An intermediate poly-dopamine layer for alginate coating on high-purity magnesium to achieve corrosion mitigation
Qingyun Fu ,Weihong Jin ,Mingcheng Feng ,Jingyo Li ,Jin Li ,Wei Li,∗ ,Zhento Yu,b,∗
a Institute of Advanced Wear & Corrosion Resistant and Functional Materials,Jinan University,Guangzhou 510632,China
b Northwest Institute for Nonferrous Metal Research,Xi’an 710055,China
Abstract Although magnesium (Mg) and its alloys are proposed as the next generation orthopedics transplanted materials,their clinical applications are limited by the fast degradation.To reduce the degradation rate,a strong adhesion poly-dopamine (PDA) layer was introduced as an intermediate layer for the subsequent alginate (ALG) spin-coating on high-purity Mg.The surface morphology and chemical composition were detected by scanning electron microscope,energy disperse spectroscopy,and Fourier transform infrared spectroscopy.The corrosion resistances of all samples were evaluated by electrochemical and 10-day immersion tests in Hanks’ balanced salt solution.Our results suggest that the thickness of the fabricated PDA/ALG composite coating is 8.58 ± 0.65 μm,and the intermediate PDA layer evidently enhances the adhesion between the substrate and ALG coating.The corrosion current density of Mg coated with the PDA/ALG composite coating decreases more than 10 times compared to that of the Mg substrate,and the charge transfer resistance is 12 times bigger than that of the bare Mg,which indicates the improved corrosion resistance.Moreover,the mechanism of corrosion protection of the composite coating is also discussed.
Keywords: Magnesium;Coating;Ploy-dopamine;Alginate;Corrosion.
1.Introduction
Magnesium (Mg) and its alloys have attracted tremendous attention as potential biodegradable orthopedic implants due to their excellent biodegradability,elastic modulus close to that of natural bones,and great biocompatibility.However,the rapid degradation of Mg-based implants leads to adverse reactions such as hydrogen release,rapid increase in the local pH,and mechanical strength reduction,which hinder their clinical applications [1–3].Hence,it is essential to develop appropriate methods to improve the corrosion resistance of Mg and its alloys.To suppress the degradation rate of Mg and its alloys,alloying and surface coating have been developed.Alloying [4,5] is a common method for the substrate treatment.Although the alloying elements can reduce the degradation rate of Mg and its alloys,alloying elements that can be applied in clinical practice are limited[6],and improvement of the corrosion resistance are limited.On the contrary,surface coating is not only a well-known strategy to efficiently decrease the degradation rate of Mg and its alloys without affecting their inherent properties,but also more convenient and economical [7,8].
Some technologies such as dip-coating [9],micro-arc oxidation [10],spin coating [11],electroless plating [12],ion implantation [13],and sol-gel method [14] are commonly applied to fabricate coatings on Mg and its alloys.Electroless Ni-P coating was prepared on the zirconiumpretreated AM60B Mg alloy by Seifzadeh et al.[12],and the continuous noise resistance calculation showed that the prepared uniform,pore-free,dense,and cauliflower-like Ni-P coating improved the corrosion resistance of the bare alloy.Jin et al.[13] performed Nd self-ion implantation into WE43 Mg alloy and the enhanced corrosion resistance was mainly attributed to the formation of the stable Nd2O3outer layer.Seifzadeh et al.[14] fabricated the oxidized fullerene incorporated sol-gel coating on AM60B Mg alloy and found that the enhanced corrosion resistance of the AM60B alloy was achieved by the hybrid sol-gel coating incorporated with 25–100 mg L-1oxidized fullerene nanoparticles.In contrast,dip coating and spin coating are widely used by researchers to produce polymer coatings due to its simple and convenient operation.
To retard the degradation of Mg and its alloys and improve their cytocompatibility,natural polymer-based coatings are considered as a valid coating strategy [1].As a natural polymer,alginate (ALG) exists in the cytoplasm and plays an important strengthening effect in the cell wall [15].Moreover,ALG have good biodegradability and is widely used in drug delivery [16],food industry [17],and tissue engineering [18].Liu et al.[19] fabricated an ALG hydrogel coating on the micro-arc oxidation-treated Mg alloy,and the platelets adhesion assay and hemolysis ratio test in vitro demonstrated that the ALG hydrogel coating can improve the biocompatibility of the Mg alloy substrate well.Although Suga et al.[20] reported that the ALG coating retarded the local alkalinity and rapid hydrogen release of biodegradable Mg-Zn-Ca alloy.However,an obvious crack occurred at the interface between the coating and substrate when the ALG coating was directly fabricated on Mg-Zn-Ca alloy surface.The weak adhesion between the ALG coating and substrate tends to cause adverse effects on long-term stability of Mg-based implants.Hence,it is important to explore a suitable intermediate layer to enhance the adhesion between the ALG layer and Mg-based substrate.
Mussel-inspired poly-dopamine (PDA) was first reported by Lee et al.[21] and then widely used in the field of biomaterials due to its great biocompatibility.The use of PDA has been considered to be an achievable strategy to obtain high-performance coatings on Mg and its alloys,which is attributed to the fact that dopamine contains imine,amino,and catechol functional groups [22–24].It is well known that the PDA film can be easily formed on almost all solid surface[15,16].Considering the special performances of PDA,Xie et al.[25] developed a PDA intermediate layer,which was used for inducing the polyether imide deposition on the pure Mg.The results indicated that the PDA intermediate layer enhanced the adhesion of the polyether imide coating to the pure Mg substrate and decreased the corrosion rate of Mg.
In this work,a composite coating composed of the PDA pretreatment layer and outer ALG protection layer were constructed on high-purity Mg for potential orthopedic applications.The sandwiched PDA and outer ALG layers were prepared by dip-coating and spin-coating techniques,respectively.The surface morphology,chemical composition,and thickness of the composite coating were investigated.The corrosion resistance was examined by the electrochemical and 10-day immersion tests in Hanks’ balanced salt solution (HBSS).The mechanism of corrosion protection of the PDA/ALG hybrid coating was also discussed.
2.Experimental details
2.1. Materials and chemicals
In this work,industrial-grade pure Mg ingots (99.99 wt% Mg;Al 0.0028;As <0.0001;Ca <0.001;Cd <0.0001;Cr 0.0002;Fe <0.0001;Cu <0.0001;Hg <0.0001;Mn 0.0004;Ni 0.0002;Zn 0.0028;Pb 0.0004;Si <0.001;Sn 0.0002;Ti 0.0003;and Zn 0.001) were obtained from Northwest Institute for Non-ferrous Metal Research,China.Dopamine hydrochloride (98%,M=189.64 g mol-1)was bought from Shanghai Macklin Biochemical Co.,Ltd,China.Sodium alginate (Viscosity=200 ± 20 mpa s) was purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd,China.All chemical reagents were of analytical grade and used without further purification.
2.2. Sample preparation
The high-purity Mg ingots were cut into plates with dimensions of 10 mm × 10 mm × 5 mm.Prior to the coating preparation,all plates were mechanically ground with silicon carbide abrasive papers successively up to 2000 grit to obtain a bright and smooth surface.The samples were then treated by ultrasound in 100% ethanol for 15 min.Afterwards,the treated samples were dried by an electric blower at room temperature.
The PDA coating was prepared by the oxidative selfpolymerization of dopamine in a mild alkaline aqueous medium with a pH of 8.5.The forming process of the PDA coating is described in Fig.1(a).First,dopamine is oxidized and self-polymerize at room temperature spontaneously and then forms 5,6-dihydroxyindole through the intermolecular cyclization.Finally,the PDA is formed by the polymerization reaction [26,27].

Fig.1.(a) Possible reaction mechanism for the dopamine polymerization.(b) Schematic diagram of preparation process of the Mg/PDA/ALG composite coating.
Fig.1(b) shows the schematic illustration of preparation process of the PDA/ALG composite coating on high-purity Mg.Briefly,dopamine hydrochloride (2 mg mL-1) was dissolved in the 50 mM Tris–HCl buffer solution (pH 8.5)to prepare a soak solution of dopamine.The Mg specimens were immersed in the soak solution with stirring for 24 h at room temperature,and the samples with the top surface deposited with PDA were then taken out,rinsed three times with deionized water,and dried [28].The PDA coated samples were labeled as Mg/PDA.Afterwards,spin coating were performed on the PDA layer using the 3 mg mL-1alginate solution at a rotate speed of 1000 rpm for 15 s and 3500 rpm for 45 s,and the samples were then immersed in the 0.1 mol L-1Ca2+solution for 15 min to enhance the alginate net structure.The obtained specimens were marked as Mg/PDA/ALG.The ALG layer was directly coated on the Mg substrate with the same method,and the sample was named as Mg/ALG as a control group.
2.3. Surface characterization
The surface morphology and thickness of the coatings were observed by scanning electron microscope (SEM,Phenom XL,Field Electron and Ion Company,Netherlands)equipped with an energy dispersive X-ray spectroscopy(EDS).Before the SEM detection,all samples were coated with gold.The thickness of the coating was calculated from three different regions in the cross-sectional SEM image.The cross-sectional samples were prepared according to the following procedures.Firstly,all samples were mechanically ground with successive silicon carbide abrasive papers (600#,1200#,and 2000#).Subsequently,the samples were polished with 1.5 and 0.5 diamond paste,respectively.Finally,the samples were cleaned ultrasonically in 100% ethanol for 5 min and dried.The chemical elements were detected by EDS.The functional groups of the coatings were investigated by Fourier transform infrared spectroscopy (FT-IR,Nicolet iS50,Thermo Fisher Scientific,America) in the wavenumber range between 4000 cm-1and 550 cm-1.
2.4. Electrochemical corrosion tests
Prior to electrochemical corrosion tests,all specimens were sealed by silicone rubber (Kafuter 704,Guangdong Hengda New Material Technology Co.LTD,China) with a working area of 1 cm2.The corrosion tests of the coated samples were conducted in HBSS (8.0 mg mL-1NaCl,0.4 mg mL-1KCl,0.1 mg mL-1MgCl2·6H2O,0.14 mg mL-1CaCl2,0.35 mg mL-1NaHCO3,0.06 mg mL-1MgSO4·7H2O,0.06 mg mL-1KH2PO4,0.06 mg mL-1Na2HPO4·12H2O,and 1.0 mg mL-1glucose) with a three-electrode system on the electrochemical workstation (PARSTAT 4000,Princeton Applied Research,America) at 37 °C.The sample,saturated calomel electrode,and platinum mesh acted as the working electrode,reference electrode,and counter electrode,respectively.The open circuit potential (OCP) was firstly monitored for 30 min to reach a relatively stable value.The frequency range of electrochemical impedance spectroscopy (EIS) was recorded from 100 kHz to 0.01 Hz with a perturbation voltage of 10 mV.The potential range of potentiodynamic polarization (PDP) was eventually carried out from-300 mV to 500 mV with a scanning rate of 1 mV s-1.All electrochemical tests were repeated at least 3 times for statistical purpose.
2.5. Immersion degradation tests
The long-term degradation behaviors were investigated by immersing the bare and coated specimens in 40 mL HBSS for 10 days at 37 °C with an exposed area of 1 cm2.The volume of released hydrogen and varied pH values of the immersion solution were recorded as indicators to evaluate the corrosion rate.For the hydrogen collection,the immersion solution was replaced every two days and a tube was used to collect the released hydrogen.The pH value of the immersion solution was recorded every day without changing the HBSS.After 10-day immersion,the surface morphology and chemical elements of corrosion products of all samples were examined by SEM and EDS,respectively.Moreover,the corrosion products were removed by putting the samples into the chromic acid solution(200 g L-1CrO3+10 g L-1AgNO3)for 7 min,and the specimens were then rinsed by deionized water no less than 3 times.After drying,the surface morphologies of the samples without corrosion products were observed by SEM again.
3.Results and discussion
3.1. Surface characterization
The SEM surface morphology of different samples and their corresponding cross-sectional views are displayed in Fig.2.There are a plenty of scratches on the bare Mg surface,which results from the mechanical grinding.For the Mg/PDA sample,the substrate surface is completely covered by an obvious membrane,but presents numerous irregular and random distributing cracks,which may be a threat to prevent the corrosive media to penetrate onto the substrate.For Mg/PDA/ALG,a fully covering,uniform,and compact ALG coating is observed on the Mg surface,and the abrasion marks are distinctly desalinated.Compared to the single ALG and PDA coatings,the PDA/ALG composite coating exhibits a more tightness and even surface,and the cracks of Mg/PDA sample are completely sealed by the ALG coating,which is beneficial for preventing the ion penetration through the coating impeding the direct contact between the electrolytes and Mg substrate.Moreover,the cross-sectional profiles of all samples are examined by SEM.
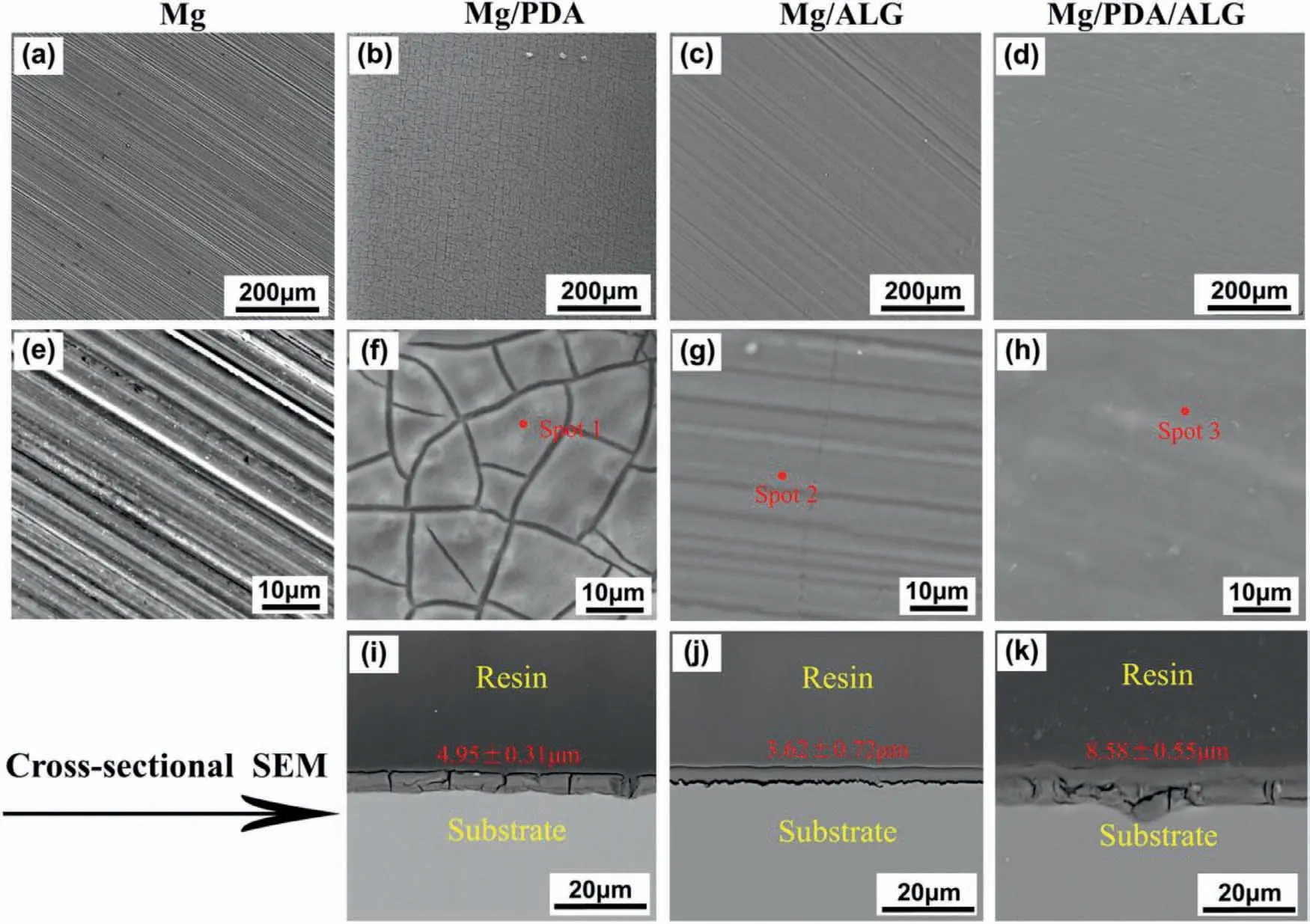
Fig.2.Representative SEM images of the surface topographies of (a,e) the bare Mg,(b,f) Mg/PDA,(c,g) Mg/ALG,and (d,h) Mg/PDA/ALG,respectively;cross-sectional SEM images of (i) Mg/PDA,(j) Mg/ALG,and (k) Mg/PDA/ALG.
Figs.2(i-k) show the SEM cross-sectional profiles of the Mg/PDA,Mg/ALG,and Mg/PDA/ALG samples,respectively.For the PDA coating,the thickness is approximately 4.95 ± 0.31 μm,slightly thicker than that of the ALG coating (3.62 ± 0.16 μm).Although the PDA coating has some cracks,it bonds to the Mg substrate closely due to the adhesion performance of PDA,which can reduce the possibility of coating delamination.As a hydrogel coating,the ALG coating shows a higher compactness than the PDA coating,which contributes to protection of the substrate in the physiological environment.However,an obvious gap between the ALG hydrogel coating and Mg substrate is observed from the cross-sectional image.On account of the poor connection between the ALG coating and substrate,it may cause the failure of coating protection.From the SEM cross-sectional image of the PDA/ALG composite coating,it can be seen that the thickness of the PDA/ALG composite coating reaches 8.58 ± 0.65 μm,and the ALG coating adheres well to the Mg substrate with the PDA pretreatment layer.These results suggest that the PDA interlayer effectively enhances the connection between the ALG layer and Mg substrate.According to the previous literature [26],the exposed amine and catechol groups of the PDA molecules result in the strong adhesion of PDA to the Mg substrate.Moreover,the PDA molecules contain numerous functional groups,which can serve as binding sites for the ALG hydrogel via Van der Waals and hydrogen-bonding interactions.
The EDS spectra of spot 1,spot 2,and spot 3 are displayed in Figs.3 (a-c).Fig.3(a) shows that the C and N elements are observed on the surface of Mg/PDA,which mainly comes from the amino and catechol of PDA,indicating the presentence of the PDA layer on the Mg substrate.For spots 2 and 3,the elements of N,C,and Ca in Figs.3(b)and 3(c) illustrate that there remains an ALG coating on the Mg substrate and Mg/PDA sample.

Fig.3.(a)-(c) EDS point analysis and (d) FTIR spectra of the Mg/PDA,Mg/ALG,and Mg/PDA/ALG samples.
To further investigate the specific composition of the coatings,the FTIR spectra of the three coated specimens are acquired as shown in Fig.3(d).It can be seen that from the spectrum of Mg/PDA,the characteristic bands of PDA at 1631 cm-1,1491 cm-1,and 1263 cm-1corresponds to the C=C,C–N,and phenolic alcohol bands,respectively [29],which demonstrates that the PDA layer is successfully fixed on the Mg substrate.Dopamine is suitable for anchoring on different types of solid material surface due to the strong covalent interaction of its catechol structure with solid materials [30].For Mg/ALG,the strong absorption characteristic bands at around 1035 cm-1,1415 cm-1,and 1607 cm-1are assigned to the six-member ring stretching vibration absorption and asymmetric and symmetric stretching vibrations of carboxylate groups of ALG,while the detected strong absorption band at 3262 cm-1is attributed to the hydroxyl group of ALG [29,30].These results indicate the fabrication of the ALG coating on the substrate.Compared to the stretching vibration band of O–H of the ALG coating,it shifts to a low wavenumber (3235 cm–1) for the Mg/PDA/ALG sample,which may arise from the overlapping bands of the hydroxyl groups on ALG and catechol and amine groups on PDA leading to the formation of intermolecular hydrogen bonds between ALG and PDA.The characteristic bands at 1597 cm-1and 1410 cm-1belong to the asymmetric and symmetric stretching vibrations of carboxylate groups emerging on the surface of the PDA/ALG composite coating,respectively.The carboxylate absorption bands of the PDA/ALG composite coating are different from those of the pure ALG layer appearing some shifts,which may due to the influence of the catechol and amine groups of PDA [31].Additionally,the band at 1262 cm-1corresponds to the phenolic alcohol bands [32],and two bands observed at 1086 cm-1and 1035 cm-1are related to the stretching of C–O and six-member ring of ALG do not change after conjugation with the PDA coating [31,33].Therefore,these results suggest that the PDA layer is successfully modified by the ALG layer.
3.2. Electrochemical corrosion behavior
The degradation of Mg is actually an electrochemical corrosion process [34].Therefore,it is suitable to study the corrosion properties by electrochemical tests conducted in HBSS at 37 °C.Fig.4 shows the PDP curves of the bare and coated samples,and the corresponding corrosion potential (Ecorr),corrosion current density (icorr),and cathode Tafel slope (bc) values are obtained by the cathodic Tafel extrapolation method [35,36] and given in Table 1.The Mg/PDA/ALG sample has the lowest cathode and anode current density.The lowest cathode current density indicates that the hydrogen evolution of cathode can be effectively hindered.Meanwhile,the lowest anode current density clarifies that the dissolution of the substrate is restrained [37].To be specific,theEcorrvalue of the bare Mg substrate is-1.78 V vs.SCE,whereas theEcorrvalues of Mg/PDA,Mg/ALG,and Mg/PDA/ALG are-1.75 V,-1.63 V,and-1.57 V vs.SCE,respectively.The three coated samples show a relatively positive potential shifts compared to the bare Mg substrate,and the Mg/PDA/ALG sample shows the largest positive potential shift.This suggests that the three types of coatings decrease thermodynamic tendency of corrosion of the bare Mg substrate to a certain extent,and the PDA/ALG composite coating has a better inhibitory effect on thermodynamic tendency of corrosion than the PDA and ALG coatings.This is due to that the compact ALG layer blocks the cracks in the PDA coating,effectively improving corrosion resistance.From the histogram oficorr,it can be seen that all coated samples have a smallericorrthan the substrate.Theicorrvalue of Mg/PDA decreases from 45.34 μA cm-2of the substrate to 35.56 μA cm-2,indicating a lower corrosion rate of the Mg/PDA sample.This is in line with those in the previous reports and demonstrates the use of PDA is a rational strategy for protecting Mg against corrosion [28,38].Theicorrvalue of the Mg/ALG is 29.85 μA cm-2,lower than that of the Mg substrate,which is assigned to that the compact ALG layer isolates the electrolyte solution from directly contacting with the substrate.For Mg/PDA/ALG sample,theicorrvalue decreases to 3.24 μA cm-2,which is approximately 13 and 11 times smaller than that of the bare Mg substrate and Mg/PDA,respectively.Compared to Mg/ALG,the value of theicorrof Mg/PDA/ALG reduces nearly one order of magnitude than that of Mg/ALG,which indicates the PDA/ALG composite coating exhibits the best protection among the coatings.From the polarization curves,all the samples show a cathodiccontrol type,conforming to the characteristic of active metal corrosion [39].The Mg/PDA/ALG sample displays the smallesticorr,which results from its lowest cathodic current density.It is worthy to note thaticorrof the Mg/PDA/ALG sample is apparently lower than that of the Mg/ALG sample,which indicates the validity of PDA as a sandwich layer to suppress the corrosion process of the Mg substrate.
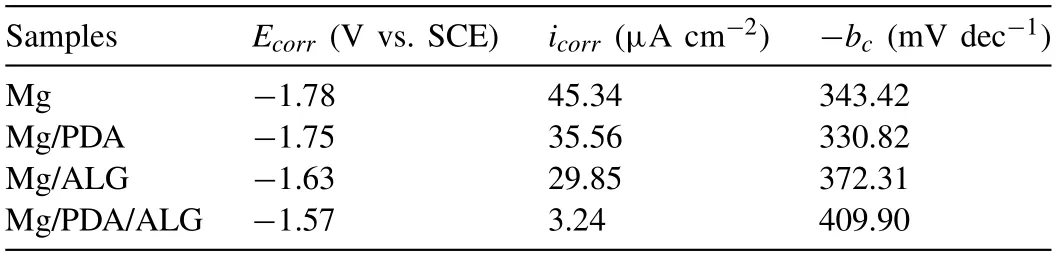
Table 1Polarization parameters of the bare and coated samples.

Fig.4.PDP curves of the bare Mg,Mg/PDA,Mg/ALG,and Mg/PDA/ALG samples.
EIS is a powerful way to decipher the kinetics of the corrosion process[40].From the Nyquist plots in Fig.5(a),it can be seen that the diameters of the capacitive loops follow the trend: Mg/PDA/ALG >Mg/ALG >Mg/PDA >Mg.Since the semi-circle loop indicates the capacitive and resistive behavior of the coating [41],the composite coating shows the best protection to the Mg substrate in HBSS [42].The bode impedance and bode phase plots are displayed in Figs.5(b)and (c).The |Z| value of Mg/PDA/ALG (1.7 × 105Ωcm2)is higher than those of Mg/PDA (1.2 × 103Ωcm2) and Mg/ALG(1.6×103Ωcm2)and much higher than that of the bare Mg (8.7 × 102Ωcm2).In the low frequency region,the greater the impedance modulus is,the better the corrosion resistance is [43].This indicates that the Mg/PDA/ALG sample owns better resistance against corrosion than the other two coated samples and bare Mg substrate.The phase angle of pure Mg is close to 53°,lower than that of three coated samples,and the Mg/PDA/ALG sample has a wider range of frequencies with an additional time constant than the two other coated samples,these reveal that the PDA/ALG composite coating is a more effective physical barrier to corrosion [37].

Fig.5.(a) Nyquist plots,(b-c) Bode plots,and (d) corresponding equivalent circuit models.
Based on the EIS plots,three sets of the appropriate equivalent circuit models in Fig.5(d) are used to fit the EIS data to understand the processes of corrosion of all samples.The model A is suitable for the fitting of the bare Mg,and the equivalent electrical circuit is proposed asRs(Qp1Rp1)(QdlRct(RL(L))).In this model,Qdldenotes the double layer capacitance.Qp1andRp1represent the capacitance and pore resistance of the corrosion layer on the Mg substrate,respectively.The low-frequency inductive loop is characterized by inductance (L) and resistance (RL),meaning the pitting corrosion of the Mg substrate.The model B,Rs(Qp1Rp1)(QdlRct),is used to fit the EIS data of the Mg/PDA and Mg/ALG samples.TheQdldenotes the double layer capacitance.Qp1andRp1represent the capacitance and pore resistance of the PDA layer on Mg/PDA and ALG layer on Mg/ALG,respectively.The model C is used to fit the EIS data of Mg/PDA/ALG and is present asRs(QP3RP3)(Qp2Rp2)(CdlRct).In this model,Qp2andRp2symbolize the capacitance and pore resistance of the PDA layer of Mg/PDA/ALG,respectively.Qp3andRp3are the capacitance and pore resistance of the ALG layer of Mg/PDA/ALG,respectively.Cdlcorresponds to the double layer capacitance.In all equivalent circuits,RsandRctrepresent the solution resistance and charge transfer resistance,respectively.
The fitted results are listed in Table 2.The value ofRctcan roughly reveal the corrosion protection effectiveness of the samples because the corrosion rate of the metallic substrate beneath the coating is inversely proportional toRct.TheRctvalue of Mg/PDA/ALG is 2563Ωcm2,which is nearly 8 times larger than 333.9Ωcm2of Mg/PDA,over 6 folds than that of Mg/ALG (416.6Ωcm2),and 12 times larger than that of the untreated Mg substrate (208.2Ωcm2),respectively.The pore resistance is an important parameter to evaluate ion penetration through pathways in the coatings.Compared to 254.8Ωcm2of the bare Mg,theRp1value of Mg/PDA rises to 3395Ωcm2,corresponding to a 13 times increase.This means that the PDA layer hinders the direct contact between the electrolyte and Mg substrate.TheRp1value of Mg/ALG increases to 5703Ωcm2,resulting from the higher compactness of the ALG coating,whereas theRp2value of Mg/PDA/ALG reaches 13,890Ωcm2,which increases about 55 times in contrary to that of the substrate.These results demonstrate that the sandwiched PDA/ALG coated Mg has good resistance against corrosion under the physiological conditions.
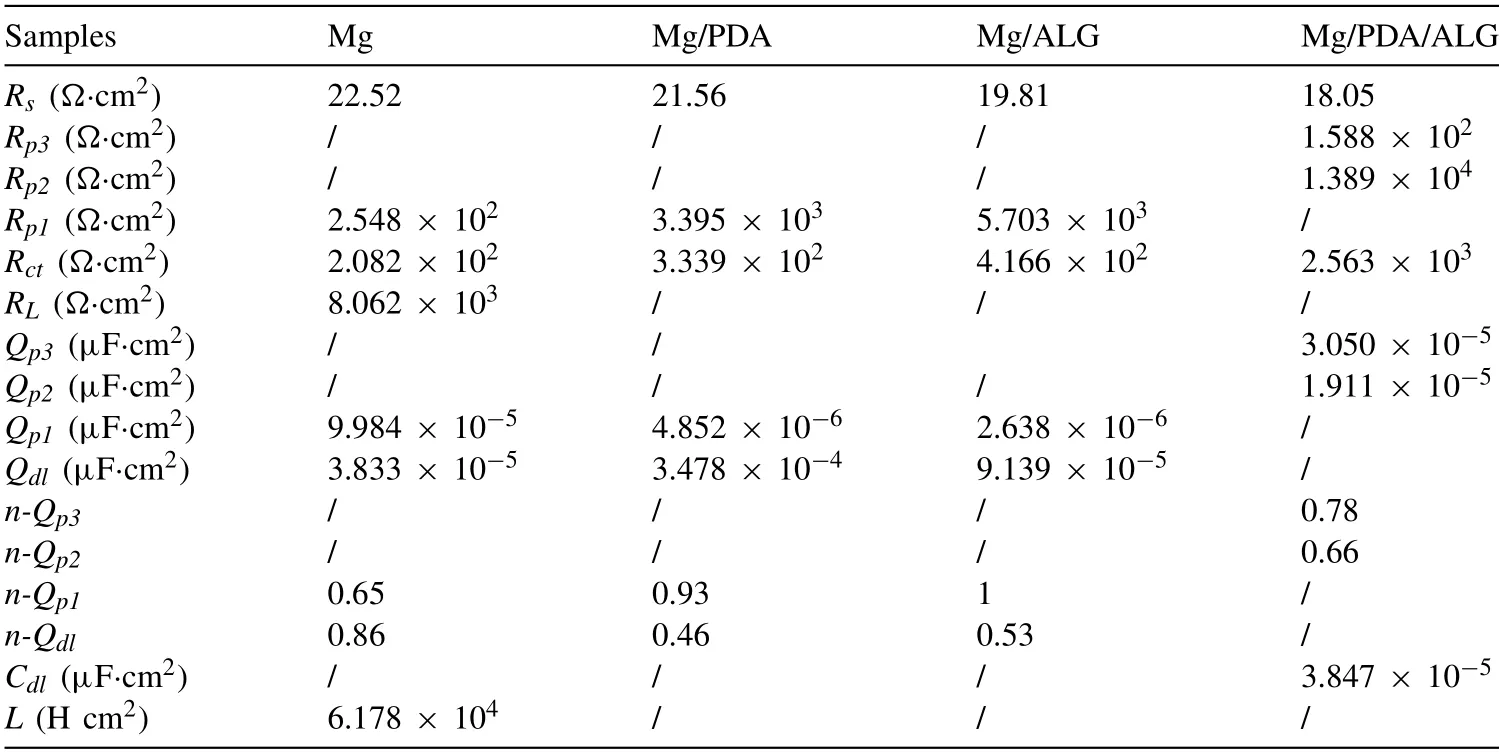
Table 2Fitted electrochemical parameters of EIS plots.
3.3. Immersion degradation behavior
The degradation behavior of an Mg-based biomaterial is a long-term and dynamic process [34].Therefore,it is of great importance to carry out immersion tests to assess long-term degradation behavior.It is well known that the mechanism of Mg degradation is Mg+2H2O →Mg2++2OH-+H2↑[44].Hence,both hydrogen evolution and pH variation can work as indicators to investigate the degradation behavior of Mg and its alloys.During 10-day immersion in HBSS,the released hydrogen is depicted in Fig.6(a).It can be noted that the Mg/PDA/ALG sample has the lowest hydrogen evolution volume over the entire period of immersion.The hydrogen evolution volume of the Mg/PDA sample is slightly smaller than that of the Mg substrate,which is attributed to the numerous cracks on the surface of the PDA layer.Because the compactness of the ALG coating is higher than that of the PDA coating,the hydrogen evolution volume of the Mg/ALG sample further reduces compared to the Mg/PDA sample.The variation of pH values of all samples during 10-day immersion in HBSS is displayed in Fig.6(b) and has a similar trend to the hydrogen evolution.These results suggest that the PDA/ALG composite coating effectively inhibits degradation of the Mg substrate.

Fig.6.Immersion degradation testing results of the samples after immersion in HBSS at 37 °C for 10 days: (a) volume of hydrogen evolution and (b) change of the pH value.
The SEM images in Figs.7(a-d) present the surface corrosion products of all samples after 10-day immersion in HBSS.The corrosion products of high-purity Mg are inhomogeneous distribution,whereas the corrosion products on the surface of Mg/PDA are evenly distributed,but has a loose structure,which may provide channels for the electrolyte solution invasion.For the Mg/ALG sample,the surface corrosion products have a plenty of cracks,which may be a big challenge for the long-term protection to the Mg substrate.The corrosion layer of the Mg/PDA/ALG sample has a similar feature as that on the corroded Mg/PDA sample,but the number of cracks decreases obviously,which is beneficial for protecting the substrate.
The SEM images after removal of corrosion products from the surface of samples presents in Figs.7 (e-h).As shown in Fig.7e,numerous deep etch pits are observed from the corroded surface of bare Mg sample,manifesting that the substrate undergoes severe attack.Figs.7(f) and 7(g) show the morphology of the corroded Mg/PDA and Mg/ALG without corrosion products,respectively.Although corrosion pits are significantly reduced,severe corrosion is still observed.On the contrary,there are only a small number of small and shallow etch pits on the surface of corroded Mg/PDA/ALG as depicted in Fig.7(h).These results imply that the extent of corrosion and rate of corrosion propagation on Mg/PDA/ALG are the smallest.

Fig.7.SEM images of the Mg,Mg/PDA,Mg/ALG,and Mg/PDA/ALG samples after soaking in HBSS at 37 °C for 10 days (a-d) with and (e-h) without corrosion products.
In the meantime,the chemical elements of surface corrosion products of all samples are also investigated.The EDS spectra of the different corroded samples and statistical results of quantitative analysis are displayed in Fig.8.For the Mg substrate,the corrosion products mainly contain the Mg,O,P,and Ca elements.For the coated samples,the detected corrosion products consist of the Mg,O,P,Ca,C,and N elements.The presence of the P and Ca elements means the deposition of Ca-P corrosion products on the surface of all samples surface.Also,the Ca/P ratios in corrosion products of the Mg,Mg/PDA,Mg/ALG,and Mg/PDA/ALG are 1.47,1.56,1.59,and 1.64,respectively.The Mg/PDA/ALG sample has the highest Ca/P ratio,it demonstrates the PDA/ALG coating having a more conducive to the formation of Ca-P products,which may be beneficial to the growth of osteoblasts.
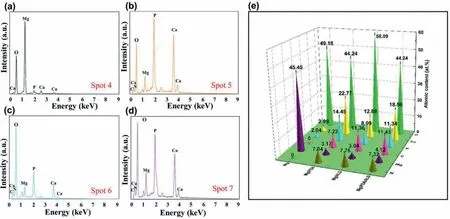
Fig.8.EDS spectra of (a) Mg,(b) Mg/PDA,(c) Mg/ALG,and (d) Mg/PDA/ALG after 10-day immersion in HBSS at 37 °C and (d) corresponding quantitative analysis.
In this work,a rational schematic to describe the mechanism of anti-corrosion provided by the PDA/ALG composite coating for the Mg substrate is shown in Fig.9.Firstly,according to the results of the EIS data,the pore resistances of Mg/PDA,Mg/ALG,and Mg/PDA/ALG are 3795Ωcm2,5703Ωcm2,and 13,890Ωcm2,larger than that of pure Mg substrate,implying that the function of coatings is to act as barriers to prevent the electrolyte contacting with substrate directly [45].During the whole corrosion progress,the high compactness of the outer ALG layer plays a crucial role in preventing the electrolyte from contacting with the Mg substrate.When the samples are soaked in HBSS for a certain time,the electrolyte penetrates into the ALG coating layer by swelling,then penetrates along the cracks in the PDA layer,and finally reaches onto the Mg substrate.Once the electrolyte contacts with the Mg substrate,the anode reaction occurs and generates electrons.The electron transfer from the anode to cathode is a prerequisite for the electrochemical corrosion.Owing to the very low conductivity of PDA [38],the resistance between the PDA coating and Mg substrate is very large [46],which is difficult for electrons to pass through the PDA layer.The cathodic reaction is effectively suppressed because of the delayed arrival of electrons from the anode,and the overall corrosion rate thus slows down.

Fig.9.Schematic illustration of corrosion mechanism of anti-corrosion provided by the PDA/ALG composite coating for the Mg substrate.
4.Conclusion
In this work,PDA was introduced as a versatile pretreated layer for the following ALG immobilization to obtain the PDA/ALG hybrid coating with a thickness of 8.58±0.65 μm on the high-purity Mg substrate.The PDA layer shows favorable adhesion to both the ALG layer and Mg substrate.The Mg/PDA/ALG sample significantly decreases theicorrfrom 45.34 μA cm2of the bare Mg to 3.24 μA cm2and also has a larger charge transfer resistance and pore resistance,which indicates the excellent anti-corrosion property of the composite coating.The 10-day immersion tests in HBSS show that a suppressed degradation rate is achieved by the PDA/ALG composite coating,compared to the single PDA and ALG coatings directly deposited on the Mg substrate.Our results prove that the strategy of PDA as a buffer layer for the deposition of the ALG coating on Mg is a promising strategy to control the corrosion rate of active Mg.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Key Research and Development Project of China (No.2020YFC1107202),Shanxi Provincial Key Research and Development Project (No.2019ZDLSF03–06),Guangdong Basic and Applied Basic Research Foundation (No.2020B1515120078),and Science and Technology Planning Project of Guangdong Province (No.2021A0505030042).
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Carbon nanotube and graphene reinforced magnesium matrix composites:A state-of-the-art review
- Stress corrosion cracking of magnesium alloys: A review
- Simultaneous enhancement of mechanical properties and corrosion resistance of as-cast Mg-5Zn via microstructural modification by friction stir processing
- Effect of wire-arc directed energy deposition on the microstructural formation and age-hardening response of the Mg-9Al-1Zn (AZ91) alloy
- Regulating local coordination environment of Mg-Co single atom catalyst for improved direct methanol fuel cell cathode
- Hydrogen-induced optical properties of FC/Pd/Mg films: Roles of grain size and grain boundary
