Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios alone or combined with prostate-specific antigen for the diagnosis of prostate cancer and clinically significant prostate cancer
Sat Prasad Nepal, Takehiko Nakasato, Takashi Fukagai,Yoshio Ogawa, Yoshihiro Nakagami, Takeshi Shichijo,Jun Morita,Yoshiko Maeda,Kazuhiko Oshinomi,Tsutomu Unoki,Tetsuo Noguchi, Tatsuki Inoue, Ryosuke Kato, Satoshi Amano,Moyuru Mizunuma, Masahiro Kurokawa, Yoshiki Tsunokawa,Sou Yasuda
Department of Urology, Department of Medicine, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo, Japan
KEYWORDS Blood parameter;Gleason score;Neutrophil-tolymphocyte ratio;Platelet-tolymphocyte ratio;Prostate cancer
Abstract Objective: We evaluated whether the blood parameters before prostate biopsy can diagnose prostate cancer (PCa) and clinically significant PCa (Gleason score [GS] ≥7) in our hospital.Methods: This study included patients with increased prostate-specific antigen (PSA) up to 20 ng/mL.The associations of neutrophil-to-lymphocyte ratio (NLR) and platelet-tolymphocyte ratio(PLR)alone or with PSA with PCa and clinically significant PCa were analyzed.Results: We included 365 patients,of whom 52.9%(193)had PCa including 66.8%(129)with GS of ≥7.PSA density (PSAD) and PSA had better the area under the curve (AUC) of 0.722 and 0.585, respectively with p=0.001 for detecting PCa compared with other blood parameters.PSA combined with PLR (PsPLR) and PSA with NLR (PsNLR) had better AUC of 0.608 and 0.610,respectively with p<0.05,for diagnosing GS≥7 population,compared with PSA,free/total PSA,NLR,PLR,and PsNPLR(PSA combined with NLR and PLR).NLR and PLR did not predict PCa on multivariate analysis.For GS≥7 cancer detection,in the multivariate analysis,separate models with PSA and NLR (Model 1: PsNLR+baseline parameters) or PSA and PLR (Moder 2:PsPLR+baseline parameters) were made.Baseline parameters comprised age, digital rectal exam-positive lesions, PSA density, free/total PSA, and magnetic resonance imaging.Model 2 containing PsPLR was statistically significant (odds ratio: 2.862, 95% confidence interval:1.174—6.975, p=0.021) in finding aggressive PCa.The predictive accuracy of Model 2 was increased (AUC: 0.734, p<0.001) than that when only baseline parameters were used(AUC: 0.693, p<0.001).Conclusion: NLR or PLR,either alone or combined with PSA,did not detect PCa.However,the combined use of PSA with PLR could find the differences between clinically significant and insignificant PCa in our retrospective study limited by the small number of samples.
1.Introduction
Prostate-specific antigen (PSA) for screening prostate cancer (PCa) lacks adequate sensitivity, specificity, and positive predictive value [1,2].Many patients undergo unnecessary prostate biopsy which has several disadvantages: financial burden and stress due to hospital admission, biopsy needle insertion, and risk of infection.Moreover, in 20% of patients with normal first biopsy results,PCa was found at the second biopsy[3].Thus,various measures have been taken to improve the diagnostic results and reduce unnecessary biopsy, such as using PSA density(PSAD),free PSA(fPSA),free/total PSA(f/t PSA),prebiopsy magnetic resonance imaging (MRI), and prostate biopsy nomograms [2,4,5].Prostate health index combining PSA,fPSA, and -2proPSA (PSA precursor), PSAD, and age, was currently used in a model to detect Gleason score (GS) ≥7 PCa, which had sensitivity and specificity of 86% and 89%,respectively [6].
Benign prostate hyperplasia(BPH)and PCa both occur in old age, are hormone-dependent, and are associated with inflammation [7].PSA elevation in both BPH and PCa is noted due to prostatic ducts damage[8].Numerous studies have shown the coexistence of inflammation and enlarged prostate [8—10].
In 1863, Virchow discovered leukocytes in cancerous lesions [11]; since then, the role of inflammation in cancer has been explored and generated interests.A worldwide incidence analysis reported that approximately 13% of global cancer is due to infections in 2018 [12].Moreover,chronic inflammation plays a major role in these infections[11].In 2018, theHelicobacter pyloriinfection, human papillomaviruses, hepatitis B virus, and hepatitis C virus were major contributors for noncardiac gastric,cervix uteri carcinoma, and hepatocellular carcinomas, respectively.Furthermore, Epstein-Barr virus, human T-cell leukemia virus type 1, human herpesvirus 8, and parasitic infections are also responsible for a large number of cancer cases[12].These infectious agents cause stimulation of inflammatory cells releasing cytokines, thus becoming chronic causes for DNA injury,blood vessel formation,growth stimulation,and cancer cell invasion [11].
The use of blood parameters in PCa diagnosis and outcomes has generated interests due to their easy availability and cost-effectiveness.The utilization of prebiopsy blood parameters to identify PCa and its outcomes remains controversial.Several previous studies have approved the use of neutrophil-to-lymphocyte ratio(NLR) and platelet-to-lymphocyte ratio (PLR) for the diagnosis of PCa and prognosis [13—18], whereas other studies disagreed that, as upon comparison, other clinical parameters such as age, PSA, PSAD, fPSA, and f/t PSA were better markers [19—21].
In this study, we investigated whether these blood parameters could detect PCa and clinically significant PCa before conducting a prostate biopsy with PSA value up to 20 ng/mL.We included NLR or PLR combined with other examinations in screening for PCa (such as PSA, digital rectal exam [DRE], f/t PSA, prostate volume [PV], PSAD,and MRI).The samples were collected before the prostate biopsy procedure.This study aimed to find out whether NLR and PLR could improve detecting PCa and clinically significant PCa (GS≥7) if used alone or in combination with PSA(PsNLR,PSA combined with NLR;PsPLR,PSA combined with PLR; PsNPLR, PSA with NLR and PLR) in our hospital.
2.Patients and methods
We included 365 patients with increased PSA up to 20 ng/mL from January 2014 to February 2018 who underwent all examinations required.There were 193 (52.9%)patients with PCa.The exclusion criteria were as follows:patients with lacking data; patients with histologically proven prostatitis,autoimmune diseases,and inflammatory diseases (rheumatoid arthritis or systemic lupus erythematosus); and patients taking corticosteroids, having other organ tumors, and having chemotherapy and radiotherapy history.
Blood samples were taken at the outpatient department of the current study for complete blood count at the patient’s initial visit 1—3 weeks before prostate biopsy.In addition, the patients underwent regular urine analysis to rule out infection.PLR and NLR were calculated by dividing platelet and absolute neutrophil counts by absolute lymphocyte counts.PsNLR, PsPLR, and PsNPLR were obtained by multiplying PSA with NLR (PsNLR = PSA × NLR),PSA with PLR (PsPLR = PSA × PLR), and PSA with NLR and PLR (PsNPLR = PSA × NLR × PLR), respectively.
For the prostate biopsy, patients were admitted in the morning of the procedure and were given fluoroquinolone antibiotics.All patients underwent 1- to 2-core MRI cognitive biopsy and 10- to 11-core standard prostate biopsies.The targeted biopsy was not labeled separately and was included with the standard biopsy in the pathological results.Prostate Imaging Reporting and Data System(PIRADS)score was not used in our study, and MRI results were interpreted as yes or no for suspicious lesions.
The PV was calculated using the formula—PV (cm3) =0.52 x length (cm) x width (cm) x height (cm).Urology residents with considerable experience and senior urologists performed the prostate biopsies.PSAD was calculated by dividing total PSA by PV.
We used two models for significant PCa: Model 1 included PsNLR, and Model 2 had PsPLR.In both models,age,DRE-positive lesions,PSAD,and f/t PSA were included.In the multivariate analysis,we created two models(Models 1 and 2) to detect GS≥7 cancer.As both the models contained age, DRE-positive lesions, PSAD, f/t PSA≤15 ng/mL,and MRI-positive lesions, these parameters will be collectively called baseline parameters.Model 1 had PsNLR and baseline parameters, whereas Model 2 had PsPLR and baseline parameters.
This study was approved by the local ethics committee of Showa University(3244—31/8/2020)and was in complete agreement with the Declaration of Helsinki.The ethics committee waived the need for informed consent due to the retrospective nature of the current study.
We used SPSS version 16.0(SPSS Inc.,Chicago,IL,USA)for analyzing the data.Except age,all the variables in our data were not normally distributed, and we used thet-test and Mann-WhitneyUtest for continuous variables to compare PCa and non-PCa patients,and clinically significant PCa and non-significant PCa population groups.Moreover,Chi-square test was used for categorical variables.Then,we performed receiver operating characteristic(ROC)curve analysis to find the area under the curve (AUC) values, withp<0.05 being considered statistically significant.Then, sensitivity, specificity, positive predictive values, and negative predictive values for clinically significant PCa (GS≥7) were calculated.Furthermore,we performed a logistic regression analysis for PCa and clinically significant PCa detection.We separated PsNLR and PsPLR due to their high correlations (Spearman’s correlation=0.868,p<0.001).Thep<0.05 was considered significant in the multivariate analysis.
3.Results
An increase in platelets (p<0.05) and PLR in the non-PCa population was noted when comparing PCa with the non-PCa group.However, the PLR difference was not statistically significant (p=0.083).Differences in age, DRE-positive lesion,f/t PSA,PV,PSAD,MRI-positive lesion,and hemoglobin were statistically significant between the PCa and the population without PCa(Table 1).

Table 1 The t-test and Mann-Whitney test for continuous variables and Chi-square test for categorical variables in relation to prostate cancer.
In the GS≥7 group, PSA and the combined markers PsNLR, PsPLR, and PsNPLR were higher with statistical significance, whereas f/t PSA was lower than those in the GS<7 population (p<0.05).MRI-suspicious lesions showed difference between the two groups (p=0.043).No differences were observed in age,DRE-positive lesion,PV,PSAD,level of hemoglobin, neutrophil, platelet, lymphocyte, albumin, NLR, or PLR between the two groups (Table 2).

Table 2 The t-test and Mann-Whitney test for continuous variables and Chi-square test for categorical variables in relation to clinically significant cancer (Gleason score ≥7).
When ROC curve analysis for PCa detection was conducted, PSAD with a cut-off of 0.175 ng/mL had an AUC of 0.722 (p=0.001), with a sensitivity and specificity 67.9%and 62.8%, respectively, and PSA with a cut-off of 4 ng/mL had an AUC of 0.585 (p=0.016) with a sensitivity and specificity of 96.9% and 5.8%, respectively.However, the AUC values for blood parameters were <0.5(Table 3).Thus,the sensitivity, specificity, positive predictive value, and negative predictive value of the blood parameters were not performed.The PSA, f/t PSA, PsNLR, PsPLR, and PsNPLR were found to have increased AUC (p<0.05) in detecting clinically significant PCa.The AUC of the variables were as follows: f/t PSA, 0.630; PsPLR, 0.608; PsNLR, 0.610; PSA,0.589; and PsNPLR, 0.604.The AUC values of NLR and PLR did not show any statistical significance (Table 4).A multivariate analysis was done using a logistic regression analysis to detect clinically significant PCa using age, DREpositive lesions, PSAD, MRI-positive lesion, f/t PSA, NLR,and PLR.In addition, neither NLR nor PLR was statistically significant (Table 5).

Table 3 ROC curve values in the detection of prostate cancer.

Table 4 ROC curve values in the detection of clinically significant prostate cancer (Gleason score ≥7).
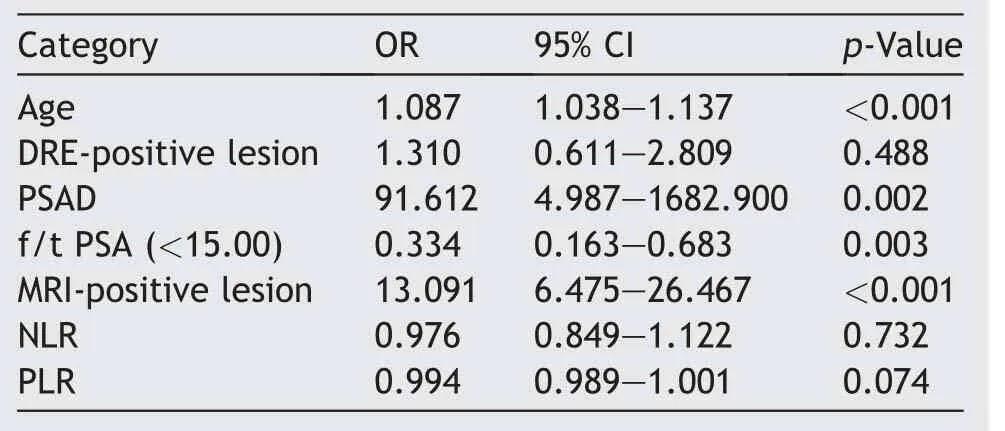
Table 5 Multivariate analysis in the detection of clinically significant prostate cancer (Gleason score ≥7).
As the ROC curve values for PSA combined with NLR or PLR were better than PSA, NLR, or PLR, we investigated whether the use of combined markers was better in predicting significant PCa by using different multivariate models.
We found PsPLR to be statistically significant (p=0.021)in detecting clinically significant PCa, but not PsNLR(p=0.055).The f/t PSA and MRI suspicious lesions were statistically significant in both models(Table 6).We did not use PSA in the models due to its high correlation with PsNLR and PsPLR (Spearman’s correlation of >0.650;p<0.001).The predictive accuracy for Model 2, baseline parameters with PsPLR (AUC=0.734,p<0.001) was more than that of baseline parameters only (without PsPLR) (AUC=0.693;p<0.001) for predicting clinically significant PCa.

Table 6 Multivariate Models 1 (PsNLR+baseline parameters) and 2 (PsPLR+baseline parameters), for detecting clinically significant PCa (Gleason score ≥7).
4.Discussion
It is hypothesized that inflammatory cells and cytokines in the tumor microenvironment have a role in the development of cancer [11,21].In a tumor microenvironment, neutrophils release reactive oxygen species (ROS) and reactive nitrogen species having mutagenic effects;furthermore,inducible nitric oxide synthase release leads to DNA and tissue injury[22].Platelets comprised P-selectin, platelet endothelial cell adhesion molecule-1, glycoprotein IIb/IIIa (GP IIb/IIIa),GP Ib/IX, and GP VI that cause platelet and cancer cell adhesion, escaping immune function, and metastasis.The releaseofthevascular endothelial growthfactorandplateletderived growth factor causes tumor cell proliferation,vascular permeability, and the formation of new blood vessels.Furthermore,the release of transforming growth factor beta results in cancer cell invasion and proliferation[23].
The link between PCa and inflammation has been explored and studied in the literature [6—36].Atrophic epithelium with stromal changes was found in the prostate biopsy of older patients[24,25].In this area,it was observed that inflammatory cells have high proliferative capacity compared with the surrounding normal prostate tissue,known as proliferative inflammatory atrophy, which may progress to high-grade prostatic intraepithelial neoplasia and early prostate adenocarcinoma [24,25].During inflammation, proliferative inflammatory atrophy is exposed to ROS; loss of protective agents against ROS might change proliferative inflammatory atrophy to high-grade prostatic intraepithelial neoplasia or PCa [26].According to meta-analyses, a history of prostatitis increases the risk of PCa with an odds ratio of 1.6[27],and there are associations between sexually transmitted infections and PCa [28].
Recently, various blood parameters (NLR, PLR, lymphocyte-to-monocyte ratio, and mean platelet volume)[13—18] have been used to predict the diagnosis of PCa,clinically significant PCa, and their outcomes.
Different studies have combined inflammatory markers like prostatic systemic inflammatory markers.Prostatic systemic inflammatory markers comprised NLR, PLR, and lymphocyte-to-monocyte ratio [29] or added NLR with PIRADS version 2 score in a multivariate model[30]to detect significant PCa.However, using these blood parameters is still a matter of debate and controversy as different studies argued that they are unreliable in detecting PCa [19,20].In this study, we assessed the ability of NLR or PLR alone or combined with PSA in detecting PCa and significant PCa.
We found platelets to have larger values in the non-PCa group (p=0.012), and PLR, although higher, was not statistically significant (p=0.083).Most of the population without cancer in the current study had large prostates.Inflammation associated with enlarged prostates is found in the literature.Of the 162 BPH samples analyzed, 98% had inflammatory cells(neutrophils,lymphocytes,plasma cells,macrophages, and epithelioid cells) [8].BPH tissue contained large amounts of interleukin-2 and interleukin-4,whereas PCa cells secreted interleukin-6 [8,9].In a histological examination of 3942 patients with BPH, 43.1% had prostatic inflammation comprising mainly chronic inflammation[10].Kaynar et al.[31]found higher PLR in the BPH group for population with PSA more than 10 ng/mL.The findings of the current study indicate the blood parameter’s role in differentiating benign and malignant prostate.
In the current study,PSAD and PSA were better than the other blood parameters in PCa detection.Similar to the current findings, Khosropanah et al.[32] and Kamali et al.[33] did not find NLR to predict PCa.Contrastingly, Kawahara et al.[15]in a study including 810 men who underwent prostate biopsy with PSA between 4 ng/mL and 10 ng/mL found NLR to be a risk factor of PCa.In Korean patients with gray-zone PSA,NLR was associated with PCa detection[16].Adhyatma and Warli [17] discovered that prebiopsy PLR is valuable in detecting PCa.Our study thus further adds to the uncertainty whether the blood parameters alone can be used for detecting PCa.
We found that PsNLR and PsPLR were high in the GS≥7 group (p<0.05) and that combined marker (PsPLR) was associated with high GS PCa using the multivariate analysis.However, NLR or PLR alone was not able to detect GS≥7 PCa.Similarly,Murray et al.[20]reported that the PLR and systemic immune-inflammation index did not differentiate between BPH, indolent cancer, and clinically significant PCa.Huang et al.[34] did not find NLR to be useful in detecting advanced PCa for patients with PSA from 4 ng/mL to 10 ng/mL.In contrast,Oh et al.[16]found that NLR with gray-zone PSA was associated with GS≥4+3 cancer detection.Furthermore, Gokce et al.[18] reported that aggressive PCa is associated with increase in NLR.However, they suggested that prostatitis limited the use of NLR in detecting PCa.
The possible reason for the inability of NLR or PLR to predict PCa or clinically significant PCa may be the uncontrolled immune response causing a general inflammatory state in old age, which did not show elevations in inflammatory markers due to PCa [18,20].Upregulation of interleukin-6, tumor necrosis factor-α, and their receptors was noted.In addition,the elevation of chemokines and Creactive protein in aged tissues was found to be involved in age-related pathogenesis [35].Furthermore, both PCa and BPH were associated with inflammation and occur together in 20% of the same prostate zone [18].
PSA and PLR were found to detect clinically significant PCa.A PSA cut-off of 4 ng/mL was generally used for prostate biopsy to detect PCa.However, it leads to unwanted prostate biopsy or detection of clinically insignificant PCa[1—3,30], and most of the insignificant PCa remains asymptomatic [30,36].Currently, attention is focused on prebiopsy MRI and MRI-targeted biopsy to find significant PCa[30].The economical and noninvasive method of the current study on small population samples can avoid prostate biopsy for insignificant PCa and future study.
This study has several limitations.Our study is a nonrandomized retrospective study from a single institution with a small population sample.Thus, various biases might affect the detection of clinically significant PCa.The use of blood parameters with PSA had better specificity.Thus,despite improving the detection of aggressive PCa, these parameters are still not ideal.Finally,PIRADS score was not used in MRI as PIRADS score criteria changed within our study duration and PIRADS version 1 (in 2012) was changed to version 2 (in 2015).
5.Conclusion
We could not find the role of NLR and PLR in detecting PCa.However,NLR and PLR combined with PSA exhibited better results in detecting clinically significant PCa than NLR,PLR,or PSA alone.PsPLR with f/t PSA and MRI suspicious lesions were statistically significant in detecting clinically significant PCa in multivariate analysis.The low sample size from a single institution is the major limitation of the current study and studies with a larger population are required.
Author contributions
Study concept and design: Sat Prasad Nepal, Takehiko Nakasato, Takashi Fukagai, Yoshio Ogawa, Yoshihiro Nakagami, Takeshi Shichijo, Jun Morita, Yoshiko Maeda, Kazuhiko Oshinomi.
Data acquisition: Sat Prasad Nepal, Tsutomu Unoki, Tetsuo Noguchi, Tatsuki Inoue, Ryosuke Kato, Satoshi Amano,Moyuru Mizunuma, Masahiro Kurokawa, Yoshiki Tsunokawa,Sou Yasuda.
Data analysis: Sat Prasad Nepal, Takehiko Nakasato, Takashi Fukagai, Yoshio Ogawa, Yoshihiro Nakagami, Takeshi Shichijo, Tsutomu Unoki, Tetsuo Noguchi, Tatsuki Inoue,Ryosuke Kato, Satoshi Amano, Moyuru Mizunuma, Masahiro Kurokawa, Yoshiki Tsunokawa, Sou Yasuda.
Drafting of manuscript: Sat Prasad Nepal.
Critical revision of the manuscript: Sat Prasad Nepal,Takehiko Nakasato, Takashi Fukagai, Yoshio Ogawa, Yoshihiro Nakagami,Takeshi Shichijo,Jun Morita,Yoshiko Maeda,Kazuhiko Oshinomi,Tsutomu Unoki,Tetsuo Noguchi,Tatsuki Inoue, Ryosuke Kato, Satoshi Amano, Moyuru Mizunuma,Masahiro Kurokawa, Yoshiki Tsunokawa, Sou Yasuda.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2023年2期
Asian Journal of Urology2023年2期
- Asian Journal of Urology的其它文章
- Radiofrequency ablation for renal tumours:A retrospective study from a tertiary centre
- The role of quick Sepsis-related Organ Failure Assessment score as simple scoring system to predict Fournier gangrene mortality and the correlation with Fournier’s Gangrene Severity Index: Analysis of 69 patients
- Role of circulating tumor cell clusters in patients with metastatic hormone-sensitive prostate cancer receiving a gonadotropin-releasing hormone antagonist: A pilot study
- Percutaneous embolization by direct puncture for the treatment of high-flow priapism
- Subadventitial resection of the ureter—new method for surgical corrections of the ureteropelvic junction and ureterovesical junction obstructions
- Tunica albuginea versus buccal mucosa graft urethroplasty for anterior urethral stricture:A prospective randomised pilot study
