Eccentric exercise-induced muscle weakness abolishes sex differences in fatigability during sustained submaximal isometric contractions
Hnn L.Jodoin,Avery Hinks,Olivi P.Roussel,Vincenzo S.Contento,Brin H.Dlton,Geoffrey A.Power,*
a Department of Human Health and Nutritional Sciences,College of Biological Sciences,University of Guelph,Guelph,ON N1G 2W1,Canada
b School of Health and Exercise Science,University of British Columbia,Kelowna,BC V1V 1V7,Canada
Abstract Background: Females are typically less fatigable than males during sustained isometric contractions at lower isometric contraction intensities.This sex difference in fatigability becomes more variable during higher intensity isometric and dynamic contractions.While less fatiguing than isometric or concentric contractions,eccentric contractions induce greater and longer lasting impairments in force production.However,it is not clear how muscle weakness influences fatigability in males and females during sustained isometric contractions.Methods: We investigated the effects of eccentric exercise-induced muscle weakness on time to task failure(TTF) during a sustained submaximal isometric contraction in young (18-30 years) healthy males (n=9) and females (n=10).Participants performed a sustained isometric contraction of the dorsiflexors at 35° plantar flexion by matching a 30% maximal voluntary contraction (MVC) torque target until task failure(i.e.,falling below 5% of their target torque for ≥2 s).The same sustained isometric contraction was repeated 30 min after 150 maximal eccentric contractions.Agonist and antagonist activation were assessed using surface electromyography over the tibialis anterior and soleus muscles,respectively.Results: Males were~41% stronger than females.Following eccentric exercise both males and females experienced an~20% decline in maximal voluntary contraction torque.TTF was~34% longer in females than males prior to eccentric exercise-induced muscle weakness.However,following eccentric exercise-induced muscle weakness,this sex-related difference was abolished,with both groups having an~45% shorter TTF.Notably,there was~100% greater antagonist activation in the female group during the sustained isometric contraction following exercise-induced weakness as compared to the males.Conclusion:This increase in antagonist activation disadvantaged females by decreasing their TTF,resulting in a blunting of their typical fatigability advantage over males.
Keywords: Fatigue;Isometric;Sex;Time to task failure;Weakness
1.Introduction
Fatigability,defined as any exercise-induced transient impairment in the ability to generate force or power regardless of whether the task can be sustained,is highly task-and population-dependent.1,2Across most studies,females are shown to be less fatigable than males during sustained isometric contractions.1This sex-related difference is most prominent at lower isometric contraction intensities3,4and becomes more variable during higher intensity isometric4,5and dynamic contractions.6-8While often less fatiguing than isometric or concentric contractions,high intensity eccentric contractions can induce greater and longer lasting impairments in force production (i.e.,owing to muscle weakness).9-13However,it is not clear how eccentric exercise-induced muscle weakness influences fatigability in males and females during sustained isometric contractions.
Repeated unaccustomed lengthening contractions of muscle results in cytoskeletal damage,14mechanical disruptions of actin-myosin bonds,impaired excitation-contraction coupling,15,16and ultimately a prolonged reduction in voluntary force production (i.e.,weakness) in both animals17and humans.18Following repetitive lengthening contractions,female rats incur less muscle damage as compared to males due to the protective effects of estrogen.19However,reports in humans addressing this sex-dependent response are equivocal,20,21with females potentially exhibiting greater muscle weakness and damage.18,20-22While sex-related differences in eccentric exercise-induced muscle weakness have been investigated extensively,20,22,23no studies have systematically assessed sex-related differences in fatigability during sustained isometric contractions following unaccustomed eccentric exercise.
Females are typically less fatigable than males for low-level submaximal isometric contractions across various muscle groups,including the ankle dorsiflexors.6,24Often,males are stronger than females;thus,for a similar relative submaximal intensity,males typically generate greater absolute forces for the upper and lower limbs.4Higher force production leads to greater blood flow occlusion to the active musculature,25and thus less oxygen delivery and metabolite removal in males than females,which would impair performance and likely explain,in part,the lower fatigability in females.1While females have a longer time to task failure (TTF) during lowlevel submaximal tasks,6when the target force was considered as a covariate in the analysis,sex-related differences were eliminated and TTF was inversely related to the absolute target force.3However,as Gabel et al.26recently reported,absolute force alone cannot fully explain these sex-related differences in fatigability.Using a model of shorteninginduced residual force depression,sex-related differences in TTF were eliminated despite females generating less absolute force.26Meanwhile,for high-intensity isometric contractions,sex-related differences in TTF are often not observed,4,5which may owe to similar levels of blood flow occlusion for females and males.27Therefore,the magnitude of the sex-related differences in fatigability is greatest at low-intensity sustained isometric contractions.3,6Other factors potentially contributing to lower fatigability in females compared to males include sexspecific differences in muscle gene expression;28greater area of type I muscle fibers and,thus,oxidative metabolism;29,30greater density and volume of mitochondria;31a greater capillary-to-fiber ratio;32and better energy substrate efficiency,33which may ultimately result in less metabolite accumulation within the muscle in females during and after exercise as compared to males.24,29However,it is unknown whether there is a sex difference in fatigability following eccentric-induced weakness when the contraction is performed at the same absolute intensity,which would represent a higher percentage of maximum force production capacity for females.
The purpose of this study was to investigate the effects of eccentric exercise-induced muscle weakness on TTF for a sustained isometric contraction in males and females.Consistent with previous findings,we expect to observe a sex-related difference,with females having a longer TTF than males.6,24However,we expect that following eccentric exercise-induced muscle weakness,when the contraction is performed at the same absolute intensity,the fatigue-resistant advantage in females would be abolished,likely owing to a weaknessinduced shift in absolute contraction forces to a greater relative contraction intensity (i.e.,the relative load is now a greater percentage of maximal force production capacity).
2.Methods
2.1.Participants
Nineteen healthy,recreationally active young adults were recruited from the University of Guelph,consisting of 10 females (age=20.2±2.5 years,height=164.6±6.4 cm,mass=62.5±4.9 kg;mean±SD)and 9 males(age=23.3±3.3 years,height=180.6±6.7 cm,mass=82.6±9.7 kg).Because our research question related to eccentric exerciseinduced muscle weakness,1 male participant was excluded from the study due to improper baseline data collection (i.e.,the participant’s maximal voluntary contraction (MVC)increased following the eccentric contraction protocol due to learning how to better voluntarily activate his dorsiflexors).One female participant was also removed from the study for not experiencing >5% muscle weakness following the eccentric exercise.Other than MVC torque34and optimal angle of torque production,indirect markers of muscle damage (i.e.,serum creatine kinase activity)were not assessed in the present study.Participants were all right-leg dominant,free from recent injuries preventing dorsiflexion of the right ankle,and reported no history of neuromuscular disease.All participants were informed of the purpose and procedures of the study and gave written,informed consent before participating.All procedures were approved by the Human Research Ethics Board of the University of Guelph(REB:#20-09-026).
2.2.Experimental set up
A HUMAC NORM dynamometer (CSMi Medical Solutions,Stoughton,MA,USA) was used for all torque,angular velocity,and position recordings.Participants were seated comfortably with their right hip and knee at 110° and 140° of flexion,respectively.The right knee was immobilized with an inelastic strap positioned superior to the knee,with padding placed on the distal portion underneath the thigh.A 4-point seatbelt harness secured the torso.The shoeless right foot was fixed to a dynamometer footplate for dorsi-and plantar flexion(PF),with 1 inelastic strap wrapped over the ankle and another at the mid-distal portion of the metatarsals.For the eccentric contraction protocol,the minimum/maximum ankle dorsiflexion angles were set to 0°PF(e.g.,neutral ankle-foot position:0°)and 40°PF,respectively,with the isometric reference angle where the isometric contractions were performed set at 35°PF.Given the broad plateau of the dorsiflexor torque-angle relationship,35,3635° PF allowed for sufficient passive tension on the dynamometer to optimize torque recordings while not disadvantaging active torque production.
A bipolar surface electromyography (EMG) configuration was used to sample tibialis anterior and soleus neuromuscular activity via self-adhering Ag-AgCl electrodes (1.5 cm×1 cm;Kendall,Mansfield,MA,USA).The soleus was chosen due to the flexed knee position limiting gastrocnemius neuromechanical contributions to torque generation.37Electrodes were placed on the tibialis anterior (TA) 7 cm inferior and 2 cm lateral to the tibial tuberosity and just proximal to the musculotendinous junction (~8 cm interelectrode distance).Electrodes were placed on the soleus 2 cm inferior to the distal aspect of the gastrocnemius,along the midline of the leg and on the calcaneal tendon (~5 cm interelectrode distance).Large inter-electrode distances were used to optimize the acquisition of evoked potentials by ensuring M-waves were representative of the majority of active motor units.38A single ground electrode was placed on the right patella.Before placing electrodes,the locations were shaved and the skin was cleaned with rubbing alcohol.
2.3.Experimental protocols
The experimental procedures are described below in chronological order.(Fig.1 depicts a graphical timeline.)
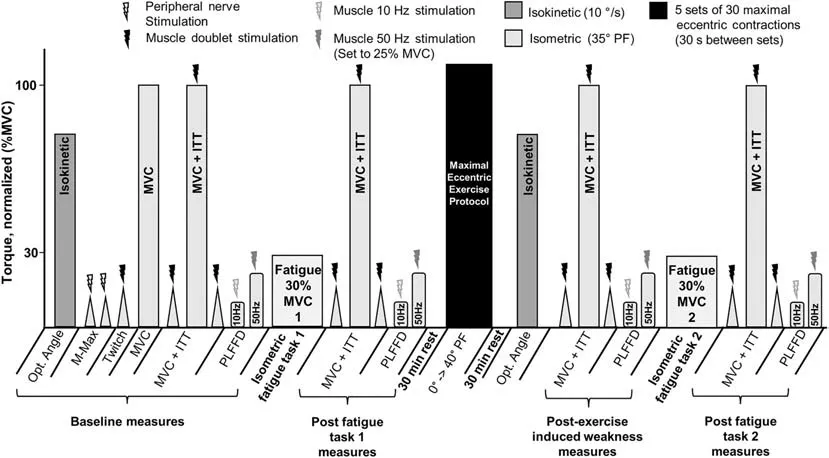
Fig.1.Experimental timeline.Participants performed a sustained isometric contraction at 30% MVC torque until task failure.Then,muscle weakness was induced via 5 sets of 30 maximal effort isokinetic eccentric contractions at 80°/s.The same sustained isometric contraction was repeated to assess the effects of eccentric exercise-induced muscle weakness on performance fatigability.DF=dorsiflexion;ITT=interpolated twitch technique;MVC=maximal voluntary contraction;Opt.=optimal;PF=plantar flexion;PLFFD=prolonged low-frequency force depression.
2.3.1.Optimal angle of torque production
Passive dorsiflexion torque was measured in 5° increments from 0°PF to maximal PF(~50°PF)to cover a large range of motion.Participants then performed a slow concentric isokinetic dorsiflexion contraction at 10°/s over the full range of motion.Passive torque was subtracted from total torque at each of the angles recorded to provide a measure of active torque.39The angle that produced the highest active torque was deemed optimal.
2.3.2.Deep branch of common fibular nerve and tibial nerve stimulation
Nerve stimulation was used to evoke maximal compound muscle action potentials (M-waves),which were recorded from the TA and soleus by percutaneously stimulating the deep branch of the common fibular nerve and tibial nerve,respectively,with a standard clinical bar electrode (Empi,St.Paul,MN,USA)coated in conductive gel.The deep branch of the common fibular nerve,which innervates the TA,was located by palpating the head of the fibula and moving posteroinferiorly until the nerve was intercepted.The tibial nerve,which innervates the soleus,was found by locating the distal tendon of the semitendinosus muscle and moving laterally while palpating deep into the popliteal fossa.All peripheral nerve stimuli were delivered as a single,square-wave pulse from a constant current,high-voltage stimulator (model DS7AH;Digitimer,Welwyn Garden City,Hertfordshire,UK).Voltage was set to a maximum of 400 V and a pulse width of 200 μs.The current was increased incrementally until a plateau was reached for the peak-to-peak amplitude of the M-wave(soleus:215.0±9.5 mA,TA:45.0±1.8 mA).
2.3.3.Muscle stimulation
For mechanical measures,electrical stimulation was delivered via 2 custom stimulatory pads(4 cm×4.5 cm to 4 cm×7.5 cm),which were constructed by taping paper towel around a flat,multi-layered piece of aluminum foil such that the top of the pad was covered by tape while the single layer of paper towel exposed on the bottom was soaked in water and coated in conductive gel.40The custom pads were placed transversely on the TA,just superior to each EMG electrode.Alligator clips were attached to the aluminum foil within the custom pads such that the proximal and distal pads acted as the cathode and anode,respectively.To mitigate the spread of current to other muscles,the size of each pad was tailored to each participant such that it covered the muscle belly from the medial to lateral boarders without excess.The stimulator(DS7AH;Digitimer)had a maximum potential of 400 V and a pulse width of 200 μs.The current was increased until the peak torque of twitch doublets (2 pulses at an interstimulus interval of 10 ms) plateaued.The current used for tetanic contractions was increased until a 1-s,50-Hz stimulation elicited a dorsiflexion torque equivalent to 30% MVC torque.This stimulation intensity was used for all tetanic contractions.Prolonged low-frequency force depression(PLFFD)was characterized by a decrease in the 10:50 Hz peak tetanic torque ratio.
2.3.4.Maximum voluntary isometric contractions and voluntary activation
Participants were encouraged verbally during all MVCs,and the torque trace was visible during each contraction.Two MVCs of 3-5 s in duration were performed.The interpolated twitch technique was used to evaluate voluntary activation(VA) via direct muscle stimulation using twitch doublets as described above.The twitch doublet performed before the MVC was used to evaluate the half relaxation time(HRT)and twitch torque amplitude.The torque of the superimposed twitch elicited during the plateau of the MVC was compared to the peak torque of the control twitch delivered 1-2 s after relaxation.The level of VA was calculated using the following formula: VA (%)=(1-(superimposed twitch torque/control twitch torque))×100%.All participants were required to and capable of reaching a minimum of 95% VA during all MVCs and were given a 3-min rest between MVCs.The PLFFD was assessed following the MVCs.
2.3.5.Determining submaximal target torque
To determine the submaximal target torque,participants were asked to perform 2 baseline MVCs with the interpolated twitch technique (as explained previously) with verbal encouragement.The 30% MVC target torque was determined using the highest torque from these baseline MVCs.Guidelines were set on the computer monitor at 30% MVC,and the cut-off line indicating task failure was set at 5% of their target torque,creating a“target window”.
2.3.6.Isometric fatigue task pre-and post-exercise induced weakness
Participants dorsiflexed at 30% MVC torque,and the target torque was displayed on the computer monitor as a horizontal line.The computer monitor was positioned 1 m from the participant,and the speed of the torque trace was displayed at a compression ratio of 10:1.The viewing window was scaled so participants could see their resting passive torque trace at the bottom of the screen and their 30% MVC guideline at the top of the screen.Participants were instructed before the task,and verbally encouraged throughout the task,to match their torque to the 30% MVC guideline as steadily as possible for as long as possible.TTF was classified as the participant’s torque falling below the target window (<5% of their target torque) for 2 s.Following task failure,participants rested in the dynamometer chair for 30 min.Then,the weakness and recovery protocols were completed,followed by a repeat of the sustained isometric contraction task.The MVC,VA,and PLFFD (10:50 Hz ratio)were assessed following both fatigue tasks(Fig.1).
2.3.7.Weakness protocol
Participants performed 5 sets of 30 maximal eccentric dorsiflexions,with each set separated by 60 s of rest.Participants were provided visual feedback of their dorsiflexion torque and instructed to resist the lowering of the footplate(velocity: 80°/s) through the 40° range of motion (0°-40° PF).After each lengthening contraction,the foot was returned to the neutral ankle starting position over a 0.5-s (80°/s) period while the participant relaxed fully.The participant was then instructed to maximally resist the lowering of the footplate again immediately until the protocol was complete.The participant was asked to point to their perceived soreness on a scale of 0-10 following each set of 30 contractions and up to 30 min of recovery.The voluntary and electrically evoked responses of the dorsiflexors were recorded 30 min after task termination.Measures following the weakness protocol were performed in the following order: (a) assessment of MVC and VA;(b) isokinetic concentric contractions to determine the optimal angle of torque production;(c) twitch torque and twitch speed;(d) perceived soreness;and(e)PLFFD(10:50 Hz ratio).
2.4.Data analysis and statistics
Torque,angular position,and stimulus trigger data were sampled at 1000 Hz using a 12-bit analog-to-digital converter(PowerLab System 16/35;ADInstruments,Colorado Springs,CO,USA).The EMG data were sampled at 2000 Hz and digitally bandpass filtered online between 10 and 1000 Hz.All data were analyzed with Labchart (Version 8;Labchart,Pro Modules 2014,Colorado Springs,CO,USA)software.
The MVC with the greatest torque amplitude was chosen to represent MVC peak torque (Nm).The twitch doublet prior to the highest MVC was assessed for peak twitch torque and HRT.The HRT was defined as the time it takes for peak twitch torque to decrease to half of peak twitch torque.The root mean squared EMG (EMGRMS) amplitude was analyzed over a 500-ms window for peak torque for each MVC.EMGRMSwas analyzed over a 500-ms window during each fatigue task within 5 s of each of the following time points: start of task,25% of total task length,50% of total task length,75% of total task length,and immediately prior to task failure.Peak-to-peak EMG analysis from the M-waves of the soleus and TA were used to normalize and quantify agonist and antagonist activation,respectively,during the sustained isometric contraction.We adhered to all normalization and reporting guidelines outlined in the review by Besomi et al.41We opted to normalize to maximal M-Wave amplitude to obtain the maximal electrical potential,even considering the possible limitations of signal phase cancellation differences between voluntary and electrically evoked contractions.MVCs were not performed for the plantar flexors,limiting normalization to MVC as a viable option.
Unpairedttests were used to compare baseline differences between males and females.A two-way analysis of variance(Sex (male,female)×Time (pre-weakness,weakness)) was used to detect any differences in:peak twitch torque and HRT,MVC torque,VA,optimal angle of torque production,TTF,PLFFD between males and females,before and following eccentric exercise-induced muscle weakness.To assess relative changes over time,a two-way repeated-measures analysis of variance(Sex(male,female)×Time(baseline,fatigue task 1,weakness protocol,fatigue task 2)) was also used to detect any sex-related differences over time for MVC torque,VA,PLFFD,and agonist and antagonist activation.The Holm-Sidak procedure was used for all pairwise multiple comparisons.Significance was based on an a=0.05.Data are presented as mean ± SD in text and as mean ± SE values with individual data points in figures.
3.Results
3.1.Baseline sex differences
Males had~58% higher twitch torque amplitude(p<0.05)than females,with no difference in HRT (p=0.13).Males were stronger as well,with an~41%higher MVC torque(p<0.001;Fig.2) than females and no sex-related difference in VA (~99%;p=0.68).Optimal joint angle of torque production also did not differ across the sexes (p=0.30;Fig.3).For the electrically evoked tetanic contractions,males had~40% greater 10-Hz and 50-Hz torque (p<0.001;Fig.4A and 4B)than females.Both males and females performed the sustained isometric contraction at 30% MVC,with absolute contraction intensities of 9.1,Nm and 5.4 Nm,respectively.As expected,females were less fatigable with a TTF that was~34%longer than that for males(p<0.05;Fig.5).
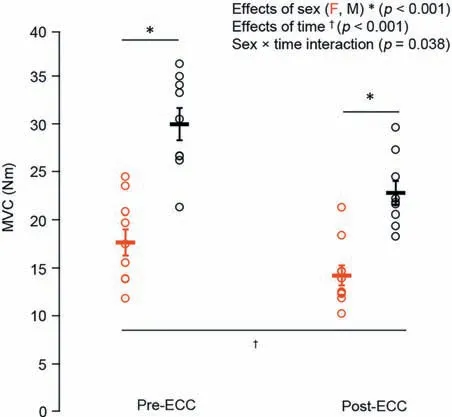
Fig.2.MVC in females(red)and males(black)before and after the eccentric exercise-induced muscle weakness.Data are presented as mean ± SE.ECC=eccentric exercise-induced muscle weakness;F=female;M=male;MVC=maximum voluntary contraction.
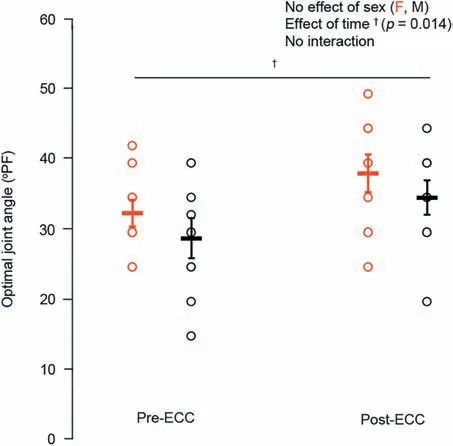
Fig.3.Optimal ankle joint angle of dorsiflexion torque production in females(red)and males(black)before and after the eccentric exercise-induced muscle weakness.Data are presented as mean ± SE.ECC=eccentric exerciseinduced muscle weakness;F=female;M=male;PF=plantar flexion.
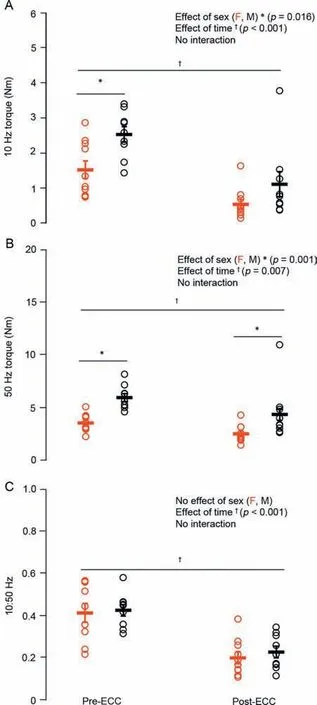
Fig.4.Electrically evoked (A) low (10 Hz) and (B) high (50 Hz) frequency tetanic contraction torque and subsequent low:high frequency ratio (C: 10:50 Hz)in females(red)and males(black)before and after the eccentric exerciseinduced muscle weakness.Data are presented as mean±SE.ECC=eccentric exercise-induced muscle weakness;F=female;M=male.
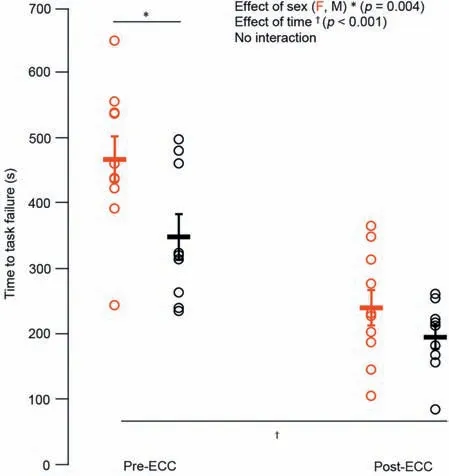
Fig.5.Time to task failure for the sustained isometric contractions performed before and after the eccentric exercise-induced muscle weakness in females(red) and males (black).Data are presented as mean ± SE.ECC=eccentric exercise-induced muscle weakness;F=female;M=male.
3.2.Eccentric exercise-induced neuromuscular impairments
The repeated unaccustomed eccentric contractions were effective at inducing impairments in neuromuscular function 30 min following the intervention.For MVC torque,there was a sex×time interaction (p<0.05;Fig.2) such that males were stronger than females at baseline;there were also main effects of time (p<0.001) and sex (p<0.001),with both groups having an~20% reduction in MVC torque following the eccentric exercise.Meanwhile,VA remained unchanged from baseline (99%;p=0.24).There was also an effect of time (p<0.05;Fig.3) on the optimal joint angle of torque production,with both groups experiencing an~19% shift to longer muscle lengths,indicating increased series compliance following the eccentric exercise.Both 10-Hz (Fig.4A) and 50-Hz (Fig.4B) torque demonstrated main effects for sex and time (p<0.05),with males being stronger than females and both groups showing~35% and~14% reductions in 10-Hz and 50-Hz torque,respectively,following the eccentric exercise.Taken together(10:50 Hz;PLFFD),there was a main effect of time (p<0.001;Fig.4C) such that both males and females experienced an~25% decrease.This indicates significant impairments in low-vs.high-frequency torque production following the eccentric contractions and significant PLFFD.
3.3.Post-weakness fatigability
While performing the sustained isometric contraction following eccentric exercise-induced weakness,the relative load of the task shifted closer to 40% for both males and females.There was an elimination of the sex difference in fatigability,as both males and females had an~45% reduction in TTF(p<0.05;Fig.5),with no difference in TTF across sexes in the weakened state (p=0.30).Given the elimination of sex differences in fatigability,a power analysis fell below the 80% threshold;thus,there is a chance of type II error.However,when viewing the group and individual data (Fig.5),it is unlikely that this affects the interpretation (i.e.,there is no difference in TTF between males and females following eccentric exercise-induced weakness).
3.4.Fatigue-and weakness-associated reductions in neuromuscular function
For reductions in MVC torque relative to baseline,there was a sex×time interaction (p<0.01;Fig.6A) for fatigue task 1,with females experiencing an~21% reduction and males an~11%reduction in MVC torque.However,there was no difference in MVC torque reductions between sexes following the eccentric exercise(~20%;p=0.38)or following fatigue task 2(~27%;p=0.71).There was an effect of time(p<0.001) as well,with both sexes experiencing reductions in MVC torque compared to baseline.There were no interactions or main effects for VA,which remained high (~99%)following task failure of both fatigue tasks and following the eccentric exercise (p=0.24;Fig.6B).Both sexes experienced similar reductions(~50%-75%)in 10:50 Hz torque(PLFFD)over time(p<0.001;Fig.6C)compared to baseline.
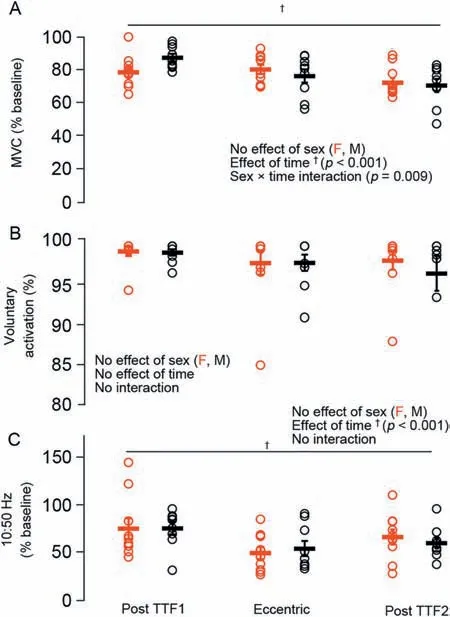
Fig.6.Relative reductions in (A) MVC torque,(B) VA,and (C) PLFFD(10:50 Hz ratio) immediately following the pre-exercise fatigue task (TTF1),30 min following the eccentric exercise,and immediately following the postexercise fatigue task (TTF2) in females (red) and males (black).Data are presented as mean ± SE.F=female;M=male;MVC=maximum voluntary contraction;PLFFD=prolonged low-frequency force depression;TTF=time to task failure;VA=voluntary activation.
3.5.EMG during both fatigue tasks
Agonist (TA) EMGRMSnormalized to maximal M-Wave amplitude increased similarly in males and females over the duration of the sustained isometric contraction (p=0.86),reaching a significant increase by 50% of TTF for fatigue task 1 and by 25% of TTF for fatigue task 2 (Fig.7A;p<0.001).Antagonist(soleus)EMGRMSnormalized to maximal M-Wave amplitude increased similarly in males and females for the fatigue task 1.However,during the second fatigue task (i.e.,post-weakness),there was a sex×time interaction (Fig.7B;p=0.008) in which females experienced almost double the antagonist activation compared to males by 75%of TTF.
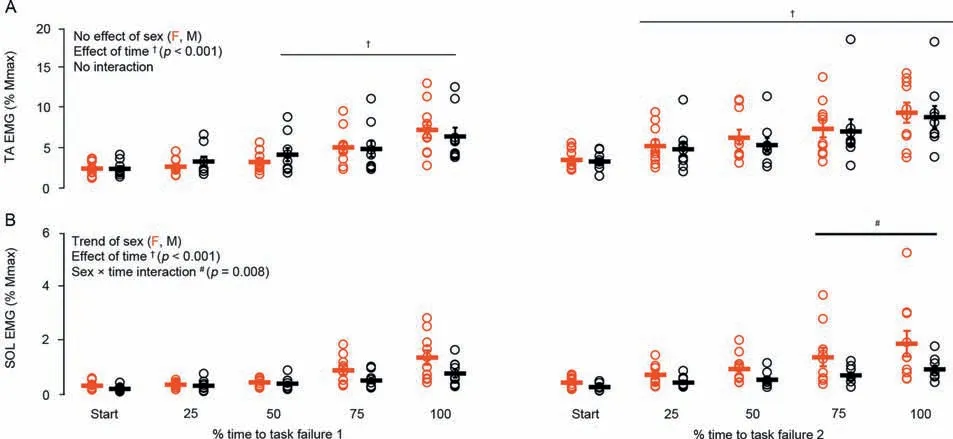
Fig.7.(A) Agonist (TA) and (B) antagonist (SOL) EMG activity during the sustained isometric contractions performed before and after the eccentric exerciseinduced muscle weakness in females(red)and males(black).Data are presented as mean±SE.EMG=electromyography;F=female;M=male;SOL=soleus;TA=tibialis anterior.
4.Discussion
Our study investigated sex-related differences in fatigability of the dorsiflexors during a sustained submaximal isometric task to task failure before and after eccentric exercise-induced muscle weakness.The main findings supported our hypothesis such that muscle weakness following the eccentric exercise protocol shifted the relative contraction intensity of the submaximal task to a relatively higher contraction intensity(from 30% to~40%)and blunted any sex-related differences.The underlying neuromuscular factors contributing to the elimination of the classic isometric sex-related performance advantage following eccentric exercise-induced muscle weakness (i.e.,the eccentric exercise protocol) appears to be a greater magnitude of antagonist muscle activation in females compared to males during the post-exercise fatigue task.This difference in neuromuscular activation across the sexes likely disadvantaged females,resulting in a blunting of their typical fatigability advantage over males.Here we highlight the importance of a divergence in neuromuscular activation strategies and antagonist activation in males and females by understanding the possible mechanisms driving sex-related differences in fatigability.
Males were~40% stronger than females for electrically evoked twitch and tetanic contractions as well as voluntary efforts (Figs.2 and 4),with no difference in VA.Prior to eccentric exercise-induced muscle weakness,and consistent with previous findings of reduced fatigability in females,24,27TTF during the sustained 30% MVC isometric contraction was~34% longer in females than in males (Fig.5).Agonist and antagonist neuromuscular EMG activity increased similarly in males and females throughout the fatigue protocol (Fig.7B).The unaccustomed eccentric exercise protocol successfully impaired neuromuscular function similarly across sexes with a 20% reduction in MVC torque,24% increase in PLFFD,and shift in optimal angle of torque production to 20% longer muscle tendon unit lengths following the fatigue task up to 30 min of recovery (Figs.3 and 4).These findings are consistent with previous reports of similar neuromuscular impairments of the ankle dorsiflexors in both males and females following eccentric exercise.8,18,23,39However,following eccentric exercise-induced muscle weakness,the reduced fatigability of females relative to males was eliminated,with both groups having an~45% shorter TTF(Fig.5).Calcium regulation of force was unlikely a contributor to the observed sex differences in fatigability,as there was no difference in PLFFD between males and females before or following the eccentric exercise (Fig.6C).While the mechanisms inherent to sex-related differences in fatigability are multifactorial,27,29,42data from the present study points to increased antagonist activation(Fig.7B)as a potential contributor to the blunting of the typically superior fatigue resistance females possess over males during submaximal isometric TTF.
It is well-accepted that females are less fatigable than males during sustained submaximal isometric contractions.1This sex-related difference in fatigability is most prominent at lower isometric contraction intensities3,4and becomes more variable during higher intensity isometric4,5and dynamic contractions.6-8As outlined in recent reviews,1,6mechanisms for sex-based differences in fatigability during submaximal sustained isometric contractions likely fall into 3 categories:(a) skeletal muscle metabolism and contractile properties,(b)muscle perfusion,and (c) VA.Assuming these 3 categories capture the underlying factors driving sex differences in fatigability,we can speculate on the possible mechanisms behind the diminution of females’ superior fatigue resistance following eccentric exercise-induced weakness/muscle damage.
(a) It has been suggested that during isometric contractions,females are less fatigable than males due to their greater proportional area of type I muscle fibers and,thus,greater oxidative metabolism.29,30This means females,as compared to males,have a greater density and volume of mitochondria,a greater rate of oxidative enzyme activity,31and a greater capillary-to-fiber ratio.32These factors enhance their ability to regenerate ATP and match ATP usage with aerobic metabolism in type I muscle fibers30as compared to males.However,in the present study following eccentric exercise-induced muscle weakness,there was a relative shift in the 30% MVC load for the sustained isometric contraction to a higher relative load (~40% of the post-eccentric exercise MVC).It is well-established4,5that with increasing contraction intensity,sex-related differences become less clear.To sustain the 30% of baseline MVC isometric load following eccentric exercise-induced muscle weakness,there would have to be a shift in the motor unit distribution to a greater proportion of higher threshold motor units,likely consisting of less oxidative muscle fibers,thus disadvantaging the females’reliance on oxidative metabolism as a potential mechanism driving sex-related differences in fatigability.
(b) Another factor thought to contribute to females’ superior fatigability over males is perfusion of the active muscle.Often,males are stronger than females;thus,for a similar relative submaximal intensity,males generate greater absolute forces,4which can lead to greater blood flow occlusion.Thus,less oxygen delivery and metabolite removal in the dorsiflexors of the males as compared to females could ultimately impair performance and explain,in part,the lower fatigability of females.1However,there have been reports of damage to not only the muscle contractile machinery following unaccustomed eccentric exercise,but also to the muscle microvasculature,which can impair microcirculation and hemodynamics.43-47Therefore,the eccentric contraction protocol used here likely disrupted the microvascular network,limiting perfusion to the muscle in both males and females,thereby blunting the female performance advantage.The lower occlusion pressure experienced by females compared to males for the same relative load43-47likely stopped being advantageous in terms of optimizing fatigability due to disruption of the microvascular network.For example,intramuscular pressure generated during isometric exercise will occlude muscular blood flow at 50%-65% MVC.25Therefore,arguably,blood flow occlusion may not be a major limiting factor in our 30% MVC fatigue task.However,the absolute target force for both fatigue tasks was the same,representing 30% of the baseline MVC values (males~9 Nm;females~5 Nm).As a result,the first fatigue task was performed at 30% of baseline MVC (male~30 Nm;female~18 Nm;Fig.2),while the task following the eccentric protocol was performed at~40% of the new post-eccentric exercise MVC (males~23 Nm;females~14 Nm;Fig.2).The higher relative intensity of the posteccentric exercise fatigue task likely made blood flow occlusion more of a limiting factor in TTF,25which under normal conditions should have made the sex differences in fatigability more pronounced.Eccentric exercise-induced damage to the microvasculature may have thereby eliminated the muscular perfusion advantage in females.
(c) There was no difference in VA during MVCs for males or females before or after the fatigue tasks or eccentric exercise.However,neuromuscular activation strategies during the sustained isometric contraction following the unaccustomed eccentric exercise diverged between males and females,with the females experiencing greater antagonist activation.Upon activation of an agonist muscle,the antagonist muscle is concomitantly activated,and this is termed coactivation.48Coactivation of the antagonist muscle results in a force opposing the agonist,49reducing overall net joint torque unless the agonist increases activation.While the literature is equivocal,evidence indicates that antagonist muscle co-activation is greater in females than in males.50-52For example,during submaximal concentric and eccentric contractions of the elbow flexors,females experienced greater antagonist activation than males.53Females were also reported to have greater antagonist hamstring and tibialis anterior activation than males during knee extension54and PF,50respectively.We are,however,not aware of any reports showing a divergence in antagonist coactivation in males and females during a sustained submaximal isometric task,and we only observed a difference in antagonist activation between females and males during the later phases(≥75%) of the sustained isometric contraction following eccentric exercise-induced muscle weakness (Fig.7B).This elevated antagonist activation in females may be mediated by greater central descending common drive or depressed reciprocal inhibition.55,56Both mechanisms lead to a greater level of excitability of the antagonist motor unit pool,thereby increasing co-activation.
It has been suggested that antagonist activation may contribute to fatigue-induced decreases in torque production.57Indeed,increases in antagonist activation during dynamic and isometric fatiguing tasks have been widely reported57for submaximal55,58,59and maximal59isometric contractions.The level of antagonist activation may also depend on muscle length such that there is an increase in antagonist activation at longer muscle lengths due to the joint being more susceptible to injuries such as hyperextension.60-63Therefore,since a relatively longer muscle tendon unit length (ankle joint angle of 35° PF) was used for the fatigue tasks in the present study,this sex-dependent difference in antagonist activation may also be muscle length/joint angle dependent.While there have been no sex-related differences in antagonist activation reported during MVCs following repeated eccentric contractions,8,10,23,39perhaps the MVC task was not sensitive enough to tease out any differences.Moreover,Pereira et al.64recently reported that TTF can be altered in response to pain.However,given the short time course of the fatigue task in the present study,it is unclear what relationships may exist between eccentric exercise-induced muscle soreness and alterations to TTF or whether these relationships would be different between sexes.
A limitation regarding our EMG normalization procedures should be noted.While the current consensus in the literature is that EMG activity should be normalized to EMG during an MVC for the corresponding muscle group,41we opted for a different approach by normalizing to maximal M-Wave amplitude.In order to normalize our EMG data to MVC,we would have needed a PF MVC in addition to our dorsiflexion MVC,which we did not obtain.Although normalizing to maximal M-Wave amplitude has some limitations (outlined by Besomi et al.41),in this case it is not unreasonable,and there is support in the literature.65,66Furthermore,during assessments of fatigue,the MVC normalization method also holds limitations,as individuals are required to provide a “true” maximum contraction,and it does not account for EMG-related alterations to fatigue.Altogether,normalization of EMG to maximal M-Wave amplitude was unlikely to confound our interpretations in the present study.Another possible limitation is our statistical power falling below the 80% threshold to detect differences in TTF between males and females post exerciseinduced muscle weakness(Fig.5).
In summary,why did females lose their fatigability advantage over males following eccentric exercise-induced muscle weakness? Although highly speculative,it seems that following the eccentric protocol,females(a)operated at a less advantageous and higher contraction intensity that was unfavorable to their superior reliance on oxidative metabolism,1,29(b) sustained more muscle damage to the microvascular network,43-47which limited their perfusion advantage over males,and(c)as indicated by our data,experienced increased antagonist activation during the sustained isometric contraction (Fig.7).These factors combined likely blunted the superior fatigue resistance typically observed in females during sustained isometric tasks.The fluctuating hormones of normally menstruating women have been shown to have potential implications in the physical performance of activities involving prolonged endurance exercise over the course of the menstrual cycle,but they have shown no effect on muscle contractile characteristics.67,68In a previous isometric study investigating fatigability in young women,it was shown that the phase of the menstrual cycle did not influence TTF when performed with the elbow flexors.69Therefore,interindividual variability in hormone fluctuations among female participants likely did not interfere with our results.
5.Conclusion
Eccentric exercise-induced muscle weakness eliminated the classic sex-related advantage reported for females when submaximal isometric tasks were performed to failure.Before eccentric exercise-induced muscle weakness of the dorsiflexors,females had a longer TTF (sustained 30% MVC isometric contraction) compared to males.However,upon inducing muscle weakness via high-intensity eccentric contractions,this sex-related difference was eliminated due to,in part,greater antagonist muscle activation in females than in males.In addition to other potential factors discussed,this difference in neuromuscular activation across the sexes likely disadvantaged females,resulting in a blunting of their typical fatigue resistant advantage over males.The effect of muscle weakness on muscle synergies contributing to sex differences requires further investigation.
Data accessibility
Supporting data are available upon request.
Acknowledgments
We would like to thank all of the participants in this study.This project was supported by the Natural Sciences and Engineering Research Council of Canada(NSERC).
Authors’contributions
All authors contributed equally to this study.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing of interests
The authors declare that they have no competing interests.
 Journal of Sport and Health Science2023年4期
Journal of Sport and Health Science2023年4期
- Journal of Sport and Health Science的其它文章
- Beyond cardiomyocytes:Cellular diversity in the heart’s response to exercise
- COVID-19:Insights into long-term manifestations and lockdown impacts
- Exercise training attenuates angiotensin II-induced cardiac fibrosis by reducing POU2F1 expression
- Specific alterations of regional myocardial work in strength-trained athletes using anabolic androgenic steroids compared to athletes with genetic hypertrophic cardiomyopathy
- Effects of exercise by type and duration on quality of life in patients with digestive system cancers:A systematic review and network meta-analysis
- Recovery shape of physical activity after COVID-19 pandemic
