Antioxidant status in patients with bladder cancer regarding cancer stage and grade
Zhid Lepr , Jsmin Ali ,, Orhn Lepr ,Hjrudin Sphovi , Almir Fjki
a Urology Clinic, Clinical Center University of Sarajevo, Bolnika 25, 71 000 Sarajevo, Bosnia and Herzegovina
b Department of Physiology,Faculty of Medicine,University of Sarajevo,ekalua 90,71 000 Sarajevo,Bosnia and Herzegovina
c Department of Pathophysiology, Faculty of Medicine, University of Sarajevo,ekalua 90, 71 000 Sarajevo, Bosnia and Herzegovina
KEYWORDS Bladder cancer;Antioxidant;Superoxide dismutase;Catalase;Serum albumin
Abstract Objective: The imbalance of antioxidants and pro-oxidants plays a crucial role in the carcinogenesis of bladder cancer(BC).This study aimed to evaluate serum antioxidant status in patients with BC and determine its potential use in the diagnosis and progression potential considerations following histopathological assessment.Methods: A cross-sectional study included 90 patients with BC,divided into Ta,T1,and T2—T4 stage subgroups,and according to cancer progression potential,into low-grade(LG)and highgrade(HG)subgroups.The control group(CG)included 30 healthy volunteers.Antioxidant status was determined using the spectrophotometric method and standard laboratory tests.Results: Serum superoxide dismutase activity was significantly higher in BC patients regarding cancer stage in comparison to the CG (p<0.001).Catalase activity was highest in T2—T4 subgroup and was significantly higher compared to the Ta (p<0.01) and T1 (p<0.05) subgroups.Serum albumin level was significantly lower in the BC group compared to the CG (p<0.001).In addition, it was significantly lower in T2—T4 subgroup compared to T1 and Ta subgroups(p<0.01).A significant negative correlation was found between tumor size and serum albumin level only(r=-0.386,p<0.01).Catalase activity was higher in HG subgroup(p=0.009),while bilirubin level was higher in LG subgroup(p=0.035).The optimal cut-off value of catalase activity in differentiating patients with LG and HG BC subgroups was ≥11.96 IU/L,and the specificity and sensitivity were 51.1% and 82.2%, respectively.Bilirubin level, for a calculated optimal cut-off value of ≥11.95 μmol/L, had a specificity of 44.1% and sensitivity of 80.0%.Conclusion: More invasive stages of BC with greater progression potential are associated with an increase in enzymatic antioxidant activity and a decrease in non-enzymatic antioxidant capacity.It may suggest a possible role of antioxidants in the prediction and monitoring of illness trajectory.
1.Introduction
Bladder cancer (BC) is the 10th most common malignancy overall with almost 550 000 new cases recorded worldwide in 2018[1].The most strongly attributable risk factor for BC in developed countries is tobacco consumption, with a population attributable risk of 50%in both men and women,followed by occupational and environmental exposure [2].
Urothelial carcinoma accounts for 90% of BCs, classified as non-muscle invasive BC(Ta,T1,and carcinomain situ)or muscle-invasive BC (T2—T4) with the latter carrying a worse prognosis.Approximately 70%—75% of BCs are non-muscle-invasive forms and managed by transurethral resection or radical cystectomy and intravesical therapy[3].
Many studies suggested that excessive production of reactive oxygen species(ROS)and reactive nitrogen species plays a crucial role in human carcinogenesis.Alterations of the balance of antioxidants and oxidants lead to oxidative stress, which results in nitration and oxidation of nucleic acids, plasma lipids, and proteins, and promotes tissue damage mediated by the reactive species [4,5].
AccordingtotheoxidativehypothesisofBCcarcinogenesis,many carcinogens including benzidine, 2-naphthylamine,β-naphthylamine, and 4-aminobiphenyl, can generate free radicals,which can cause cell damage and create conditions for malignant transformation.This process takes place through at least three stages—initiation, promotion, and progression[5].
Antioxidants play a protective role in tissue protecting them from oxidative damage.These substances form preventative or repair systems of endogenous or exogenous origin and can be classified as primary or secondary, hydrosoluble or liposoluble, and natural or synthetic.Total antioxidant status can be observed through two separate categories: enzymatic, slow-acting antioxidants, such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and other enzymes, and non-enzymatic,fast-acting antioxidants, such as albumin, bilirubin, uric acid, glutathione, β-carotene, bioflavonoids, tocopherols,and ascorbic acid [6].SOD converts the superoxide anion to hydrogen peroxide,which is also a substrate for catalase and GPx.Catalase metabolizes hydrogen peroxide to water and oxygen, while GPx, in reaction with glutathione, reduces both hydrogen peroxide and organic hydroperoxides [6—8].
Antioxidants have been shown to inhibit both initiation and promotion in carcinogenesis, protect cells against the excessive production of ROS and reactive nitrogen species,and prevent the accumulation of hydrogen peroxide in bladder tissue.Numerous experimental and clinical studies have demonstrated the correlation of oxidative stress markers with the development and progression of BC.The decreased antioxidant enzyme expression and activity in cancerous bladder tissue have been reported, which emphasizes their possible role in the carcinogenesis of BC[4—7].
This study aimed to evaluate serum antioxidants status in patients with BC, as well as to determine its potential role in the diagnosis of the disease and progression potential considerations following histopathological assessment.
2.Patients and methods
2.1.Patients
The cross-sectional study included 90 patients, both genders with confirmed BC, operatively treated at the Urology Clinic, Clinical Center University of Sarajevo.The patients in the BC group were divided into three stage subgroups with 30 patients each, based on pathological findings after transurethral resection of bladder tumor: non-invasive,superficial BC subgroups (Ta and T1), and invasive BC subgroup(T2—T4).The control group(CG)included 30 healthy volunteers who were age- and gender-matched to participants in the BC group.Patients in the BC group were also divided into grade subgroups according to cancer progression potential and were presented as low-grade(LG)BC and high-grade (HG) BC after histopathological analysis.
Due to the potential impact of inflammatory conditions, patients and control participants with acute or chronic inflammatory diseases, asthma, Crohn’s disease,ulcerative colitis, liver cirrhosis, multiple sclerosis, pituitary tumor, or other malignant neoplasia were excluded.The study was approved by the Clinical Center University of Sarajevo Ethics Committee (approval number:0207.52115), after obtaining informed consents from all individuals.
2.2.Methods
After carrying out diagnostic procedures, which included physical examination, urological examination, ultrasonography of the bladder, intravenous urography, and urethrocystoscopy, a definite indication for surgery was given.
After histopathological analysis, the definitive selection of patients was performed.The grade and stage of tumors were determined according to the tumor nodules metastases classification of malignant tumors based on histopathological examination.Non-muscle invasive BC is defined as neoplasia confined to the mucosa (including Ta and carcinomain situ) or lamina propria (T1).Muscle-invasive BC includes muscle layer invasive forms(T2) and higher stage cancers which invade beyond the muscle layer into perivesical tissue (T3) or surrounding organs (T4).
2.3.Measurement of antioxidants
Due to the possible influence of surgery and diagnostic procedures on the serum antioxidants level, blood sampling was performed before carrying out any diagnostic procedures.The blood sample was collected during the routine biochemical tests that were part of the diagnostic protocol.
After spontaneous precipitation of the blood sample for 20 min, it was centrifuged for 5 min at 3000 rpm, and 0.2 mL of serum was extracted for SOD and catalase analysis.The serum was stored at -80°C until analysis.
SOD and catalase levels were determined using the Lambda 25 ultraviolet or visible spectrophotometer (Perkin-Elmer, Norwalk, CT, USA), while standard laboratory tests were used for albumin, bilirubin, and uric acid serum levels.
2.4.Statistical analysis
Data were provided as median and interquartile range,and the Shapiro-Wilk test was used for the data distribution analysis.Depending on the distribution of the variables,comparisons between the groups were performed by the ANOVA,Student’st-test,andKruskal-Wallsor Mann-WhitneyUtest.Additionally,the Chi-square test was used for categorical variables.Since variables were not normally distributed, correlations were assessed by Spearman’s test.To determine optimal cut-off values of SOD and catalase for differentiation between BC and CG, as well as for differentiation of patients with LG and HG subgroups,receiver operating characteristic (ROC) curves and their corresponding areas under the curve (AUC) were used.The accuracy rate for ROC curve was calculated with a 95%confidence interval (95% CI).Thep-values of <0.05 were considered statistically significant.Statistical analyses were performed using IBM SPSS 23.0 statistical software system (IBM Corporation, Chicago, IL, USA).
3.Results
The baseline characteristics of the study groups are shown in Table 1.In terms of gender distribution,men were dominant in both groups (53.3%vs.46.7% [CG], 72.2%vs.27.8% [BC group]).Median tumor size was 2.0 (interquartile range:0.5—4.0) cm.In terms of the distribution of patients by tobacco consumption habit,smokers were dominant in the BC group, with a statistically significant difference (p<0.001).

Table 1 Baseline characteristics of patients in CG and BC group.
The SOD activity (median) in BC group was significantly higher compared to the CG (50.28 IU/mLvs.32.62 IU/mL,p<0.001), while albumin level was significantly lower compared to the CG (38.0 g/Lvs.41.0 g/L,p<0.001).No significant difference was observed in catalase activity,uric acid level, or bilirubin level between BC group and CG(Table 2).

Table 2 Comparative analyses of antioxidants in CG and BC group.
The serum SOD activity(median)in Ta(52.10 IU/mL),T1(51.16 IU/mL), and T2—T4 subgroups (48.29 IU/mL), was significantly higher than in CG (32.62 IU/mL) (p=0.001,p<0.005, andp=0.004, respectively).No statistically significant difference was observed in SOD activity between individual BC subgroups (Ta, T1, or T2—T4) (Table 3).
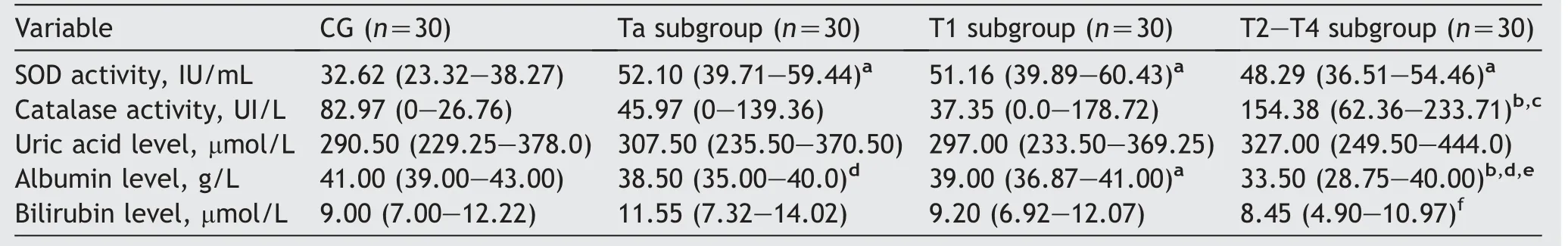
Table 3 Comparative characteristics of patients in CG and bladder cancer subgroups.
The serum catalase activity (median) in T2—T4 was significantly higher than in Ta (154.38 IU/Lvs.45.97 IU/L,p<0.01) and T1 subgroups (154.38 IU/Lvs.37.35 IU/L,p<0.05),but not significantly higher when compared to the CG.No statistically significant difference was observed between the median catalase activity in BC subgroups or CG.Furthermore, the median serum catalase activity,although higher than in the CG, was not statistically significant in BC subgroups (Table 3).
The serum levels of uric acid in Ta, T1, T2—4 subgroups, were higher, but not statistically significant,compared to the CG and between individual subgroups as well.Serum albumin level (median) in T2—T4 subgroup(33.50 g/L) was significantly lower compared with T1 subgroup (39.00 g/L,p<0.01)and Ta subgroup (38.50 g/L,p<0.01).The median albumin level in BC group(38.00 g/L) was significantly lower than in the CG(41.00 g/L,p<0.001).The median serum bilirubin level in T2—T4 was significantly lower compared with Ta subgroup(8.45vs.11.55 μmol/L,p<0.05).Although it was lower,no statistically significant difference was observed between the serum bilirubin levels in BC subgroups and in CG(Table 3).
No statistically significant correlation was observed between enzymatic antioxidants (SOD and catalase) activity or tumor sizes in individual BC subgroups(data not shown).A statistically significant negative correlation was found between tumor size and serum albumin level in BC patients(r=-0.386,p<0.01).However, a significant correlation was not found between non-enzymatic antioxidants (uric acid, bilirubin, or albumin) or tumor sizes in individual BC subgroups (data not shown).The multiple linear regression method showed that the serum albumin level was independently negatively related to tumor size (β=-0.189,p=0.038).
Comparative analysis of antioxidants levels in LG and HG BC subgroups has shown a significant difference in catalase activity and bilirubin level only(Table 4).Catalase activity (median) was higher in HG subgroup (138.00 IU/Lvs.8.77 IU/L,p=0.009),while bilirubin level(median)was higher in LG subgroup (10.20 μmol/Lvs.8.90 μmol/L,p=0.035).

Table 4 Comparative analysis of antioxidants in LG and HG bladder cancer subgroups.
The optimal cut-off value of catalase activity determined by the ROC curve in differentiating patients with LG versus HG BC was ≥11.96 IU/L.AUC for determined cut-off value of catalase activity was 0.656 (95% CI 0.546—0.775)(LGvs.HG,p=0.009).For a calculated optimal cut-off value of ≥11.96 IU/L, the specificity was 51.1% and sensitivity was 82.2%(Table 5).AUC for determined cut-off value of serum bilirubin level was 0.630 (95% CI 0.513—0.747)(p=0.035).For a calculated optimal cut-off value of≥11.95 μmol/L, the specificity was 44.1% and sensitivity was 80.0%.
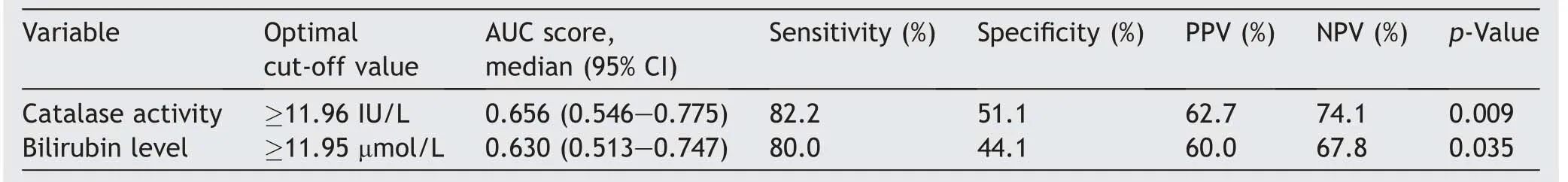
Table 5 Optimal cut-off,AUC with 95%CI,sensitivity,specificity,PPV,and NPV of serum catalase activity and bilirubin level in differentiating between patients with LG and HG bladder cancer.
4.Discussion
Numerous studies have shown that oxidative stress plays an important role in the pathogenesis and progression of cancer.It is characterized by an imbalance between increased exposure to free radicals and antioxidant defenses.Under normal conditions, ROS and nitric oxide synthase are maintained by a balance due to antioxidant defences.Loss of this homeostasis leads to structural cell damage, mutations, and chromosomal aberrations, or inactivation of tumor suppressor genes, which ultimately promote carcinogenesis.This could be particularly significant in the bladder epithelium,which is constantly exposed to carcinogens excreted by the kidneys [4—7].
Changing oxidative status in malignancies is a complex problem that requires different research approaches,ranging from enzyme activity testing to gene expression.Research should confirm the link among changes in oxidative status, onset, and course of the disease [9].In carcinomatous tissue,the activities of antioxidant enzymes have been observed and altered.This mainly applies to SOD and catalase, although other antioxidant enzymes can be altered, such as GPx, glutathione reductase, and glutathione transferase [8,9].Certainly, the reduced activity of these enzymes contributes to the accumulation of free radicals and the consequent propagation of this disease.SOD genes expression has been reported with a variety of mechanical, chemical, and biological stimuli, and under conditions of hypoxia or the action of some antitumor drugs such as mitomycin C [10].
The studyin vitroby Ha et al.[7] found a relative increase of intracellular prooxidants and a relative decrease of intracellular antioxidants in both normal and carcinomaaltered bladder cells.The authors did not find a difference in the catalase activity, while the SOD-2 activity and the hydrogen peroxide level were significantly higher in BC cells.In addition,a significant positive correlation has been found between the oxidative stress index and the expression of genes responsible for cancer progression, such as matrix metalloproteinase-9 and vascular endothelial growth factor.Furthermore, experimentally increased catalase activity leads to decrease in the expression of these genes [7].
Savic-Radojevic et al.[8] have demonstrated significantly higher SOD and GPx activity in BC cells compared to healthy mucosal cells but without a significant difference in SOD activities among different tumor grades.They suggested that the increased activity of SOD and GPx in BC was a measure of their effectiveness in inactivating superoxide and hydroperoxide in order to maintain prooxidant and antioxidant balance.
Given the very small number of studies that have assessed markers of oxidative status in different stages of BC,it is not sufficiently known whether disease progression from the initial to the most severe stages is accompanied by changes in oxidative status.The mutational effect of ROS is in dynamic equilibrium with antioxidant protection and the cellular system that repairs damaged tissue.In fact, the pronounced oxidative stress is caused by a decrease in the potential of antioxidant defense.By analyzing the expression of SOD and catalase in the mucosa and muscle layer of the bladder in rabbits, Fitzpatrick et al.[11] found higher enzyme expression in the mucosa layer.If this was proven in humans as well, it would explain why the SOD and catalase activity is higher in superficial bladder tumors,and lower in more invasive forms [11].
Another study demonstrated lower expression of SOD and catalase in invasive BC compared to superficial forms.The authors hypothesized that decreased SOD and catalase expression may be associated with tumor progression and that pharmacological control of enzymes activity could become an important way to prevent or treat cancer [12].Hempel et al.[13] found a correlation between increased SOD activity and BC grade, as well as increased activity of this enzyme in metastatic BC.They also found higher catalase activity in BC patients compared to the CG,as well as a negative association between catalase activity and matrix metalloproteinase-9 [13].
The results of our study showed that the SOD activity in the BC group and all individual subgroups was significantly higher compared to the CG.It may suggest an increase in antioxidant protection in order to decrease oxidative stress as a risk factor for the progression of the disease.However,ROC curve analysis did not confirm the use of SOD as a marker of grade differentiation and the progressive potential of BC.Furthermore,our research found significantly higher serum catalase activity in T2—T4 subgroup of patients when compared to Ta and T1 subgroups.This finding suggests the antioxidant response of the body at this stage of the highest levels of oxidative stress.The ROC curve analysis showed the possibility of applying catalase activity in assessing the grade and progressive potential of BC.Sensitivity was 82.2%, specificity 51.1%, and positive and negative predictability 62.7% and 74.1%, respectively.Catalase activity was higher in the HG subgroup(p=0.009),which means that oxidative injury might be involved in cancer progression.
Our results are consistent with the findings from several studies which demonstrated significantly higher catalase activity in subjects with BC and suggested that the enzymatic mechanism of antioxidant defense is not reduced in tumor tissue.This emphasizes an enhanced defense response through antioxidant protection and suggests a potential use of antioxidant enzymes level in monitoring the progression of BC [14,15].
Contrary to our results, a certain number of previous studies have shown that SOD and catalase activity in different types of tumors are significantly lower compared to the CG.Catalase activity is also lower in subjects with more invasive forms of BC.This practically means that with the progression of cancer, the antioxidant defense decreases [12,16,17].Unlike the cancer stage, the cancer grade was not related to the expression of any of these enzymes [12].Zainal et al.[18] have demonstrated significantly lower erythrocyte catalase activity in patients with BC compared to healthy subjects.The authors believed that the decline in catalase activity can be explained by increased production of free radicals and consumption of catalase in the fight for their elimination, as well as tissue damage and loss of cellular enzymes.The main role of catalase is the decomposition of hydrogen peroxide by catalase and/or peroxidase type.Catalase activity is manifested only at higher levels of hydrogen peroxide, which means that catalase is solely responsible for the decomposition of peroxides under conditions of oxidative stress[9,19].Considering this, catalase activity can serve as a good indicator of oxidative stress level and antioxidative status as well.It could be said that a significant increase in catalase activity in the most aggressive forms of this cancer corresponds in a similar way to SOD.
Non-enzymatic antioxidants in a very diverse way prevent lipid peroxidation and the formation of free radicals,repair the damage caused by their action at the molecular level,or restore certain components of antioxidant defense by cooperation.One of the modes of action of a nonenzymatic antioxidant is the primary retention of potentially dangerous iron and copper ions in their inactive form.In this way, they prevent their participation in the production of free radicals [20].Uric acid reduces and neutralizes free radicals and oxidizes itself to allantoin.Thus,the ratio of uric acid to allantoin level may be a parameter to assess the formation and action of free radicals underin vivoconditions[21].Bilirubin is a well-known antioxidant with a role in protection against cancer and cardiovascular diseases when it is present in a slightly elevated concentration.Unconjugated bilirubin also possesses antigenotoxic potential preventing oxidative DNA damage[22].It can stimulate apoptosis of carcinoma cellsin vitro,through activation of the mitochondrial pathway [23].
Our results showed no statistically significant difference in uric acid and bilirubin levels between patients with BC group and CG.However, bilirubin level was higher in LG subgroup(p=0.035),which suggests a possible link between low bilirubin level and increased progression potential of BC.Unfortunately, comparative studies are lacking.
Oxidative stress,as well as weak antioxidant defenses or both, may be considered important risk factors for the development of hypoalbuminemia in the pathogenesis of cancer[24].Our study showed that the serum albumin level was highest in the CG, and was significantly higher compared to patients with BC, regardless of the disease stage.We revealed a significantly lower serum albumin level in the patients with the most severe stage.This may suggest a decrease in non-enzymatic antioxidant capacity in the more invasive stages, which contributes to a higher risk of disease progression.Also, a significant negative correlation between tumor size and serum albumin level was found in patients with BC.The decline in serum albumin level is contributed by the activation of the systemic inflammatory response to the tumor, proinflammatory cytokines, and growth factors that have profound catabolic effects [25].
We should not forget the influence of age, gender,lifestyle, and nutrition on the level and activity of antioxidants.Also, care should be taken in interpreting the findings following these features and controlling them, as they may act as confounding factors.It is shown that an increased supply of nutrition products with antioxidant properties may increase the efficiency of antioxidant uptake and minimize cancer progression [26].
The main limitation of our study is the relatively small number of respondents included in the final cohort.The reason is the rigorous inclusion and exclusion criteria,which we tried to eliminate potential confounding factors.The CG was reduced and homogenized to fit the BC group according to age, gender, smoking habits, and comorbidities.Moreover, as the bladder mucosa is directly stimulated by urine,the detection of the level of antioxidants in urine may more accurately reflect the antioxidant status in BC patients.Further prospective research should aim to assess this aspect of antioxidant status evaluation.
This is one of the first reports demonstrating a link between oxidative stress and the progression potential of BC based on histopathological analysis.The observed prooxidants and antioxidants imbalance in BC as a result of altered activity and levels of both enzymatic and nonenzymatic antioxidants may have an impact on cancer incidence, development, and progression potential.It is inevitable to point out that measuring their blood level could also play a potential biomarker role.Therefore, the role of antioxidants status in BC progression remains unclear.However, we believe that their introduction as an additional biochemical test could have certain clinical implications both in the diagnosis and the therapy of BC.Further research should aim to clarify the ways in which pharmacological and molecular control of antioxidant status will be achieved with a goal of cancer prevention,monitoring, and treatment.
5.Conclusion
Cancer progression potential associated with an increase or a decrease in level and activity of antioxidants suggests their possible role in prediction and monitoring of illness trajectory.Significantly higher serum SOD and catalase activity may suggest an increase in antioxidant protection with the goal of reducing oxidative.Significantly lower level of serum albumin in patients with the most severe stage may suggest a decrease in non-enzymatic antioxidant capacity in the more invasive stages of illness.
Author contributions
Study concept and design:Zahid Lepara,Jasmin Ali,Orhan Lepara.
Data acquisition:Zahid Lepara, Orhan Lepara, Hajrudin Spahovi.
Data analysis:Orhan Lepara, Jasmin Ali, Almir Fajki.
Drafting of manuscript:Jasmin Ali, Zahid Lepara, Orhan Lepara.
Critical revision of the manuscript:Jasmin Ali, Zahid Lepara.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2023年2期
Asian Journal of Urology2023年2期
- Asian Journal of Urology的其它文章
- Role of circulating tumor cell clusters in patients with metastatic hormone-sensitive prostate cancer receiving a gonadotropin-releasing hormone antagonist: A pilot study
- Tunica albuginea versus buccal mucosa graft urethroplasty for anterior urethral stricture:A prospective randomised pilot study
- Subadventitial resection of the ureter—new method for surgical corrections of the ureteropelvic junction and ureterovesical junction obstructions
- Percutaneous embolization by direct puncture for the treatment of high-flow priapism
- Analysis of the effect of holmium laser flexible ureteroscopic intrapelvic drainage in the treatment of parapelvic renal cysts
- The role of quick Sepsis-related Organ Failure Assessment score as simple scoring system to predict Fournier gangrene mortality and the correlation with Fournier’s Gangrene Severity Index: Analysis of 69 patients
