Specific alterations of regional myocardial work in strength-trained athletes using anabolic androgenic steroids compared to athletes with genetic hypertrophic cardiomyopathy
Antoine Grndperrin,Frédéri Shnell,Erwn Donl,Elen Glli,Christophe Hedon,Olivier Czorl,Stéphne Nottin,*
a Avignon University,LaPEC UPR 4278,Avignon F-84000,France
b Rennes University,CHU Rennes,Inserm,LTSI-UMR 1099,Rennes F-35000,France
c Montpellier University,PhyMedExp,INSERM,CNRS,Physiology and Experimental Heart and Muscle Medicine,Montpellier 34295,France
Abstract Background:Strength-trained athletes using anabolic androgenic steroids(AAS)have left ventricular(LV)hypertrophy and myocardial fibrosis that can lead to sudden cardiac death.A similar feature was described in athletes with hypertrophic cardiomyopathy(HCM),which complicates the diagnosis for clinicians.In this context,we aimed to compare the LV function of the 2 populations by measuring global and regional strain and myocardial work using speckle-tracking imaging.Methods: Twenty-four strength-trained asymptomatic athletes using AAS (AAS-Athletes),22 athletes diagnosed with HCM (HCM-Athletes),and 20 healthy control athletes (Ctrl-Athletes) underwent a resting echocardiography to assess LV function.We evaluated LV global and regional strains and myocardial work,with an evaluation of the constructive work(CW),wasted work,and work efficiency(WE).Results:Compared to Ctrl-Athletes,both AAS-Athletes and HCM-Athletes had a thicker interventricular septum,with majored values in HCM-Athletes.LV strain was reduced in AAS-Athletes and even more in HCM-Athletes.Consequently,global WE was significantly diminished in both AAS and HCM-Athletes (93%±2% in Ctrl-Athletes,90% ± 4% in AAS-Athletes,and 90%±5% in HCM-Athletes (mean ± SD); p<0.05).Constructive work and WE regional analysis showed specific alterations,with the basal septal segments preferentially affected in AAS-Athletes,and both septal and apical segments affected in HCM-Athletes.Conclusion:The regional evaluation of myocardial work reported specific alterations of the major LV hypertrophy induced by the regular use of AAS compared to the LV hypertrophy due to HCM.This finding could help clinicians to differentiate between these 2 forms of pathological hypertrophy.
Keywords: Anabolic androgenic steroids;Hypertrophic cardiomyopathy;Left ventricular function;Myocardial work;Speckle-tracking echocardiography
1.Introduction
Intensive exercise training is associated with numerous morphologic and functional changes known as exerciseinduced cardiac remodeling.1These physiological adaptations,consecutive to intermittent increased hemodynamic load,are characterized by mild morphologic cardiac changes and do not raise clinical concern.2However in some athletes,left ventricular (LV) remodeling is more pronounced and can raise the suspicion of a pathologic adaptation,either due to use of performance-enhancing drugs or due to an underlying genetic cardiac disease.3,4Moreover,previous investigations defined a“gray zone”in which it is difficult for clinicians to distinguish between the extreme expressions of exercise-induced cardiac remodeling and a mild pathological or drug-induced phenotype.5
Among strength-trained athletes,the consumption of supraphysiologic doses of illicit anabolic androgenic steroids(AAS)is more and more widespread.6The regular use of AAS induces important LV mass increases,4,7-9LV systolic and diastolic dysfunctions,coronary artery disease,and myocardial fibrosis10-13that can lead to ischemic stroke or sudden cardiac death.7,8,14The increase of interstitial myocardial fibrosis induced by AAS was also observed in animal models15and was correlated with an activation of the renin-angiotensin system,which is known to be involved in cardiac pathological remodeling.16
Besides being consistent with AAS use,abnormal cardiac muscle thickening can be caused by a genetic disorder called hypertrophic cardiomyopathy (HCM).17HCM is characterized by an important LV hypertrophy,which is frequently not uniform,affecting specific regions of the myocardium such as the septum or lateral wall.18HCM is associated with myocardial disarray,energetic dysfunction of the LV myocytes,and interstitial myocardial fibrosis.3,19These structural and functional abnormalities can produce fatigue,dyspnea,chest pain,palpitations,and syncope.Sudden cardiac death represents the most devastating presenting manifestation,especially in young patients.
The purpose of the present study was to compare the regional function of pathological hypertrophy of strength-trained athletes reporting long-term and regular use of AAS with that of athletes diagnosed with HCM.They were compared with healthy highly-trained athletes with physiological LV hypertrophy.Importantly,recent development of echocardiography proposes to evaluate myocardial work as an innovative and non-invasive method for measuring myocardial performance.It requires a combination of longitudinal strain and noninvasive blood pressure measurements to obtain pressurestrain loops during the cardiac cycle,20-22and,thus,represents a more accurate assessment of myocardial function by considering cardiac afterload.22It differentiates myocardial constructive work (CW) and myocardial wasted work (WW) to estimate myocardial work efficiency(WE)and can be assessed regionally.We hypothesized that(a)the 2 forms of pathological remodeling would be associated with a decrease in myocardial CW and a decrease in myocardial WE,and (b)regional alterations would be different between pathological remodeling induced by athletes reporting AAS consumption and athletes with HCM.
2.Materials and methods
2.1.Study populations
Twenty-four strength-trained asymptomatic athletes actively taking AAS(AAS-Athletes),and who were known to participate in bodybuilding competitions,were recruited in French gymnasiums.The inclusion criteria required a training history of at least 5 h of training per week for a minimum of 5 years.To be included in this study,AAS-Athletes needed to self-report a documented history of AAS use for at least 3 courses of 2 weeks in the past 2 years.All were on drugs,i.e.,had administered their last AAS dose during the past 2 days.
Twenty-two athletes with HCM (HCM-Athletes) were retrospectively selected from a large database of the Regional Competence Center of Genetic Disease (Rennes Hospital,Rennes,France).We included only asymptomatic athletes with a moderate wall thickness hypertrophy(13-20 mm).The preliminary cardiac investigations resulting in the diagnosis were conducted during a period when these athletes were still actively engaged in competitive sports and before any decision regarding disqualification or cessation of exercise.HCM,especially in the case of borderline gray-zone wall thickness(13-15 mm),was diagnosed in the context of a combination of features,including (a) identification of HCM in a first-degree relative or an established pathogenic gene mutation;(b)nonconcentric patterns of LV hypertrophy (especially apical hypertrophy);(c) dynamic LV outflow tract obstruction;(d)late gadolinium enhancement on cardiac magnetic resonance;or (e) atrial or ventricular arrhythmias.3In this group,each subject underwent clinical examination,standard and speckletracking 2D-transthoracic echocardiography,late gadolinium enhancement by cardiac magnetic resonance imaging,48-h Holter monitoring,and cardiopulmonary exercise testing to diagnose HCM,according to current guidelines.23Exclusion criteria were as follows: obstructive cardiomyopathy at rest,concomitant moderate or severe valvular heart disease,myocardial storage disease,hypertension,coronary artery disease,or left bundle branch block.
Twenty healthy athletes (Ctrl-Athletes) from endurance(n=10)or strength(n=10)activities serving as controls were prospectively included.Ctrl-Athletes had a self-reported history of never taking AAS or other doping substances and a training history of at least 5 h of training per week for a minimum of 5 years.
Exclusion criteria for Ctrl-Athletes and AAS-Athletes were congestive heart failure,moderate or severe valvular heart disease,hypertension,respiratory or metabolic disorders,or active cigarette smoking.All subjects included in the study were aged 18-50 years.The study was conducted in accordance with the Code of Ethics of the World Medical Association as laid down in the Declaration of Helsinki and reviewed by an independent committee.The inclusion of AAS-Athletes was approved by the French Ethics Committee in Sports Science (CERSTAPS n°2018-04-10-28).All patients gave their written informed consent to participate in the study.
2.2.Echocardiographic recordings
Echocardiography was carried out with the subject in the left lateral decubitus position with Vivid ultrasound systems(GE Healthcare,Horten,Norway)using a 3.5-MHz transducer(M4S probe;GE Healthcare).Cine loops were recorded in parasternal long axis and apical (5,4,2,and 3 chambers)views and saved for blinded offline analysis (EchoPAC,BT203 Version,GE Healthcare).Grayscale images were saved at a frame rate of 80-90 frames/s.Two-dimensional echocardiographic measurements were performed in accordance with the guidelines of the American Society of Echocardiography.24
2.3.Echocardiographic analysis
2.3.1.LV morphology and global function
Interventricular septum thicknesses were measured from the parasternal long axis view.LV diastolic function was assessed by doppler from peak early (E) and atrial (A) transmitral flow velocities.LV volumes and ejection fraction (EF)were assessed using the Simpsons biplane method.Stroke volume and cardiac output were assessed from a 5-chamber view and then indexed to body surface area.
2.3.2.LV global and regional longitudinal strain
The global longitudinal strain (GLS) was assessed from apical 4-,3-,and 2-chambers views as previously described.25Data were normalized to percentage of systolic duration to avoid differences in heart rate and cine loops frame rates.Systolic and diastolic strain rates were then obtained from time-derivation of strains.The diastolic longitudinal strain rate was used as an indicator of LV relaxation.26Then we used an 18-segments model from apical 4-,2-,and 3-chambers views to assess LV regional strains and time to peak (TTP) strains in accordance with the nomenclature of Lang et al.24To assess LV mechanical dispersion,the maximum delay was calculated as the difference between TTP (in ms) of the last and the first segment reaching their peak strains over the 18 segments (Maximum delay18S).27,28The standard deviation (SD) of the TTP (in ms) over the 18 segments was also calculated(SD18S).
2.3.3.Myocardial work quantification
Myocardial work and related parameters were estimated using the Automatic Function Imaging(AFI)of the Echo PAC software(Version 203;GE Healthcare).Myocardial work was estimated as a function of time throughout the cardiac cycle by the combination of LV strain data (recorded on the apical 4-,3-,and 2-chambers views) obtained by STE and a non-invasively estimated LV pressure curve as previously described and validated.20,21,29Peak arterial pressure measured a few minutes before the echocardiography with a cuff-manometer was assumed to be equal to peak systolic and diastolic LV pressures and to be uniform throughout the ventricle.20Myocardial work was then quantified by calculating the rate of segmental shortening by differentiating the strain curve and multiplying the resulting value by the instantaneous LV pressure.The result was integrated over time to obtain myocardial work as a function of time.
Myocardial work was calculated from mitral valve closure until mitral valve opening as previously described.22,29The work performed by the myocardium during segmental shortening represents CW,whereas the work performed by the myocardium during stretch or segmental lengthening represents the energy loss,which is defined as WW.The work performed by the myocardium during segmental shortening,which does not promote LV relaxation,was considered segmental WW.On the other hand,the work performed by the myocardium during segmental lengthening was considered segmental CW.Ultimately,the global or segmental WE was obtained as follows:
Strain and myocardial work evaluation was done by a single investigator.We selected 30 subjects at random for the assessment of intraobserver reproducibility of the regional and myocardial work parameters.Measurements were made 48 h apart on the same echocardiographic images.For global parameters,intraobserver reliability was good,with a coefficient of variation of 1.2% and 2.8% for global WE and global CW,respectively.Finally,for regional parameters,intraobserver reliability was good as well,with a coefficient of variation of 2.9%for regional WE(from 1.5% on the mid-septal segment to 5.1% on the basal anterior segment) and a coefficient of variation of 8.5% for regional CW (from 5.7% on the mid-septal segment to 12.1%on the basal anterior segment).
2.4.Statistical analysis
All values were expressed as mean ± SD.Statistical analyses were performed using MedCalc Statistical Software Version 20.013 (MedCalc Software Ltd.,Ostend,Belgium;https://www.medcalc.org;2021).Normality of the distribution was checked by the Shapiro-Wilk test,and homogeneity of the variances was checked by the Bartlett test.One-way analysis of variance was used to compare groups,and Fisher’spost hoctest was used when appropriate.Statistical significance was defined as apvalue of less than 0.05.Correlations were determined between CW,WE,and functional or mechanical dispersion parameters using Pearson single linear regression test.
3.Results
Clinical data of the study populations and HCM characteristics are presented in Table 1.Body mass and body surface area were higher in AAS-Athletes compared to both Ctrl-Athletes and HCM-Athletes.Diastolic blood pressure was significantly higher in AAS-Athletes compared to Ctrl-Athletes.Stroke volume index was statistically lower in AAS-Athletes compared to Ctrl-Athletes.Information about AAS consumption reported by AAS-Athletes is presented in Table 2.
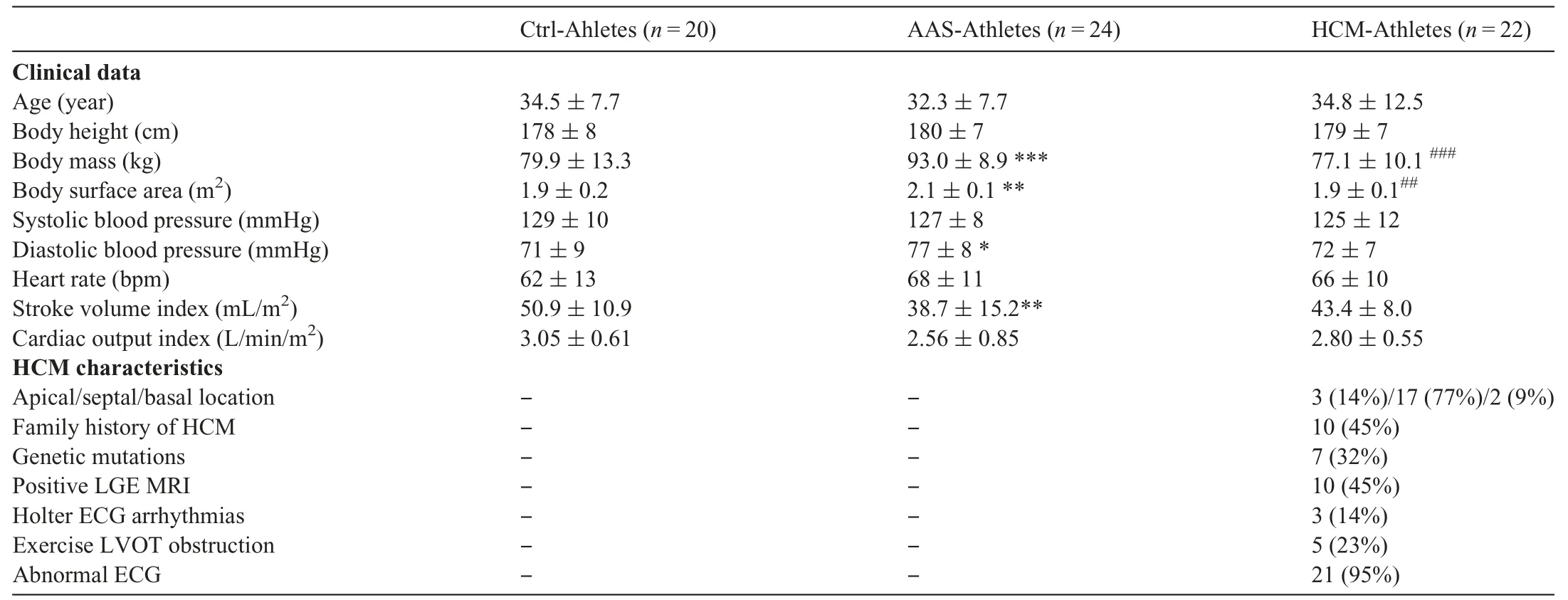
Table 1 Characteristics of the study population(mean±SD or n(%)).
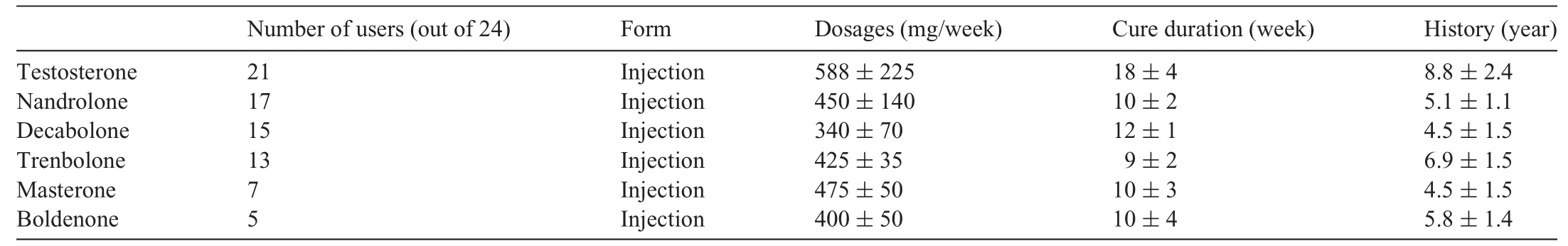
Table 2 AAS consumption reported in the 24 AAS-Athletes(mean±SD).
3.1.LV morphology and function
LV morphology and function results are presented in Table 3.Maximal wall thickness was higher in both AASAthletes and HCM-Athletes in comparison to Ctrl-Athletes.Among patients with pathological hypertrophy,HCM-Athletes had significantly thicker walls than did AAS-Athletes.Of note,most of our HCM-Athletes (64%) had a maximal wall thickness in the “gray zone” (13-15 mm).End-diastolic volume index was higher in Ctrl-Athletes compared to the other groups.The EF was lower in AAS-Athletes compared to Ctrl-Athletes,whereas E wave was lower in AAS-Athletes than in both other groups.
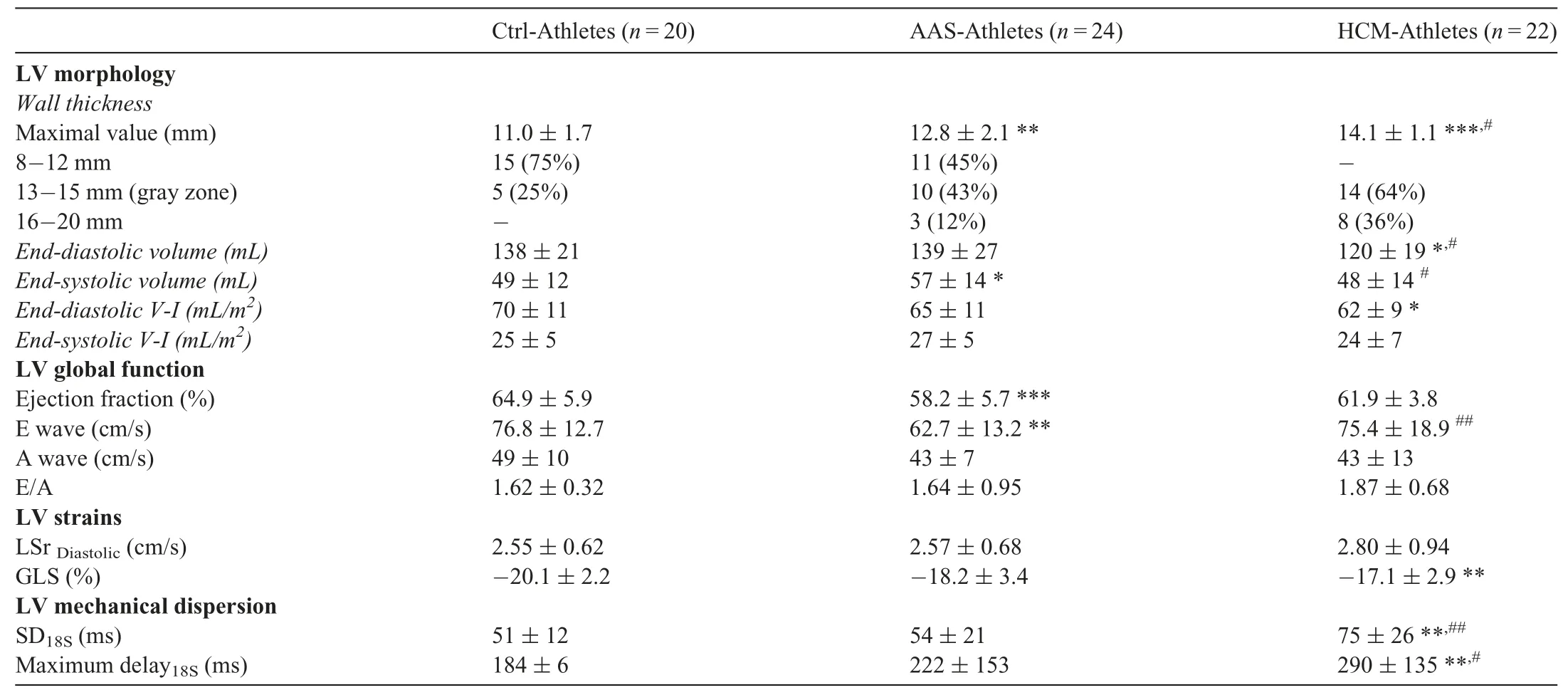
Table 3 Left ventricular morphology and function(mean±SD or n(%)).
3.2.LV strains and mechanical dispersion
Global strains and strain rates are presented in Table 3.The GLS was significantly lower (in absolute values) in HCM-Athletes and tended to be lower in AAS-Athletes(p=0.08) compared to Ctrl-Athletes.Both SD18SandMaximum delay18S,which are indexes of mechanical dispersion,were significantly higher in HCM-Athletes.Regional peak and TTP strains are presented using bullseyes in Fig.1A and 1B,respectively.Compared with the Ctrl-Athletes,the AAS-Athletes exhibited significantly lower LV strains on 1 basal segment (basal anterior) and 1 segment at the mid-wall level (mid inferoseptum).The HCM-Athletes showed more segmental dysfunctions with a significant drop in LV strains on 2 basal segments (basal anterior and basal anteroseptum),3 mid-wall segments (mid inferoseptum,mid anterolateral,and mid anterior),and 3 apical segments(apical septum,apical lateral,and apical inferolateral).AASAthletes had only 1 delayed segment on the mid-wall (mid inferoseptum),whereas HCM-Athletes had 6 delayed segments: 1 at the base (basal anterolateral),4 at the midwall (mid inferoseptum,mid inferior,mid anterolateral,and mid anterior),and 1 at the apex(apical lateral).
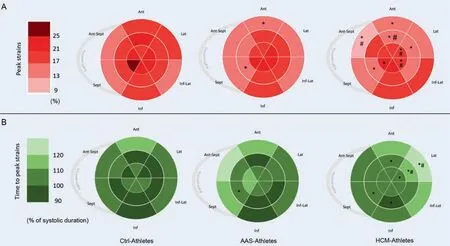
Fig.1.Distribution of strains and time to peak strains.Color coded bullseye maps for(A)average longitudinal segmental strains(regional peak strains)and(B)average segmental time to peak strains,using an 18-segment model.Sept and Lat represent the apical 4-chamber view walls;Inf and Ant,the apical 2-chamber view walls;and Ant-Sept and Inf-Lat,the 3-chamber view walls.* p<0.05,significantly different from Ctrl-Athletes;# p<0.05,significantly different from AAS-Athletes.AAS=anabolic androgenic steroids;Ant=anterior;Ant-Sept=antero-septal;Ctrl=control;HCM=hypertrophic cardiomyopathy;Inf=inferior;Inf-Lat=infero-lateral;Lat=lateral;Sept=septum.
3.3.LV myocardial work
Myocardial work parameters are presented in Fig.2.The global CW was significantly reduced in HCM-Athletes compared to Ctrl-Athletes (Fig.2A and its graphical representation with the pressure-strain loops in Fig.2B).The global WW did not differ between groups (Fig.2C).However,the global WE was significantly reduced in both AAS-Athletes and HCM-Athletes compared to Ctrl-Athletes(Fig.2D).Significant correlations were found between SD18S(index of LV mechanical dispersion) and global WE(Fig.3A)and global CW(Fig.3B)as well as between EF and global WE(Fig.3C).
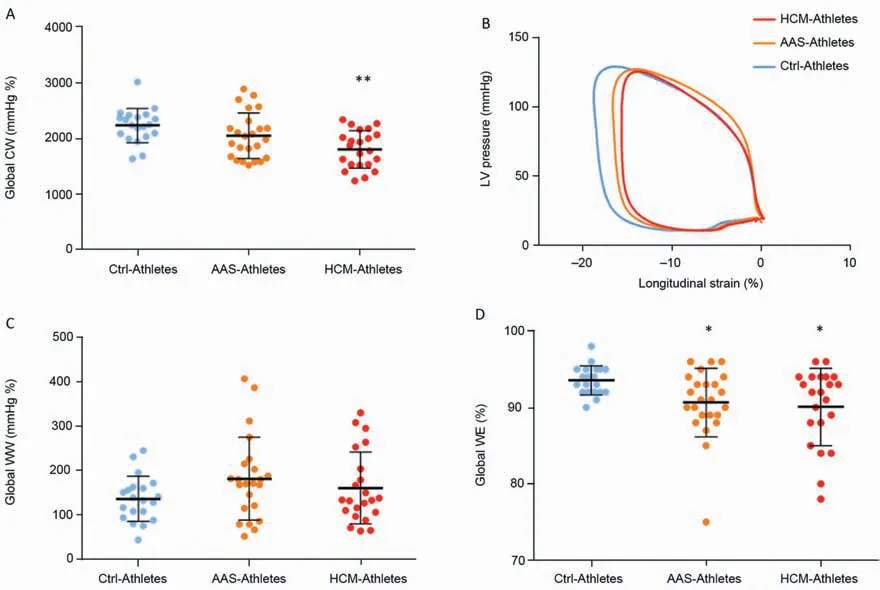
Fig.2.Myocardial work parameters.(A)the global CW,(B)pressure-strain curves over the 3 groups,(C)global WW,and(D)global WE.*p<0.05,**p<0.01,significantly different from Ctrl-Athletes.AAS=anabolic androgenic steroids;Ctrl=control;CW=constructive work;HCM=hypertrophic cardiomyopathy;LV=left ventricular;WE=work efficiency;WW=wasted work.
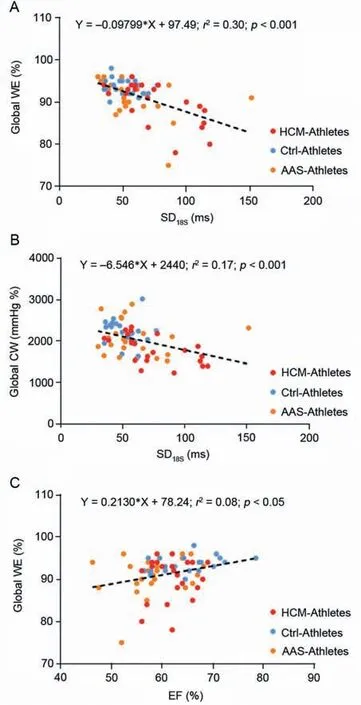
Fig.3.Correlations between myocardial work parameters and left ventricular function or mechanical dispersion parameters.(A) The correlation between global WE and SD18S,(B) the correlation between global constructive work and the SD of time to peak strains over the 18 segments,and (C) the correlation between the WE and the EF.AAS=anabolic androgenic steroids;Ctrl=control;CW=constructive work;EF=ejection fraction;HCM=hypertrophic cardiomyopathy;SD18S=standard deviation of time to peak strains over the 18 segments;WE=work efficiency.
The regional CW and WE calculated over the 18 segments of the LV are presented in Fig.4.Compared to Ctrl-Athletes,AAS-Athletes exhibited a drop of CW on 3 basal segments(basal inferoseptal,basal anterior,and basal anteroseptal),while HCM-Athletes had lower CW on 1 basal segment(basal anteroseptal),3 mid-wall segments (mid inferoseptal,mid anterolateral,and mid anterior),and 5 apical segments (apical lateral,apical anterior,apical inferolateral,apical lateral,and apical anterior).The overview of the WE index reveals that the higher reductions are observed in the base of the LV in AAS-Athletes,with significant decreases over the same 3 basal segments as those exposed for CW.However,HCM-Athletes exhibited a significantly lower WE on 1 basal segment(basal anteroseptal),1 mid-wall segment (mid anterolateral),and 2 apical segments(apical septal and apical lateral).
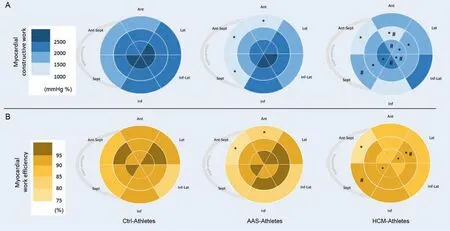
Fig.4.Distribution of myocardial constructive work and WE.Color-coded bullseye maps for(A)average segmental constructive work and(B)average segmental WE,using an 18-segment model.Sept and Lat represent the apical 4-chamber view walls;Inf and Ant,the apical 2-chamber view walls;Ant-Sept and Inf-Lat,the 3-chamber view walls.* p<0.05,significantly different from Ctrl-Athletes;# p<0.05,significantly different from AAS-Athletes.AAS=anabolic androgenic steroids;Ant=anterior;Ant-Sept=antero-septal;Ctrl=control;HCM=hypertrophic cardiomyopathy;Inf=inferior;Inf-Lat=infero-lateral;Lat=lateral;Sept=septum;WE=work efficiency.
4.Discussion
In the present study,we compared the myocardial function of 2 models of pathological hypertrophy,namely genetic and pharmacological,with healthy subjects in a context of high exercise training.We used global and regional STE and myocardial work analysis,a new echocardiographic tool,to characterize the specific adaptations of these different forms of LV remodeling in athletes.Our main findings were that (a)global CW was lower in HCM-Athletes only,whereas LV global myocardial WE was decreased in both AAS-Athletes and CMH-Athletes as compared to Ctrl-Athletes,and (b) the regional analysis of CW and WE revealed specific patterns of regional dysfunction,with the basal septal segments preferentially affected in AAS-Athletes and both septal and apical segments affected in HCM-Athletes.
4.1.LV morphology and global function
The populations of the present study were rigorously selected.The Ctrl-Athletes and AAS-Athletes reported many years of training with more than 5 h of practice per week.AAS-Athletes were included only if they reported regular high doses of AAS consumption for several years.Interestingly,we observed a major hypertrophy in this group compared to Ctrl-Athletes,confirming the additional effect of AAS.Indeed,it has been demonstrated that AAS exposure at supraphysiologic doses stimulates cardiomyocytes hypertrophy.30HCM-Athletes were retrospectively selected from a large database of athletes diagnosed with HCM,31from whom we chose only young athletes with moderate wall-thickness hypertrophy.32Despite these specific inclusion criteria,HCMAthletes exhibited significantly higher maximum septal thickness compared to Ctrl-Athletes and AAS-Athletes.
The LV remodeling of athletes consuming high doses of AAS was associated with a decrease in LV systolic and diastolic functions.4,9Our AAS-Athletes showed lower EF and E wave compared to both other groups,with lower GLS despite values not reaching statistical significance(p=0.08).In HCM-Athletes,results were different because EF remained unchanged and GLS was reduced—a finding that has been frequently observed elsewhere33-35and that is potentially explained by the compensatory increase of LV circumferential strain in HCM patients,as suggested by Stokke et al.36
In the present study,we used the recently developed noninvasive technique of myocardial work estimation,which integrates LV strains and LV intraventricular pressure curves during the cardiac cycle.Neither the negative work during systolic lengthening nor the positive work during post-systolic contractions in a dyssynchronous ventricle makes a contribution to CW and LV ejection;therefore,both are considered wasted energy.Our results indicated that global CW was lower in HCM-Athletes compared to the other groups.Global WW seemed slightly higher in AAS-Athletes compared to Ctrl-Athletes,but the difference failed to reach statistical significance.As a result,global WE was lower in both AASAthletes and HCM-Athletes compared to Ctrl-Athletes.In athletes using AAS and in patients diagnosed with HCM,previous magnetic resonance imaging (MRI) or post-mortem studies provided evidence that cardiac remodeling is associated with an increase in myocardial fibrosis,7,37which has adverse effects on myocyte excitability,cell-to-cell coupling,and the regulation of intracellular calcium.These myocardial adverse alterations could increase mechanical dispersion and the occurrence of post-systolic shortenings,resulting in myocardial inefficiency.38,39Recently,our group published a study focused on mechanical dispersion in AAS-Athletes and provided evidence of an increase in post-systolic shortenings in this population.28Therefore,these specific myocardial alterations probably explained,at least in part,the drop in WE.Along the same lines,recent investigations observed that CW and WW were lower in HCM40and predicted LV fibrosis.40On the other hand,myocardial WE was found to be closely related to exercise capacity in athletes.41The lower WE observed in our AAS-Athletes,similar to that observed in our HCM-Athletes,offers additional evidence of a pathological LV remodeling in relatively young athletes using AAS.Our significant (but moderate) correlation between global WE and SD18Ssuggests that an increase in mechanical dispersion could be partly responsible for the lower WE observed in our AASand HCM-Athletes.
4.2.LV regional myocardial work
We used a regional analysis of strains and myocardial work,which might be helpful in identifying the specific differences of LV hypertrophy related to AAS consumption or to HCM.Indeed,previous studies showed the usefulness of regional analysis in diagnosing various pathological conditions in patients,such as HCM,42or in response to cardiac resynchronization therapy.43LV strains were decreased on 2 segments in AAS-Athletes and on 8 segments in HCM-Athletes.Similar results were observed on TTP strains,since 1 segment was delayed in AAS-Athletes and 6 were delayed in HCMAthletes,suggesting that more segments were altered in HCMAthletes than in AAS-Athletes.
Analysis of LV peak strains is inherently limited because both intra-ventricular pressure and residual myocardial contraction after ejection are not considered.However,these limitations are overcome with myocardial work analysis.21Interestingly,our results strongly supported regional differences between AAS-and HCM-Athletes.In AAS-Athletes,both CW and WE were significantly reduced on 3 segments localized at the basal-septal level,whereas in HCM-Athletes they were diminished both on apical and septal segments,in line with the location of their HCM.Recently,with a concomitant evaluation of myocardial work by echocardiography and of myocardial fibrosis by magnetic resonance imaging,Galli et al.40showed an association between CW decrease and presence of interstitial fibrosis.Previous postmortem studies reported fibrosis development predominantly situated on the interventricular septum of AAS-users,7,8which could explain the drop of CW in AAS-users.Furthermore,our bullseyes showed an increase of myocardial work heterogeneity from Ctrl-Athletes to HCM-Athletes:Ctrl-Athletes exhibited a base-to-apex myocardial work gradient,which was less present in AASAthletes and totally abolished in HCM-Athletes.Taken together,these results showed that AAS consumption led to specific regional dysfunctions that were distinct from those observed in HCM-Athletes.The evaluation of regional myocardial work,a novel approach to assess the myocardial function,might help clinicians to distinguish the cardiac remodeling of athletes who are using AAS from those who are suffering from HCM,in complement with genetic analysis in selected cases.44
4.3.Study limitations
The study population was relatively small,but AASAthletes and HCM-Athletes were rigorously selected according to our inclusion criteria.Ctrl-Athletes and HCMAthletes practiced endurance,strength,or mixed sports,whereas AAS-Athletes practiced only strength exercises because the use of AAS was mainly observed in strength activities as a way of improving muscle mass.6Despite the recruitment of strength-trained athletes reporting extended use/abuse of high doses of AAS was very difficult,we included a relatively large population of bodybuilders from many gymnasiums.Inevitably and for ethical reasons,information about the intake of AAS was self-reported,and the type of drugs and dosages differed between athletes.Other studies on strengthtrained athletes using AAS are needed,especially to link the drop in their LV myocardial work with the likely increase in myocardial fibrosis.Finally,current software for non-invasive myocardial work assessment does not take myocardial wall thickness and curvature into account.
5.Conclusion
Based on myocardial work assessments,our study brings additional evidence of pathological LV remodeling in strength-trained athletes reporting regular use of AAS.Importantly,regional analysis of myocardial work highlighted that depressed myocardial function was observed at the basalseptal level in AAS-Athletes,whereas it was observed mainly at the septal and apical levels in HCM-Athletes.These results underlined the benefit of myocardial work analysis compared to myocardial strain analysis alone.In complement to other investigations(e.g.,genetic evaluation),these new parameters could be useful for the clinician to better distinguish the major LV hypertrophy induced by regular use of supraphysiologic doses of AAS from LV hypertrophy due to HCM.
Acknowledgments
This work was supported by YAKHA Sport,France and by the Platform 3A,funded by the European Regional Development Fund,the French Ministry of Research,Higher Education and Innovation,the Provence-Alpes-C^ote-d’Azur region,the Departmental Council of Vaucluse,and the Urban Community of Avignon.
Authors’contributions
AG submitted the project to the ethics committee,recruited participants,performed the physical and anthropometrical evaluations of the participants,the echocardiographic data analysis,and statistical analysis,and wrote and approved the manuscript;EG,ED,and CH supervised the research and the echocardiographic acquisitions;OC performed the statistical analysis and the critical analysis of the echocardiographic data,revised the manuscript,and approved the final version of the manuscript.SN and FS supervised the research,the echocardiographic acquisitions,the echocardiographic data analysis,and wrote and approved the final version of the manuscript.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
 Journal of Sport and Health Science2023年4期
Journal of Sport and Health Science2023年4期
- Journal of Sport and Health Science的其它文章
- Beyond cardiomyocytes:Cellular diversity in the heart’s response to exercise
- COVID-19:Insights into long-term manifestations and lockdown impacts
- Exercise training attenuates angiotensin II-induced cardiac fibrosis by reducing POU2F1 expression
- Effects of exercise by type and duration on quality of life in patients with digestive system cancers:A systematic review and network meta-analysis
- Recovery shape of physical activity after COVID-19 pandemic
- Sports compression garments improve resting markers of venous return and muscle blood flow in male basketball players
