali Sina University,Hamedan,Iran
bFaculty of Chemistry,Bu-Ali Sina University,65174,Hamedan,Iran
c Department of Physical Chemistry,Faculty of Chemistry,Bu-Ali Sina University,Hamedan,Iran
d Department of Industrial and Engineering Chemistry,Institute of Chemical Technology,Mumbai-Marathwada Campus,Jalna 431203,Maharashtra,India
e CEET,Nanotechnology Centre,VŠB–Technical University of Ostrava,17. listopadu 2172/15,708 00 Ostrava-Poruba,Czech Republic
Abstract Hydrogen with high energy density is an environmental alternative to fossil fuels which can respond to the demand for energy considering environmental conditions.It can be stored on porous materials employing physical interaction (e.g.adsorption process).The H2 storage capacity of materials can be evaluated through electrochemical methods.Therefore,a fast and straightforward approach was employed to fabricate magnesium oxide/chitosan/Au nanoparticles (MgO/CS/Au) nanocomposites with porous structure for electrochemical hydrogen storage.Herein,laser ablation in water as a fast and green method was utilized to obtain Au nanoparticles (Au NPs).The obtained Au NPs were loaded on MgO/CS nanocomposite through physical mixing.Structural and morphological investigation of nanocomposites display spherically shaped Au NPs with a diameter of 49–58 nm agglomerated on the MgO/CS.Drop casting,the fast and cost-effective method was deployed to deposit the benign,and reusable MgO/CS/Au-x (x is Au NPs weight percentage of 1,3 and 5 wt.%) nanocomposites on stainless steel mesh and their electrochemical hydrogen storage were measured by cyclic voltammetry (CV),indicating good stability and significant hydrogen storage capacity (28 C/g) after 300 CV scans for MgO/CS/Au-1 sample.
Keywords: Magnesium oxide (MgO);Chitosan (CS);Gold nanoparticles (Au NPs);Hydrogen storage.
1.Introduction
Human life is mainly based on using fossil fuel energies widely employed to simplify life in many aspects.Although it helps to reach better quality of living,causes global pollution.Global warming and producing greenhouse gasses,two main environmental problems,cause irreparable damage to the environment [1–3].Over the course of recent decades,renewable and green energies appeared to be prominent and exquisite substitutes for fossil fuel energies.H2is the most eco-rich and abundant molecule all over the world.It is an achromatic,tasteless,and non-poisonous molecule[4,5].Considering the outstanding properties of hydrogen owning the highest energy capacity of all common fuels,it sounds to be a superior energy carrier and favorable replacement energy source[6–8].It can use as eco-friendly fuel that does not produce greenhouse gasses [9].However,it is not found purely on earth and its production is a complex process.H2can be obtained from different materials through various techniques such as electrolysis,photocatalytic water splitting,thermolysis,electrochemical process andetc.[10–12].The storing of H2is one of the important issues in H2application.Materialbased sorption of H2is an affordable and fast technique,in which H2is adsorbed during the interaction with porous materials.Solid-state materials are interesting candidates for adsorbing H2[13–15].Recently,the electrochemical methods attracted various attention because they can be used to evaluate of H2storage properties(such as storage capacity,stability and kinetics) in ideal solid-state materials [16].Electrochemical method is an efficient direct H2storage technique in ambient pressure and temperature.In electrochemical measurement,hydrogen atoms adsorb on the electrodes decorated with porous materials during the electrochemical decomposing of an aqueous solution (electrolyte) [17].In this method,the H2storage capacity depends on the physical-chemical properties of electrode materials,acidic or alkaline electrolytes and cutoff potential [18].Electronic Impedance Spectroscopy (EIS),Galvonastatic technique of charge-discharge,Cyclic Voltammetric (CV),chronoamperometric and linear voltammetric methods are the techniques to evaluate electrochemical hydrogen storage.CV is an interesting and popular electrochemical method for the evolution of the reduction and oxidation of molecular species.The current of the redox reaction of electrode materials as a function of potential is observed in CV technique [19,20].
The application of active and suitable electrode materials is an important issue for achieving high efficiency in electrochemical energy storage.Metal oxides are an interesting class of nanomaterials for different applications such as electrochemistry,sensors and biomedical [21,22].They have also shown a high life cycle for the insertion/extraction of hydrogen atoms [23].MgO with different morphologies including nanosheets,nanotubes and nanoflakes has stood out as a catalyst or catalyst support [24].It is earth-abundant,costeffective,eco-friendly and rich with plenty of surface attractive surface features such as crystal edges,ion vacancies and defects [25].Moreover,MgO can be produced by facile synthetic methods and shows high surface reactivity [26].The oxygen vacancy-rich MgO can facilitate the decomposition of H2O molecules,providing H+groups [27].
CS is an abundant polysaccharide acquired chitin,consisting of active amino and hydroxyl groups.It has significant properties such as hydrophilicity,biocompatibility,biodegradability,high specific capacitance,low-cost and nontoxicity.The presence of–OH and–NH2groups on the CS surface has led to its facile incorporation with different materials[28–32].These groups can provide electron-rich reactive sites for various applications [33].
Metal NPs are conspicuous due to their uniform physical and chemical features and have been widely utilized as catalysts for the reason of their high surface area-to-mass ratio[34,35].In addition to the mentioned unique properties,metal NPs can enhance electrochemical performance due to their high surface area and electrical conductivity [36].Besides,metallic NPs can facilitate H atom adsorption by spillover mechanism,which consists of three main steps: (i) dissociation of H2molecules to H atoms on the metallic NPs,(ii) immigration of H atoms to the accepter surface and (iii)releasing of H atoms on the accepter surface [37].Among them,gold nanoparticles (Au NPs) with the size of 1–100 nm are biocompatible nanoparticles.Nontoxicity,large surface-tovolume ratio,good conductivity,outstanding oxidation resistance and unique optical are dominant properties of Au NPs.In the last decades,Au NPs have gained more attention for catalytic reactions as compared to the expensive catalysts of Pt and Pd.In addition,Au NPs are known as effective catalysts with significant efficiency and selectivity even at low temperatures,attributed to the high fraction of their atoms present on the surface.However,the physicochemical properties of Au NPs are strongly dependent on their size and shape which can be controlled by a selection of a suitable synthetic method [38–40].Au NPs can be fabricated through different chemical,biological and physical methods [41–43].Though chemical procedures have the potential to be utilized for mass production,using poisonous material and hazardous byproducts would be obvious downsides.Laser ablation in liquid(LAL) is an easy-to-use method,in which nanomaterials can generate at a laboratory scale.The LAL has shown a high potential to fabricate nanostructures with various morphology,size,surface defect and metastable phases.It has been widely employed to synthesize noble metals-based composites for hydrogen production [44].The LAL is an affordable,fast,and clean physical synthesis for the preparation of Au NPs.In LAL,the size and shape of Au NPs are controllable by adjusting laser parameters such as wavelength,repetition rate and irradiation time [42,45-48].
Herein,the effect of the laser-assisted synthesized Au NPs on the electrochemical hydrogen storage performance of MgO/CS was surveyed through CV analysis.Au NPs were synthesized via the fast and physical method of LAL in water and then deposited on the MgO/CS by magnetic stirring to achieve the mentioned goal.The CV was then performed in an alkaline electrolyte (1 M KOH) at the scanning rate of 0.1 V/s.After 300 cycles,the hydrogen storage capacity of the MgO/CS/Au-1 electrode was achieved 28 C/g.In addition,excellent stability was observed for the mentioned electrode compared with the MgO electrode due to the adhesion property of the CS.
2.Experimental
2.1. Reagents and instruments
The chemical materials were purchased from Merck Chemical Co.To probe the structural characterization of the MgO and MgO/CS/Au nanocomposite,X-ray diffraction (XRD,Italstructure,ADP200) was utilized in the 2θrange of 30–90°at the wavelength of 0.154 nm.To identify different kinds of chemical bonds and functional groups,Fourier transform infrared (FT-IR,Thermo Nicolet) was applied.The morphology of the nanocomposite was surveyed via scanning electron microscopy (SEM,TESCAN-MIRA3-XMU) analysis.X-ray photoelectron spectroscopy (XPS) and energy-dispersive Xray spectrometry (EDX) technique were deployed to determine the elemental composition of the nanocomposites and the presence of elements,respectively.Moreover,element map images were used to explore the distribution of the elements.All the electrochemical H2storage measurement was performed using Autolab (PSTAT204) device.
2.2. Synthesis of MgO nanosheets
Herein,MgO nanosheets (Fig.1a) were prepared through refluxing and calcination routes.Initially,43 mL of ethylene glycol and 3 g of magnesium nitrate were added to 33 mL of urea solution with a concentration of 6.05 M.The resultant solution was refluxed for 11 h in an oil bath at 115–120 °C and then was cooled at ambient conditions.Afterward,the resultant suspension was washed with DI water and ethanol,respectively,and dried at 115 °C for 5 h.The acquired white powder was calcinated using an electric furnace for 5 h at 450 °C under the air atmosphere.Fig.1a is a representation of the MgO synthesis setup.
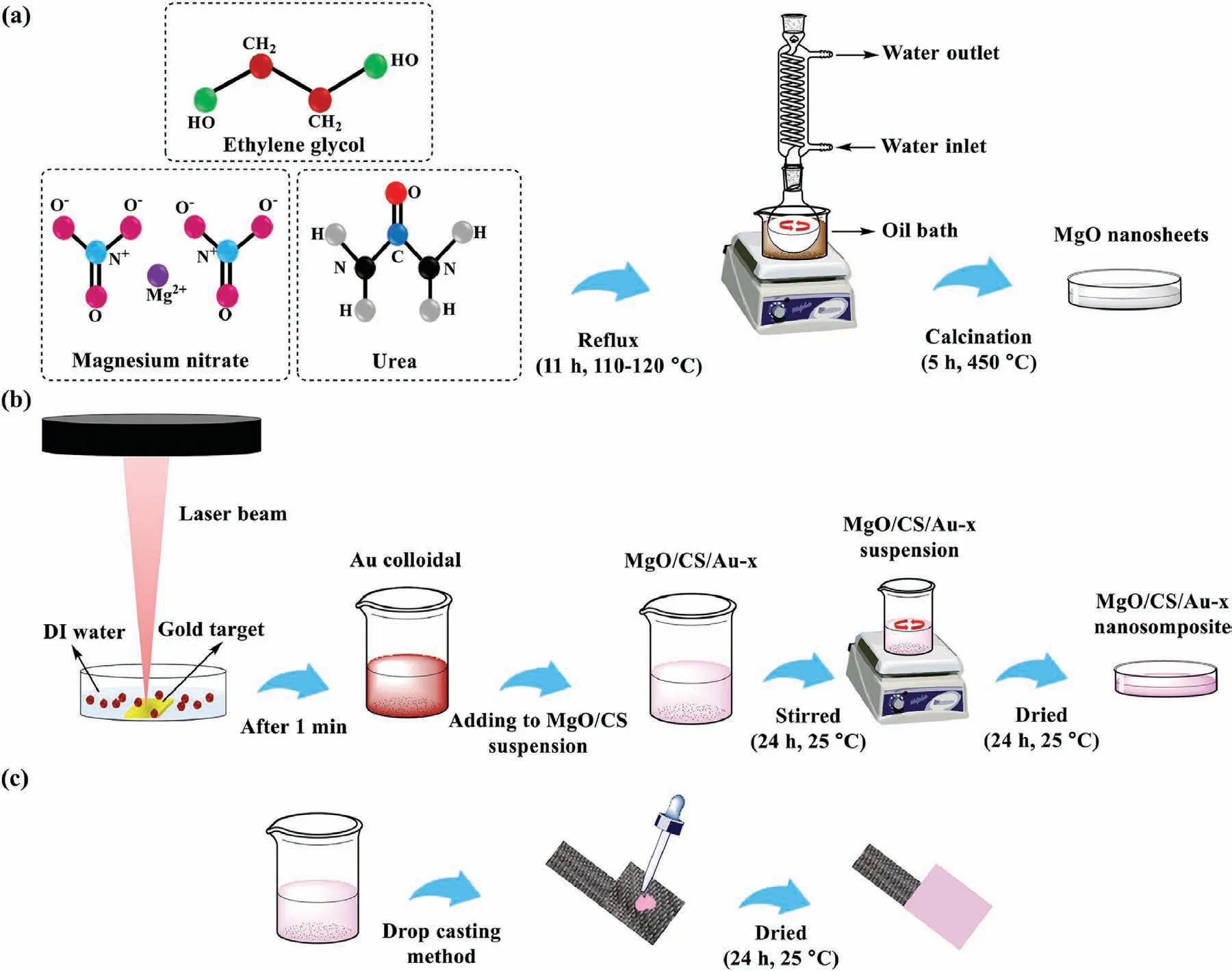
Fig.1.Schematic representation of the synthesis of (a) MgO nanosheets,(b) MgO/CS/Au-x nanocomposite and (c) the preparation of electrodes.
2.3. Synthesis of Au nanoparticles
To prepare Au NPs,a fiber laser (RFLP30Q) with a wavelength of 1064 nm and maximal power output of 30 W was employed.Firstly,the gold plate (target) was washed with acetone and deionized water (DI) water,respectively.The metal gold target was fixed in the small petri dish filled with DI water as ∼5 mm of it enveloped the surface of the piece of gold.Then,the laser beam with a frequency of 20 kHz and scanning space of 200 mm/s was aimed on the 8 mm×5 mm area of the target.The color of DI water was changed to red over time,indicating Au NPs production.To prevent power reduction and agglomeration of Au NPs,laser irradiation was stopped every 1 min and the Au NPs colloid was poured into another glass vessel.Then,LAL process was again accomplished in fresh DI water.LAL process was performed for three different weight percentages.
2.4. Synthesis of MgO/CS/Au nanocomposite
To prepare MgO/CS/Au nanocomposite,0.05 g MgO was dispersed in DI water.0.005 g CS was dissolved in 3% acetic acid solution.The obtained CS solution was added to MgO suspension.In order to protect MgO nanosheets from being dissolved by acetic acid,1 M solution of sodium hydroxide was added to the suspension of MgO before adding CS solution to it [49].Finally,the prepared Au NPs colloid were added to the MgO/CS suspension and stirred for 24 h at ambient temperature and pressure.The resultant homogenous suspensions were dried at ambient conditions and denoted as MgO/CS/Au-x.Fig.1b illustrates the steps of MgO/CS/Au nanocomposite synthesis.
2.5. Preparation of electrodes
As depicted in Fig.1c,drop-casting was used as a simple method for the fabrication of electrodes.Firstly,stainless steel mesh was cut in the area of 0.36 cm2.These electrodes were respectively rinsed with acetone and DI water.0.1 mL of MgO,MgO/CS and MgO/CS/Au-x suspensions were dropped on the electrodes (2 times) and dried at room temperature for 24 h.To determine the weight of the deposited materials,the mass of all electrodes was measured before and after dropcasting.
2.6. Hydrogen storage measurement
CV technique was used as a simple and fast instrument to investigate the H2storage capacity of MgO,MgO/CS and MgO/CS/Au-x products.A three-electrode cell was assembled including Ag/AgCl as the reference,Pt plate as the counter and a working electrode in 1 M KOH electrolyte.Firstly,asprepared working electrodes were activated in the potential window of-1.5–0.5 V with 0.1 V/s scanning rate,leading to finding the best electrode with a higher H2storage capacity.The best electrode was then optimized in the same potential window.Afterward,the H2storage capacity and cyclic life performance of the selected electrode were measured in the potential window of-1.5–0 V with 0.1 V/s scanning rate.
3.Results and discussion
3.1. Characterization of MgO/CS/Au nanocomposite
To ascertain the crystal structure of MgO nanosheets and MgO/CS/Au-x nanocomposites,the XRD analysis was exerted.As depicted in Fig.2a,the strong and sharp peaks observed in XRD stated that MgO is crystalline in nature.The MgO nanosheets peaks were observed at 36.8°,42.7°,62.2°,and 78.3° assigned to reflection planes of (111),(200),(220),and (222),respectively (JCPDS 089–4248).Four diffraction peaks located at 38.2°,44.4°,64.8° and 77.5° are attributed to planes of Au NPs (JCPDS 04–0784) which are increased with increasing Au NPs percentage.The peaks located at 38°,58.8° and 81.6° indicate diffraction of (101),(110) and (202)planes of Mg(OH)2(JCPDS 07–0239).The obtained peaks correspond to Mg(OH)2that achieved by MgO and water reaction [50].

Fig.2.(a) XRD patterns and (b) FTIR spectra of MgO nanosheets and MgO/CS/Au-x nanocomposites.
The FT-IR analysis,which identifies the characteristics and nature of functional groups,was applied to investigate the structure of MgO/CS/Au-x nanocomposites,as depicted in Fig.2b.A broad absorption peak in the wavenumber range of 2800-3700 cm-1corresponds to–OH stretching and water molecules vibration.According to MgO FT-IR spectrum,the bands 1445 cm-1and 853 cm-1are due to carbonate species and C=O stretching vibrations,respectively.Observed peaks in the range of 550–670 cm-1are ascribed to Mg-O bond[51–54].Considering MgO/CS/Au-x nanocomposites spectra,three absorption peaks at 1426 cm-1,1350 cm-1and 1564 cm-1are assigned to carbonate species,CH2and N–H bands of CS,respectively [53,55,56].In addition,it is worth mentioning that no Au-based band was observed in MgO/CS/Au-x nanocomposites.Therefore,the physical adding Au NPs has no clear influence on MgO/CS structure.
The chemical composition of MgO/CS/Au-1 was scrutinized through XPS spectra,as depicted in Fig.3.The C1s spectra is deconvoluted into four following peaks of C–C/C–H,C–O,C=O and CO32-at binding energy of 284.3 eV,286.1 eV,288.1 eV and 289.6 eV,respectively [57,58].The O 1s peak exhibits four peaks located at 532.7 eV,533.8 eV,530.9 eV and 529.6 eV,are attributed to the Mg–OH,H2O/C–O,NH–C=O and Mg–O,respectively[59].For Mg 2p spectrum,two peaks at 49.6 eV and 50.8 eV belong the binding of Mg with hydroxyl groups(Mg–OH)and oxygen atoms (Mg–O),respectively [59].The significant part of the N 1s consists of non-protonated amine or amide located at 399.7 eV.In addition,a small part located at 401.1 eV is observed due to protonated amine [60,61].It is worth mentioning that N 1s peak appeared in behalf of the presence of CS.The peak corresponding to Au NPs consists of two major peaks Au 4f7/2(83.9 eV) and Au 4f5/2(87.6 eV).The synthesized Au NPs are in metallic state and this is on account of Au (0) presence [62,63].The percentages of various bonds are listed in Table 1 and Table 2.

Table 1The different C 1s and O 1s bonds percentages.

Table 2The different Mg 2p,N 1s and Au bonds percentages.
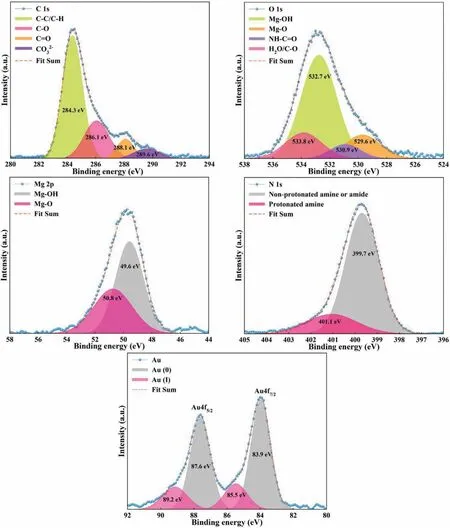
Fig.3.XPS spectra of C 1 s,O 1 s,Mg 2p,N 1 s and Au.
The shape and D-spacing of Au NPs were evolved by HRTEM images.The black area indicates spherically shaped Au NPs agglomerated on the MgO nanosheets (gray area)(Fig.4a,b).As depicted in Fig.4c,D-spacing was acquired 0.23 nm,attributing to(111)plane of Au NPs.To evaluate the crystallinity of MgO/CS/Au-1,selected area electron diffraction (SAED) pattern was employed.The obtained planes of(111),(200),(220) and (311) are ascribed Au NPs,which are in good settlement with XRD pattern.For further investigation of the surface morphology of MgO/CS/Au-1 nanocomposite,FESEM analysis was employed.The porous structure of the synthesized nanocomposite can be observed in Fig.4d,indicating that the structure of spherical ball-shaped MgO includes nanosheets which are covered by CS.As it is illustrated in Fig.4e,the MgO nanosheets were formed in a spherical ball-shaped which is enveloped by CS.The Au NPs with spherical shape and a diameter of 49–58 nm are loaded on the MgO/CS surface (Fig.4f).EDS and elemental mapping of MgO/CS/Au-1 nanocomposite is represented in Fig.5.The existence of the C,N,O,Mg and Au elements achieves from the EDS spectrum (Fig.5a).A uniform distribution of these elements over the prepared nanocomposite is observed thorough mapping images (Fig.5b).
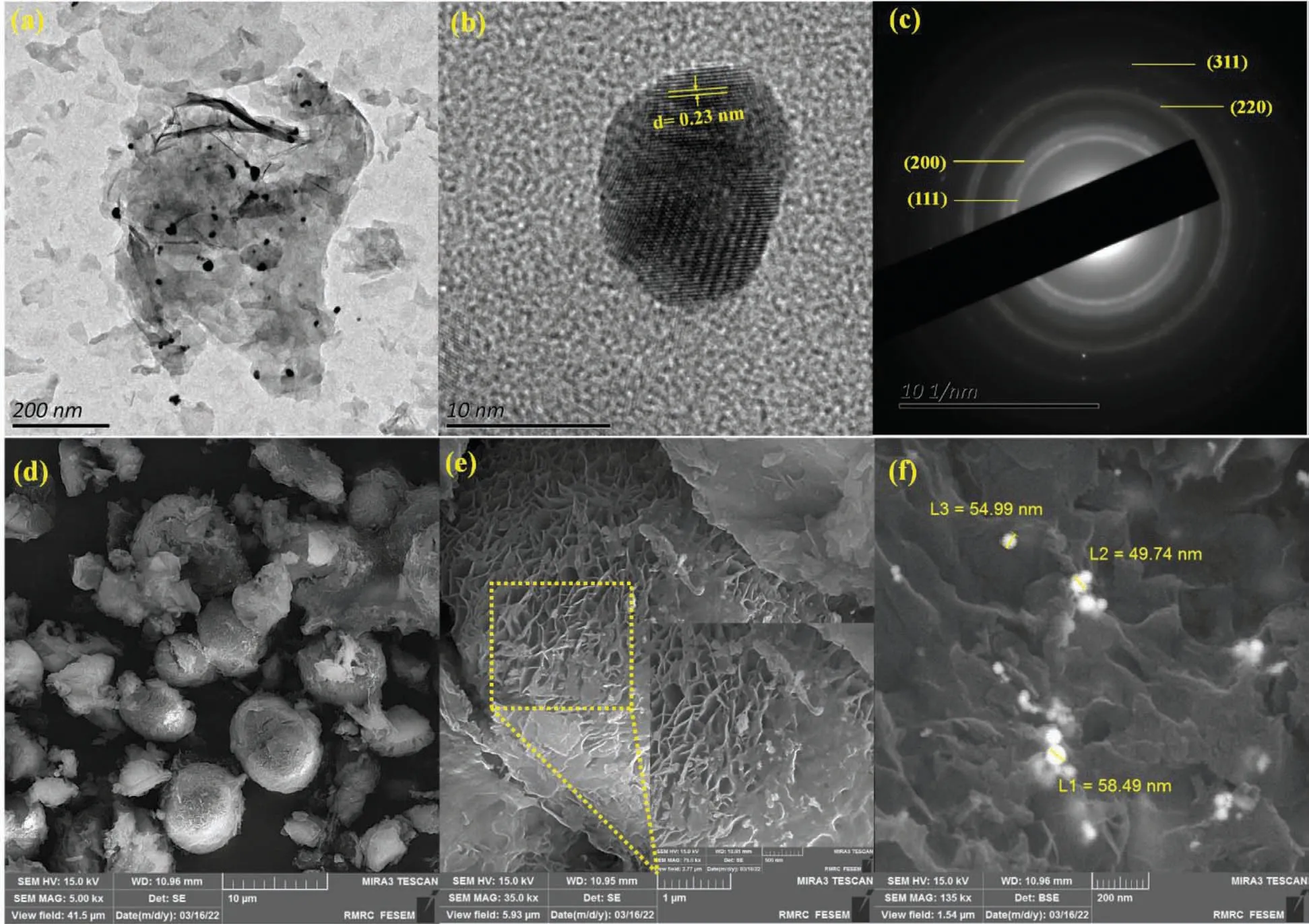
Fig.4.(a and b) HRTEM,(c) SAED and (d-f) FESEM images of MgO/CS/Au-1 nanocomposite on different magnification.
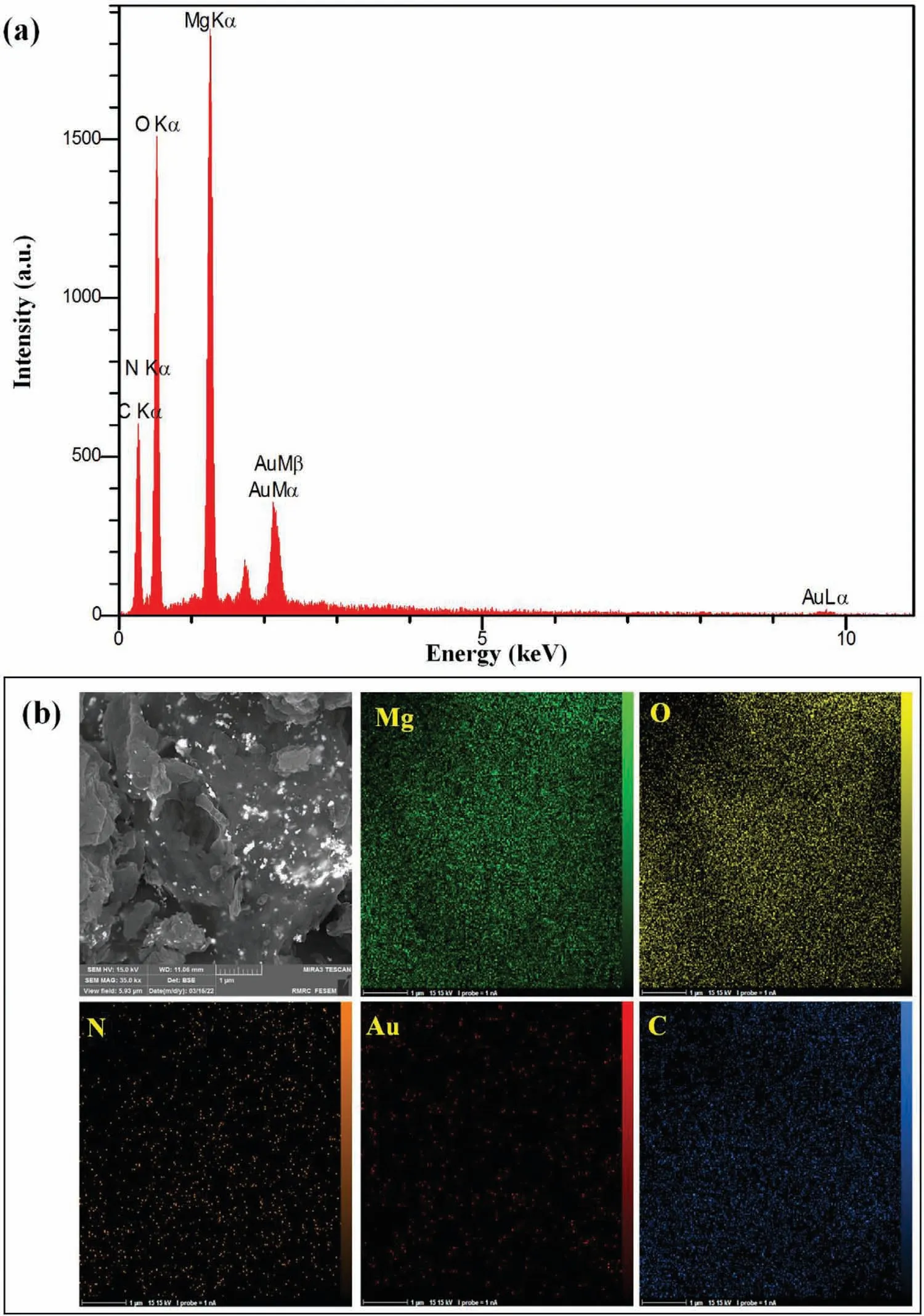
Fig.5.(a) EDS spectrum and (b) elemental mapping images of MgO/CS/Au-1 nanocomposite.
3.2. Electrochemical H2 storage study
To determine of H2storage performance of the electrode materials,CV technique was employed at room temperature.Firstly,the deposited electrodes were activated in the potential of-1.5–0.5 V during 20 cycles and the best electrode with the highest H2adsorption capacity was selected (Fig.6).As depicted in Fig.6,the faradic peaks,cathodic peak (A) and anodic peak (B),appeared for all the prepared electrodes,indicating the hydrogen adsorption and desorption processes of the electrodes’ surface.In the cathodic direction,H atoms are released via the water molecule’s dissociation.With regard to the adsorption of H atoms on the working electrode surface,a reduction peak (cathodic peak,A) is observed at-1.04 V.In opposite direction (anodic direction),an oxidation peak (anodic peak,B) is appeared at-0.72 V,indicating hydrogen ions desorption from the working electrode.The second appearance anodic peak at 0.22 V shows the oxidation of the nanostructured materials and the start of the oxidation process.Table 3,presented the comparison of the cathodic and anodic currents of the electrodes after 20 cycles.According to the results,CS and Au NPs can significantly improve oxidation peak current,indicating higher hydrogen adsorption.CS containing,amino groups,can interact with hydrogen ions which improved hydrogen adsorption current [30].Adding Au NPs can increase hydrogen adsorption owning to their electrical nature and H2spillover mechanism.However,the oxidation peak was decreased by the high content of Au NPs due to blocking the host active sites.It is worth mentioning that MgO electrode displayed low stability and deposited MgO gradually detached from its surface with increase of the number of cycles.In contrast,the MgO/CS electrode was significantly stable due to the adhesion of the CS.

Table 3Current of the cathodic (IA) and anodic (IB) peaks of the electrodes after 20 cycles activation.
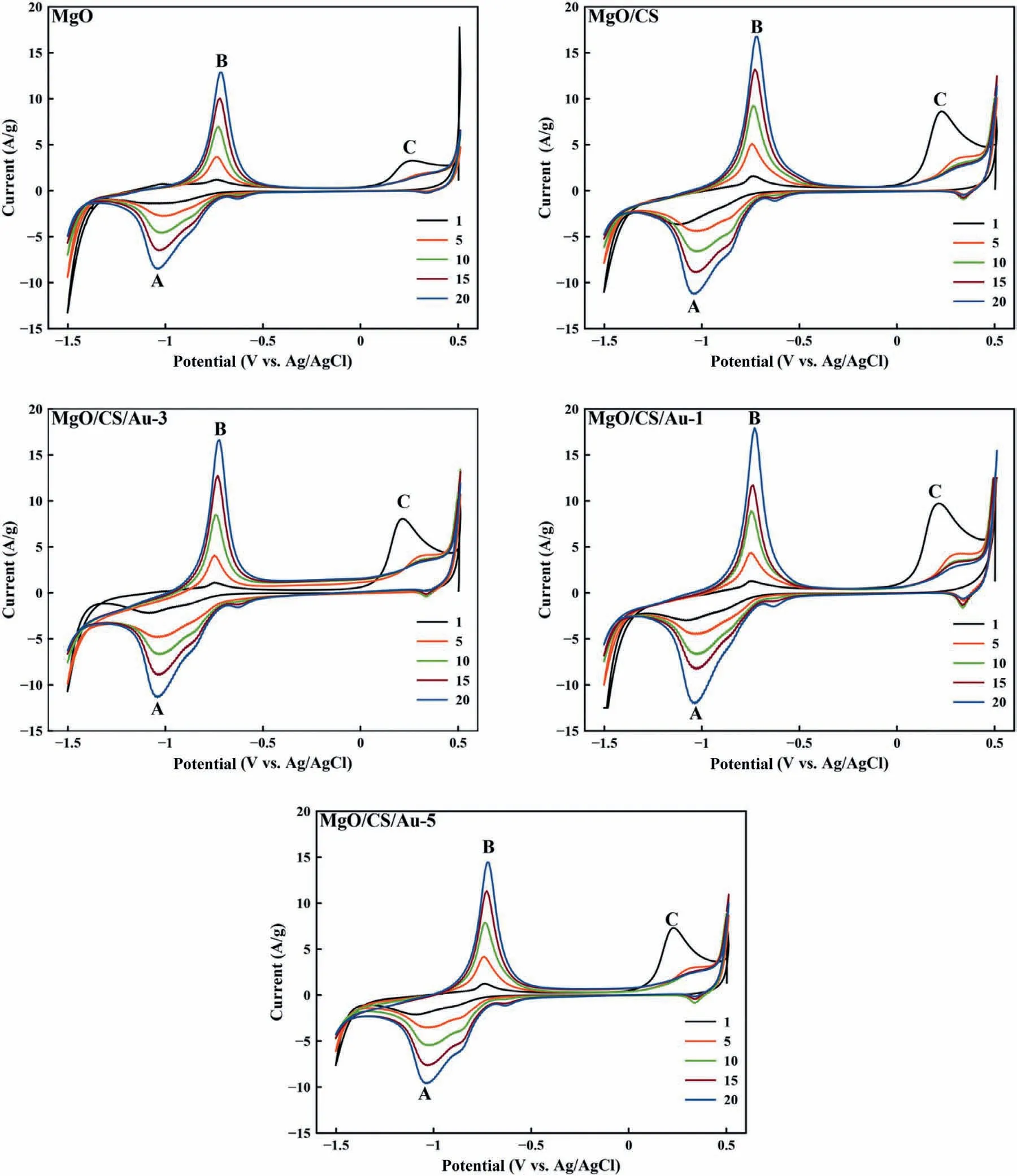
Fig.6.CV curves of MgO,MgO/CS,MgO/CS/Au-1,MgO/CS/Au-3 and MgO/CS/Au-5 samples after 20 cycles in 1 M KOH at sweep rate of 0.1 V/s.
The MgO/CS/Au-1 cycles are depicted in Fig.7.This electrode is selected as the best electrode,and it has been activated through more cycles.The oxidation peak current steadily increased from 1st to 163rd cycle.Afterward,the oxidation peak current was reduced from 164th to 169th cycle due to the oxidation of the electrode surface.The integral of the anodic peak can give the amount of stored hydrogen that is as follow:
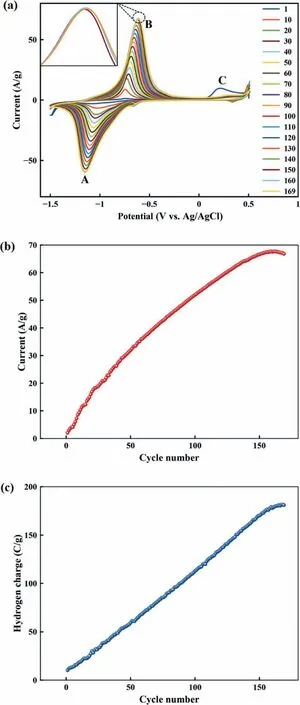
Fig.7.(a) The MgO/CS/Au-1 electrode CV curve after 169 cycles in 1 M KOH at sweep rate of 0.1 V/s,(b) B current as a function of cycle number and (c) the cyclic life performance of activated MgO/CS/Au-1 electrode.
Where,ϑindicates the scan rate,dE,IandIdlare the potential window,the anodic peak current and double layer current,respectively [64].The plotted hydrogen charge as a function of cycle number indicated that the highest hydrogen capacity is achieved after 165 CV scans.Then the optimization was stopped and the MgO/CS/Au-1 electrode hydrogen storage was examined in the potential range of-1.5–0 V at the same scanning rate.
The hydrogen adsorption/desorption ability of MgO/CS/Au-1 was studied during 300 CV scans and the B current changes,represented as a function of cycle number,were plotted,as shown in Fig.8b.A fast decay in the current intensity can be observed during the first five cycles that attribute to electrode oxidation.After 192 cycles,the current turned to be constant at approximately 4 A/g.The MgO/CS/Au-1 electrode hydrogen charge was calculated from Eq.1 and plotted as a function of cycle number,Fig.8c.A fast reduction was observed over the five first cycles and then became constant at approximately 28 C/g.The MgO/CS/Au-1 performance has been compared with other nanocomposites consisting of Au NPs in Table 4.
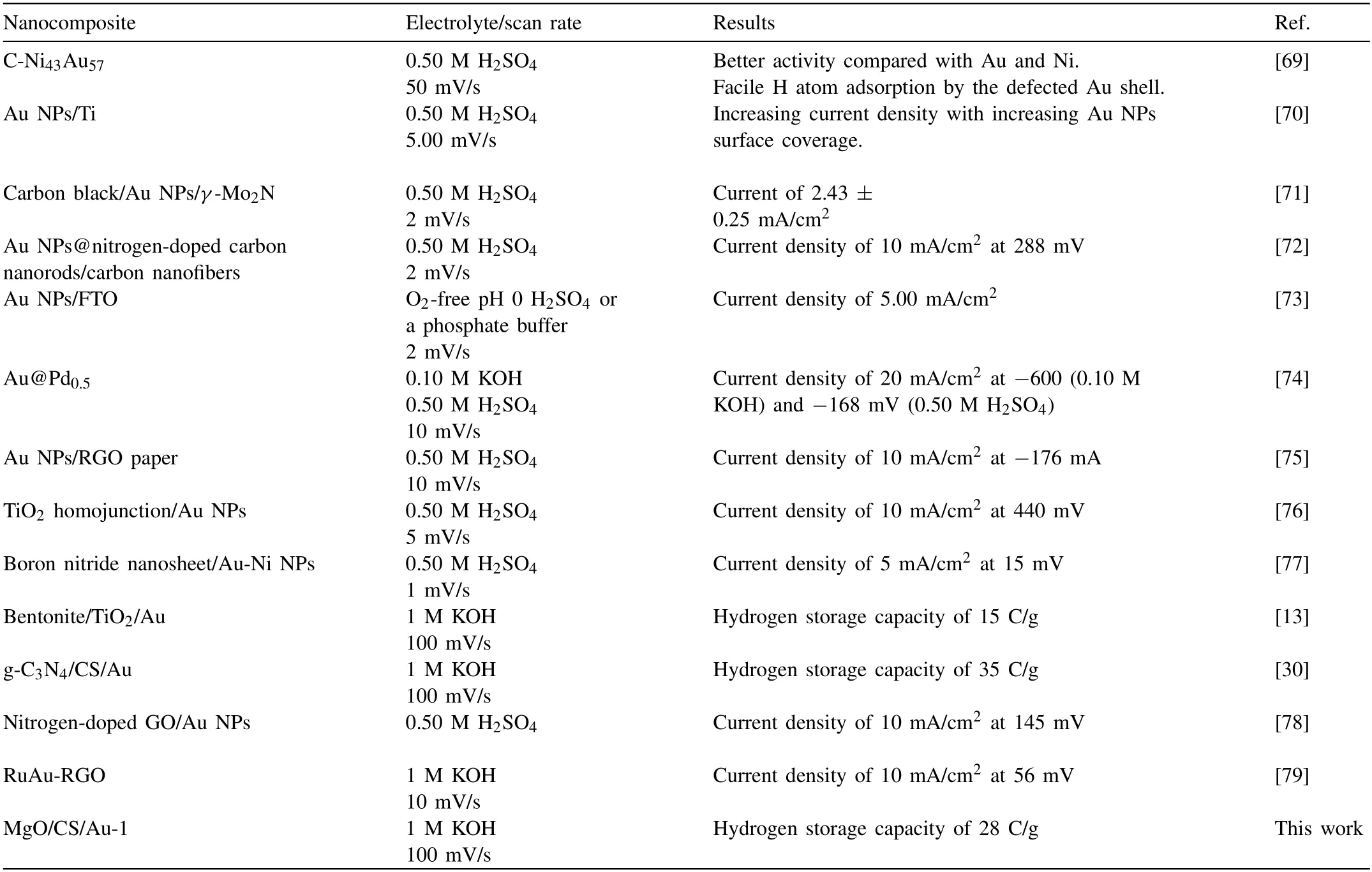
Table 4Comparison of electrochemical hydrogen storage ability of MgO/CS/Au-1 with other nanocomposites consisting of Au NPs.
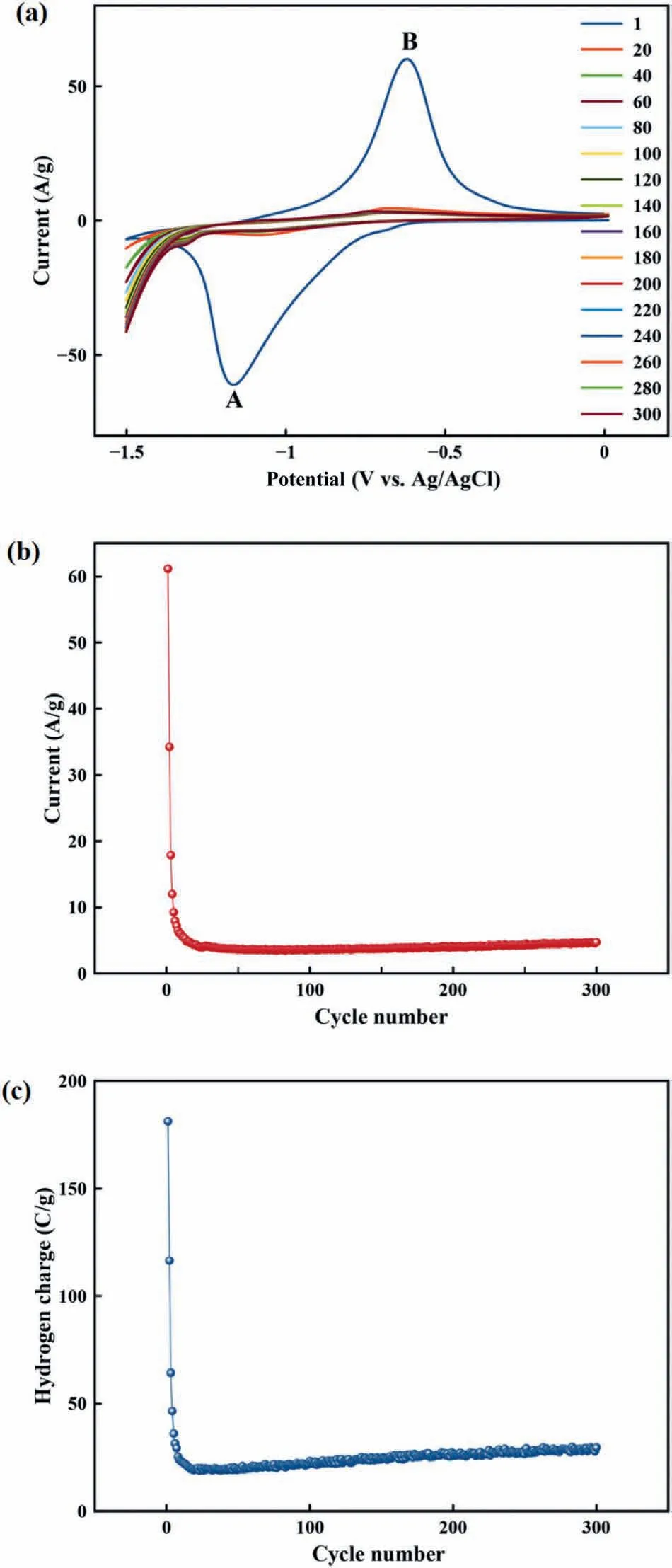
Fig.8.(a) The activated MgO/CS/Au-1 electrode CV curve for hydrogen adsorption/desorption in 1 M KOH at sweep rate of 0.1 V/s,(b) B current as a function of cycle number and (c) the MgO/CS/Au-1 electrode cyclic life performance.
FE-SEM images of the MgO/CS/Au-1 electrode before and after CV scans indicated that the pure stainless steel mesh surface has successfully been deposited by nanocomposite(Fig.9a and b).In addition,MgO/CS/Au-1 electrode represents significant stability after CV analysis (Fig.9c and d).
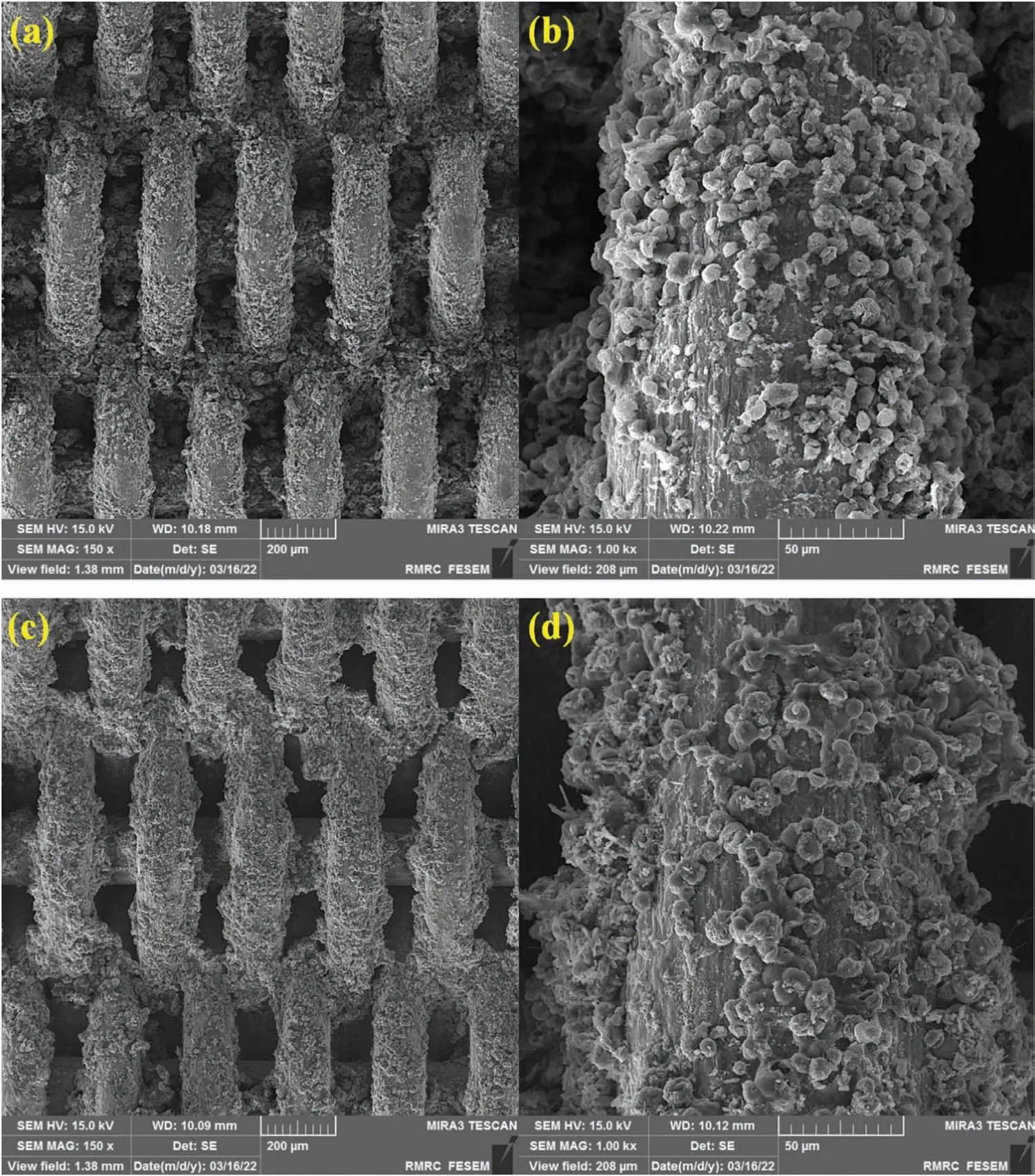
Fig.9.FESEM images of MgO/CS/Au-1 electrode (a and b) before and (c and d) after CV analysis.
3.3. The hydrogen adsorption/desorption mechanism
The three chemical reactions of Volmer,Tafel,and Heyrovsky are the best definition to introduce the mechanism of hydrogen storage on the working electrode [19,65-68].
Through applying potential,water molecules dissociate or reduce during the Volmer reaction in the first step.The released hydrogen atoms adsorb onto the surface of MgO/CS/Au-1 electrode due to electrical polarization.
The adsorbed hydrogen diffuses into the host materials of the MgO/CS/Au-1 electrode.
The hydrogen atoms recombination during the Tafel or Heyrovsky reactions causes the formation of hydrogen gaseous.
This step can be physically observed as bubbles in proximity to the MgO/CS/Au-1 electrode.In the anodic direction,the oxidation peak has appeared as the following reaction:
These reactions can repeat since the water molecule is the by-product.
4.Conclusion
Laser ablation in water is a fast and clean method for the fabrication of nanoparticles from metallic targets.Hence,laser-assisted synthesized Au NPs were loaded on the MgO/CS to prepare MgO/CS/Au nanocomposites.The structural and morphological investigation of MgO/CS/Au demonstrated that Au NPs had been deposited on the covered spherical ball-shaped MgO by CS.The CV analysis in 1 M KOH at the scanning rate of 0.1 V/s was used to study the electrochemical hydrogen storage performance of the drop-casted MgO/CS/Au-x nanocomposite on the working electrode.The CV curves suggested that MgO/CS/Au-1 electrode has a significant performance for hydrogen storage.The hydrogen storage of the mentioned electrode was obtained 28 C/g after 300 cycles,which was higher than MgO and MgO/CS electrodes.Moreover,MgO/CS and MgO/CS/Au-1 showed better stability during CV analysis because of the adhesion property of the CS.
Declaration of competing interest
The authors declare no conflict of interest.
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Ameliorating the re/dehydrogenation behaviour of MgH2 by zinc titanate addition
- Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2Y (in wt.%) alloy
- PEO coating on Mg-Ag alloy: The incorporation and release of Ag species
- Underlying mechanisms of variation in yield asymmetry and strain hardening behavior of extruded pure Mg with Gd addition
- Edge crack damage analysis of AZ31 magnesium alloy hot-rolled plate improved by vertical roll pre-rolling
- High sintering and dielectric performance: The improved (Mg,Zn)3B2O6 ceramics with the help of the DFT calculation
