基于网络药理学探究黄连调控巨噬细胞干预动脉粥样硬化斑块稳定性的潜在机制
刘婷 韩慧子 向磊 赵梦涵 俞琦


【摘要】目的探究黃连调控巨噬细胞干预动脉粥样硬化斑块稳定性的潜在机制。方法对GEO数据库中的动脉斑块相关数据集进行差异分析得到巨噬细胞干预动脉粥样硬化斑块相关基因。从TCMSP数据库获取黄连的有效成分和潜在靶点。对两者取交集后找出发挥作用的化学成分和潜在靶点。对潜在靶点进行蛋白质-蛋白质相互作用(PPI)、基因本体(GO)、京都基因与基因组百科全书(KEGG)分析,探究黄连调控巨噬细胞干预动脉粥样硬化斑块稳定性的作用机制。结果稳定斑块组和破裂斑块组差异分析共找到892个差异基因。通过TCMSP共找出黄连中的11个化学成分,251个靶点。两者取交集后得到16个黄连调控巨噬细胞的潜在靶点。PPI结果显示,DPP4、TNFAIP6、IL6ST、POR、RUNX1T1、HMOX1、CAV1等16个交集基因之间有较强的相互作用关系,且DPP4、HMOX1、CAV1和VCAM1处于PPI网络的枢纽位置。GO结果表明,生物学过程(BP)与对脂多糖的反应、对细菌来源分子的反应、对T细胞激活的正向调节等有关。细胞组成(CC)与膜筏、膜微区、膜区等细胞器有关。分子功能(MF)参与肽酶活化剂活性、趋化因子活性等分子功能的调节。KEGG结果与流体剪切应力及动脉粥样硬化、NF-kappa B信号传导途径有关。结论黄连内槲皮素可能通过调节DPP4、HMOX1、CAV1等靶点影响斑块内巨噬细胞的信号传导途径,进而干预斑块稳定性。
【关键词】黄连;巨噬细胞;动脉粥样硬化;斑块稳定性;网络药理学
中图分类号:R543.5文献标志码:ADOI:10.3969/j.issn.1003-1383.2023.08.002
Exploration on the potential mechanism of Coptis chinensis Franch in regulating macrophages
to intervene the stability of atherosclerosis plaque based on network pharmacology
LIU Ting, HAN Huizi, XIANG Lei, ZHAO Menghan, YU Qi
(School of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, Guizhou, China)
【Abstract】ObjectiveTo investigate the potential mechanisms of Coptis chinensis Franch in regulating macrophages to intervene the stability of atherosclerosis plaque. MethodsDifferential analysis of arterial plaque related datasets from GEO database was performed to obtain macrophage intervention in atherosclerotic plaque related genes. The active ingredients and potential targets of Coptis chinensis Franch were obtained from TCMSP database, and the intersection of the two was taken to identify the chemotactic components and potential targets that play a role. Protein-protein interactions (PPI), Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed on the potential targets to investigate the mechanism of action of Coptis chinensis Franch in regulating macrophages to intervene the stability of atherosclerosis plaque. ResultsA total of 892 differential genes were found in the differential analysis of the stable and ruptured plaque groups. 11 chemical components and 251 targets in Coptis chinensis Franch were identified by TCMSP, and 16 potential targets of Coptis chinensis Franch were obtained after taking the intersection of the two. PPI results showed that there were strong interactions between 16 intersecting genes, including DPP4, TNFAIP6, IL6ST, POR, RUNX1T1, HMOX1 and CAV1, etc. And DPP4, HMOX1, CAV1 and VCAM1 were at the pivotal position of the PPI network. GO results showed that biological process (BP) was associated with response to lipopolysaccharide, response to molecules of bacterial origin, and positive regulation of T cell activation. Cell composition (CC) was associated with organelles such as membrane rafts, membrane microregions, and membrane zones. Molecular function (MF) was involved in the regulation of molecular functions such as peptidase activator activity and chemokine activity. KEGG results were related to fluid shear stress and signal transduction of atherosclerosis and NF-kappa B signal transduction pathway. ConclusionQuercetin within Coptis chinensis Franch may affect the intraplaque macrophage signaling pathway by regulating DPP4, HMOX1, CAV1 and other targets, and thus intervene plaque stability.
【Key words】Coptis chinensis Franch; macrophages; atherosclerosis; plaque stability; network pharmacology
动脉粥样硬化(atherosclerosis,AS)是一种慢性炎症性疾病,是心脑血管疾病、外周动脉疾病等的病理基础[1]。巨噬细胞是调控炎症反应、调节免疫的重要角色,可通过分泌基质金属蛋白酶,降解斑块细胞外基质中的胶原纤维,导致AS患者斑块破裂、出血、血栓形成[2],并释放大量介质和酶,影响动脉粥样硬化患者的预后[3]。在临床实践中,现有治疗手段重在改善临床症状及防止不良事件发生,多采用介入或使用他汀类药物降低患者血脂水平[4]。但由于介入手术价格较为昂贵,他汀类药物有较高的毒副作用,因此患者多倾向于中医保守治疗。中国传统医学治疗AS有独到的见解和优势,中医理论将AS归于“脉痹”“脱疽”“胸痹”等范畴。治疗上秦景明、李中梓等众多医家重视火热之邪所致胸痹而痛,反复强调寒凉之品的使用。黄连(Coptis chinensis Franch)是临床中治疗AS的常见配伍药材,虽在诊治过程中取得了较好的疗效,但具体作用机制不清楚[5]。网络药理学可基于“药物-成分-靶基因-疾病”交互作用网络,系统性观察药物及其有效成分对疾病靶基因的干预与影响,从而揭示中药作用于人体的机理。因此,本文采用网络药理分析方法[6]探索黄连作用于AS患者动脉斑块内巨噬细胞所介导的斑块破裂的分子机制。
1材料与方法
1.1活性成分的药代动力学评价通过中药系统药理学分析平台TCMSP(https://www.tcmsp-e.com/)检索并收集黄连中各个药物所含的化学成分,根据毒药物动力学(ADME)原理,以TCMSP最新筛选标准:口服利用度(OB)≥30%,类药性(DL)≥0.18为筛选条件,筛选组方中所含有的有效化学成分[7]。基于TCMSP将筛选得到的有效成分进行靶点蛋白获取,去重后借助Uniprot数据库(https://www.uniprot.org/)对靶点蛋白标准化处理。
1.2巨噬细胞在不同状态斑块中的基因表达差异GEO数据库(http://www.ncbi.nlm.nih.gov/geo/)的GSE41571数据集含有5个稳定斑块组织和6个破裂斑块组织内巨噬细胞的表达谱数据。通过“limma”包,找出两组样本的差异基因。并绘制火山图和热图。对药物靶点及差异基因取交集找出黄连影响巨噬细胞介导斑块稳定性的潜在靶点。
1.3绘制成分-靶点网络图将交集基因与所对应的化学成分导入Cytoscape 3.8.1进行可视化。
1.4蛋白质-蛋白质相互作用(PPI)网络通过STRING数据库(https://www.string-db.org/)对交集基因进行蛋白互作分析,找出不同蛋白间的相互作用。
1.5基因本体(gene ontology,GO)和京都基因与基因组百科全书(Kyoto encyclopedia of genes and genomes,KEGG)通路富集分析黄连的潜在作用靶点通过“org.Hs.eg.db”包进行ID转换后,使用“clusterProfiler”包进行GO分析与KEGG富集分析。其中GO分类富集分析包括生物学过程(biological process,BP)、分子功能(molecular function,MF)、细胞组成(cellular component,CC),選取BP、MF、CC排名前20的条目进行可视化,KEGG通路富集根据富集在通路上基因数目以及与疾病的相关性,用“ggplot2”包进行可视化。
2结果
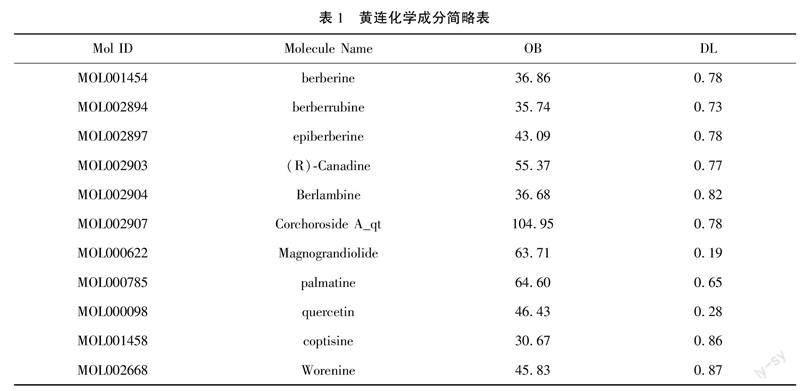
2.1黄连成分和靶点的提取通过TCMSP共找出黄连中的11个化学成分,251个靶点。见表1。
2.2GEO芯片差异分析通过“limma”包对稳定斑块组和破裂斑块组差异分析,我们共找到892个差异基因。其中,上调基因376个,下调基因516个。分别提取上调基因和下调基因的前20个基因绘制热图(见图1A),对所有差异基因绘制火山图(见图1B)。
2.3韦恩图的绘制通过对药物靶点和巨噬细胞在不同斑块间的差异基因去交集后,得到16个黄连调控巨噬细胞的潜在靶点(见图2)。
2.4绘制成分-靶点网络图绘制成分-靶点网络图后发现,黄连治疗巨噬细胞的潜在成分主要为槲皮素。槲皮素可通过调节DPP4、TNFAIP6、IL6ST、POR、RUNX1T1、HMOX1、CAV1、GJA1、VCAM1、CXCL8、ABCG2、GSTP1、COL3A1、CXCL2、CTSD、PCOLCE影响AS患者体内斑块的稳定性(见图3)。
2.5PPI绘制PPI结果显示,DPP4、TNFAIP6、IL6ST、POR、RUNX1T1、HMOX1、CAV1、GJA1、VCAM1、CXCL8、ABCG2、GSTP1、COL3A1、CXCL2、CTSD、PCOLCE之间有较强的相互作用关系,且DPP4、HMOX1、CAV1和VCAM1处于PPI网络的枢纽位置,表明这些基因可能是影响动脉粥样硬化斑块稳定性的核心(见图4)。
2.6富集分析GO结果表明,BP与对脂多糖的反应、对细菌来源分子的反应、对T细胞激活的正向调节、对白细胞-细胞黏附的正向调节、对营养物质的反应、气体稳态、T细胞激活、缝隙连接组装、白细胞迁移、细胞-细胞黏附的积极调节等生物学过程有关(见图5A)。CC与膜筏、膜微区、膜区、三级颗粒管腔、小窝、早期内体、细胞顶端、质膜筏等细胞器有关(见图5B)。MF参与肽酶活化剂活性、趋化因子活性等分子功能的调节(见图5C)。KEGG结果与流体剪切应力及动脉粥样硬化、病毒蛋白与细胞因子和细胞因子受体的相互作用、糖尿病并发症中的AGE-RAGE信号通路、阿米巴病、NF-kappa B信号传导途径、疟疾、卡波西肉瘤相关的疱疹病毒感染、军团菌病、脂质和动脉硬化、幽门螺杆菌感染中的上皮细胞信号传导有关(见图5D)。
3讨论
斑块由免疫细胞、间充质细胞、脂质和细胞外基质组成,随着病情的发展,斑块会逐渐破坏血管的内部弹性层、中膜、外部弹性层和外膜[8]。巨噬细胞在AS斑块稳定性方面有决定性作用[9]。有研究表明,巨噬细胞是动脉粥样硬化斑块中炎症和代谢信号的关键整合者,它可通过巨胞饮作用、吞噬作用和清道夫受体介导脂蛋白的摄取和转运,其内部的脂质含量通过增加Toll样受体对其配体的敏感性和激活NLRP3炎性体来促进先天免疫反应和炎症[10]。而易损斑块具有脆弱的薄纤维帽、扩张的脂质核心、斑块内出血、免疫激活、促炎介质(细胞因子、趋化因子、金属蛋白酶)的产生增加,以及某些巨噬细胞亚型的强活性等特性[11-13]。因此,巨噬细胞可通过多种方式影响斑块的稳定性。
本研究表明,黄连干预不同状态动脉斑块内巨噬细胞的成分为槲皮素(Quercetin)。槲皮素是一种黄酮类化合物,已被证明具有心血管保护作用和抗动脉粥样硬化作用[15]。槲皮素可通过抑制活性氧(ROS)产生和激活PI3K/AKT信号通路来抑制高果糖喂养的C57BL/6小鼠的动脉粥样硬化斑块发展[15],也可明显改善高脂肪饮食的APOE-/-小鼠的动脉粥样硬化斑块的面积、脂质积累水平,并增加了动脉粥样硬化斑块中的胶原纤维[16]。此外,槲皮素还可调节 MST1介导的RAW264.7细胞自噬,抑制氧化型低密度脂蛋白(ox-LDL)诱导的泡沫细胞形成[17]。这些证据表明,槲皮素不仅对AS的斑块有改善作用,还对巨噬细胞有较好的调节作用。
PPI显示,DPP4、HMOX1、CAV1和VCAM1是槲皮素调节斑块内巨噬细胞的核心基因。其中二肽基肽酶4(DPP4)是炎症和代谢的调节剂,可能与动脉粥样硬化疾病的发展有关[18]。抑制DPP4可减少单核细胞在TNF-α和可溶性DPP4的作用下向动脉粥样硬化斑块迁移[19]。它还上调发挥抗炎作用的脂联素表达[20]。HMOX1在动脉粥样硬化中的高表达与铁死亡的发生有关,并导致MMP释放和M0巨噬细胞浸润[21]。小窝蛋白-1(CAV1)是小窝细胞器的标记蛋白,可直接结合胆固醇,在小窝功能中起着复杂的作用[22]。CAV1具有促进肿瘤生长和迁移、脂质转运和炎症调节等多种生物学功能[23]。先前的研究确定CAV1是脂肪细胞中主要的质膜脂肪酸结合蛋白,与AS动脉斑块的形成有关[24]。VCAM1是参与嗜酸性粒细胞、基底细胞、单核细胞和淋巴细胞黏附的细胞黏附分子,它使单核细胞与内皮细胞黏合,单核细胞进入内皮下[25]。不仅能加重体内的炎症损伤,还能影响斑块的状态,促进AS的发生和发展[26]。因此,槲皮素调节DPP4、HMOX1、CAV1等基因有利于改善患者的斑块稳定性。KEGG结果与流体剪切应力及动脉粥样硬化、NF-kappa B信号传导途径、脂质和动脉硬化等信号传导有关。易损AS斑块是随时间动态变化的不稳定结构。它们更常发生在颈动脉或冠状动脉的分叉处等剪应力不均匀的狭窄区域[27-28],因此流体剪切应力会对已经生成的斑块直接施加生物应力。NF-κB主要通过调节逆向胆固醇转运参与胆固醇稳态和斑块的炎症反应[29]。故这些通路均对动脉斑块稳定性和患者的炎症反应高度相关。
综上所述,本研究运用网络药理学和生物信息学的方法在一定程度上揭示了AS患者的稳定斑块与破裂斑块巨噬细胞的相关基因以及黄连发挥延缓或抑制这一生物学过程的功效作用机制。未来本课题组将持续关注这一学术领域,开展相关体内体外实验,對这一结果进行验证。总之,黄连内部的槲皮素可能通过调节DPP4、HMOX1、CAV1等靶点影响斑块内巨噬细胞的信号传导途径,进而干预斑块稳定性。参考文献[1] FAN J L, WATANABE T. Atherosclerosis:known and unknown[J].Pathol Int, 2022, 72(3):151-160.
[2] TABARES-GUEVARA J H, VILLA-PULGARIN J A, HERNANDEZ J C. Atherosclerosis:immunopathogenesis and strategies for immunotherapy[J].Immunotherapy, 2021, 13(14):1231-1244.
[3] KONG P, CUI Z Y, HUANG X F, et al. Inflammation and atherosclerosis:signaling pathways and therapeutic intervention[J].Signal Transduct Target Ther, 2022, 7(1):131.
[4] LIBBY P, BURING J E, BADIMON L, et al. Atherosclerosis[J].Nat Rev Dis Primers, 2019, 5(1):56.
[5] 赵立凤,于红红,田维毅.中药单体调控血管内皮细胞自噬干预动脉粥样硬化的研究进展[J].中华中医药学刊,2021,39(11):117-120.
[6] NOGALES C, MAMDOUH Z M, LIST M, et al. Network pharmacology:curing causal mechanisms instead of treating symptoms[J].Trends Pharmacol Sci, 2022, 43(2):136-150.
[7] RU J L, LI P, WANG J N, et al. TCMSP: a database of systems harmacology for drug discoveryfrom herbal medicines[J].J Cheminformatics, 2014, 6:13.
[8] DING H J, WANG C G, MALKASIAN S, et al. Characterization of arterial plaque composition with dual energy computed tomography:a simulation study[J].Int J Cardiovasc Imaging, 2021, 37(1):331-341.
[9] MUSHENKOVA N V, SUMMERHILL V I, ZHANG D W, et al. Current advances in the diagnostic imaging of atherosclerosis:insights into the pathophysiology of vulnerable plaque[J].Int J Mol Sci, 2020, 21(8):2992.
[10] MOORE K J, SHEEDY F J, FISHER E A. Macrophages in atherosclerosis:a dynamic balance[J].Nat Rev Immunol, 2013, 13(10):709-721.
[11] KYRIAKIDIS K, ANTONIADIS P, CHOKSY S, et al. Comparative study of protein expression levels of five plaque biomarkers and relation with carotid plaque type classification in patients after carotid endarterectomy[J].Int J Vasc Med, 2018, 2018:1-8.
[12] PAPAIOANNOU T G, KALANTZIS C, KATSIANOS E, et al. Personalized assessment of the coronary atherosclerotic arteries by intravascular ultrasound imaging:hunting the vulnerable plaque[J].J Pers Med, 2019, 9(1):8.
[13] WANG F, LI T W, CONG X F, et al. Association between circulating big endothelin-1 and noncalcified or mixed coronary atherosclerotic plaques[J].Coron Artery Dis, 2019, 30(6):461-466.
[14] DUAN H, ZHANG Q, LIU J, et al. Suppression of apoptosis in vascular endothelial cell,the promising way for natural medicines to treat atherosclerosis[J].Pharmacol Res, 2021, 168:105599.
[15] LU X L, ZHAO C H, YAO X L, et al. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway[J].Biomed Pharmacother, 2017, 85:658-671.
[16] JIA Q L, CAO H, SHEN D Z, et al. Quercetin protects against atherosclerosis by regulating the expression of PCSK9,CD36,PPARγ,LXRα and ABCA1[J].Int J Mol Med, 2019, 44(6):893-902.
[17] CAO H, JIA Q L, YAN L, et al. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells[J].Int J Mol Sci, 2019,20(23):6093.
[18] DUAN L H, RAO X Q, XIA C, et al. The regulatory role of DPP4 in atherosclerotic disease[J].Cardiovasc Diabetol, 2017, 16(1):76.
[19] SHAH Z, KAMPFRATH T, DEIULIIS J A, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis[J].Circulation,2011,124(21):2338-2349.
[20] BARBIERI M, MARFELLA R, ESPOSITO A, et al. Incretin treatment and atherosclerotic plaque stability:role of adiponectin/APPL1 signaling pathway[J].J Diabetes Complicat, 2017, 31(2):295-303.
[21] WU D Q, HU Q A, WANG Y Q, et al. Identification of HMOX1 as a critical ferroptosis-related gene in atherosclerosis[J].Front Cardiovasc Med, 2022, 9:833642.
[22] GOKANI S, BHATT L K. Caveolin-1:a promising therapeutic target for diverse diseases[J].Curr Mol Pharmacol, 2022, 15(5):701-715.
[23] RAUDENSKA M, GUMULEC J, BALVAN J, et al.Caveolin-1 in oncogenic metabolic symbiosis[J].Int J Cancer, 2020, 147(7):1793-1807.
[24] HOU K, LI S, ZHANG M,et al. Caveolin-1 in autophagy: a potential therapeutic target in atherosclerosis[J].Clin Chim Acta,2021,513:25-33.
[25] THAYSE K, KINDT N, LAURENT S, et al. VCAM-1 target in non-invasive imaging for the detection of atherosclerotic plaques[J].Biology, 2020, 9(11):368.
[26] TRONCOSO M F, ORTIZ-QUINTERO J, GARRIDO-MORENO V, et al. VCAM-1 as a predictor biomarker in cardiovascular disease[J].Biochim Biophys Acta BBA Mol Basis Dis, 2021, 1867(9):166170.
[27] CHIEN C S, LI J Y, CHIEN Y, et al. METTL3-dependent N6-methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium[J].Proc Natl Acad Sci U S A,2021,118(7):e2025070118.
[28] BRYNIARSKI K L, WANG Z, FRACASSI F, et al. Three-dimensional fibrous cap structure of coronary lipid plaque―ST-elevation myocardial infarction vs.stable angina[J].Circ J, 2019, 83(6):1214-1219.
[29] SHI S, JI X, SHI J, et al. Andrographolide in atherosclerosis: integrating network pharmacology and in vitro pharmacological evaluation[J].Biosci Rep, 2022,42(7):BSR20212812.
(收稿日期:2022-12-01修回日期:2023-02-15)
(編辑:潘明志)

