Necrosis zone depth after bipolar plasma vaporization and resection in the human prostate
Clr Breitling , Hns Nenning , Jrg Rssler ,
a Department of Urology, St.Elisabeth Hospital, Leipzig, Germany
b Institute for Pathology “Am Elsapark”, Leipzig, Germany
KEYWORDS Transurethral resection of prostate;Bipolar enucleation;Plasma resection;Plasma vaporization;Necrosis depth
Abstract Objectives: To compare the depth of thermal necrosis after use of bipolar resection and vaporization technique comparing intra-individually bipolar loop and bipolar button electrodes.Methods: Transurethral resection and vaporization of the prostate was performed in 55 male patients (260 specimens in total).In a standardized procedure, a bipolar resection loop was used for resection, and a bipolar button electrode was used for vaporization.Both electrodes were applied in each patient, either in the left or in the right lateral lobe.The depth of necrotic zones in the resected or vaporized tissue of each patient was measured in a standardized way by light microscopy.Results: The mean depth with standard deviation of thermal injury caused by the loop electrode was 0.0495±0.0274 mm.The vaporization electrode caused a mean thermal depth with standard deviation of 0.0477±0.0276 mm.The mean difference of necrosis zone depths between the two types of electrodes (PlasmaButton—resection loop) was -0.0018 mm(p=0.691).Conclusion: For the first time, we present directly measured values of the absolute necrosis zone depth after application of plasma in the transurethral treatment of benign prostatic hyperplasia.The measured values were lower than in all other transurethral procedures.Standardized procedures of measurement and evaluation allow a statistically significant statement that the low necrosis depth in bipolar procedures is independent of the applied electrodes.
1.Introduction
Benign prostatic hyperplasia (BPH) is a frequent condition in elderly men.In spite of its benign character, even fatal complications such as urinary retention with recurrent infection or secondary hydronephrosis may develop without treatment.
For a long time, the gold standard treatment among the minimal invasive procedures was monopolar transurethral resection of the prostate (TURP).Bipolar TURP, which has developed since the late 1990s, is considered to be equivalent[1]or even superior to monopolar resection due to its reduced risk of complications such as TURP syndrome and lower comorbidity [2—4].Moreover, bipolar application allows transurethral plasma vaporization of the prostate.Beside a good functional outcome, which is similar with monopolar and bipolar treatments [1,3], minimization of side-effects such as bleeding, urgency, or prolonged hematuria is an important aim of each transurethral treatment of the prostate.These side-effects are assumed to be linked to the size of necrotic zones [5].However, data on the dimension of necrotic zones after bipolar intervention vary considerably.
Published values for absolute depth of necrotic zones after bipolar treatment in human prostate tissue range from 0.14±0.02 mm [1] over 2.40±0.84 mm [6] up to 3.20±1.53 mm [7].Comparisons between monopolar and bipolar resection techniques showed controversial results.In many animal andin vitrostudies, bipolar TURP was clearly superior to monopolar utilization with respect to the histopathological size and severity of necrotic zones[8—10].In other models,smaller necrotic zones were observed with monopolar than with bipolar resection [11,12].The discrepant results concerning the depth of necrotic zones may be explained by the high diversity of different study designs.For reliable measurement of necrosis zone depth,standardized measurements are indispensable.Another possible reason for the inconclusive results of necrosis depth is the use of different bipolar electrodes, namely loop electrodes or button electrodes.Application of bipolar current induces formation of plasma at the loop.Interaction of plasma with tissue vaporizes the tissue and leaves a necrosis.From the physical point of view, the depth of this necrotic zone should be independent of the type of bipolar electrodes.However, this has never been directly measured.In a pilot study, we performed bipolar resection in eight patients with a loop electrode (further referred to as “resection”) on one hand, and with an button electrode(further referred to as “vaporization”) on the other hand[13].The necrosis depth values measured in this pilot study were in line with published results from animals and patients [8,9,14], but there were still remarkable differences to other patient studies [6,7].
Therefore, we designed anin vivostudy with standardized measurements of necrosis zone depth in patients.Standardization included resection procedure,definition of resection area, collection of specimens, processing of histologic specimens, and histopathologic evaluation.We compared the depth of thermal necrosis after use of bipolar resection and vaporization technique comparing intraindividually bipolar loop and bipolar button electrodes.
2.Patients and methods
2.1.Cohort
A single center, prospective randomized trial was performed, aiming to compare intra-individually the histopathological necrosis depth after plasma vaporization of the prostate with two different bipolar electrodes in BPH patients with indication for surgical treatment.The terms of the study agreement have been reviewed and approved by the Ethics Committee in accordance with its policy on objectivity in research.The study was approved by the local ethics and research committee and registered in the International Clinical Trials Registry Platform (DRKS00005448).
Between October 2013 and December 2015,a total of 66 patients with BPH were preselected for transurethral operative treatment within the study.Only patients routinely planned for the transurethral procedure and fulfilling the inclusion criteria described below were preselected.
Patient inclusion criteria were the following:(1)patients suffer from a BPH with indication for surgical treatment;(2)prostate volume >30 cm3;(3)age of more than 18 years;(4)a signed informed consent.Indication for surgical treatment was given in case of recurrent urinary retention, hematuria, urinary infection, or hydronephrosis caused by BPH as well as cystolithiasis.
Exclusion criteria were the following: (a) previous local treatment of the prostate (e.g., previous TURP or biopsy of prostate); (b) previously diagnosed prostate cancer;(c) transurethral catheterization more than 1 week preoperatively.
Each patient was resected with two bipolar electrodes,i.e., the resection loop and the button electrode(PlasmaButton, Olympus Europe, Hamburg, Germany).Both methods were performed either in the right or in the left lateral lobe according to a randomization list(randomization 1) that was generated by a computer before the recruitment into the study.This allowed for direct comparison of the two electrodes without patientbased bias.A second randomization list was generated for blinding the pathologist for the method of treatment in order to rule out potential bias in measuring necrosis depth of the specimen (randomization 2).
According to internal processes of the hospital (e.g., no study physician present in the operation room), nine patients had to be excluded after randomization due to unavailable specimens.Additional two cases were excluded due to “false randomization”.Thus, the final analysis data set included 55 patients.
Anthropometrical(age and body mass index),laboratory(prostate-specific antigen, prostate-specific medication,prostate volume, and blood clotting inhibitor), and clinical data of the patients are given in Table 1.
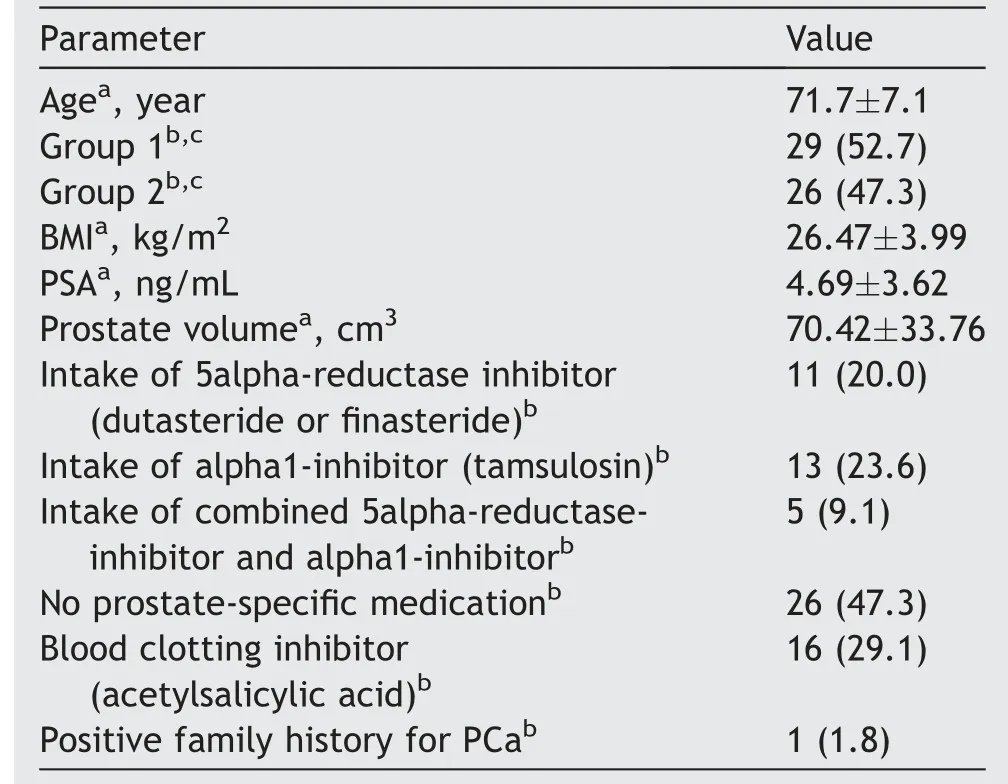
Table 1 Patients’ data (n=55).
2.2.Procedures
Using the high frequency generator “ESG-400” (Olympus Europe, Hamburg, Germany), the OES Pro bipolar resectoscope (Olympus Europe, Hamburg, Germany), and saline continuous flow irrigation, standard TURP was performed with a medium sized (diameter 5.5 mm) transurethral resection-in-saline resection loop.The standard transurethral vaporization was performed with the PlasmaButton electrode (Olympus, Berlin, Germany) with an area of 2.9 mm2in sodium chloride solution.Settings of the electrosurgical generator “ESG-400” were standardized for all procedures and set up to “Cut 180, Effect 2” and“Coag 110, Effect 2”.In addition, the resection or vaporization velocity was standardized.When the shaft was in a stationary position, the maximum possible distance of electrode movement was 23 mm.This distance had to be covered in 2 s corresponding to a defined resection or vaporization velocity of 11.5 mm/s.
In this study,one surgeon(Rassler J)with many years of experience conducted all operative procedures.Collection of study specimens was performed by another surgeon(Breitling C) in a standardized method from an intraoperatively created standardized resection and vaporization surface (Fig.1): (a) a mucosa-free layer of one lateral lobe was resected at 11.5 mm/s with the resection loop forming a standardized resection area (side I); those specimens were not used for the study and were rinsed out;(b) from the standardized resection area (side I) study specimens were taken with the resection loop; of those,three to five of the most representative specimens concerning size and potential interpretability were collected for the study;(c)the contralateral lobe was vaporized with the PlasmaButton on the mucosa-free area in the same defined speed per “cut” to create the required standardized surface; from the standardized vaporization area study, specimens were taken by using the resection loop;again, three to five of the most representative specimens were collected for the study.According to a randomization list, the resection loop was used in the right lateral lobe and the PlasmaButton was used in the left lateral lobe(Group 1) or vice versa (Group 2).After removal of the study specimen, bipolar TURP was conducted independently of the study.
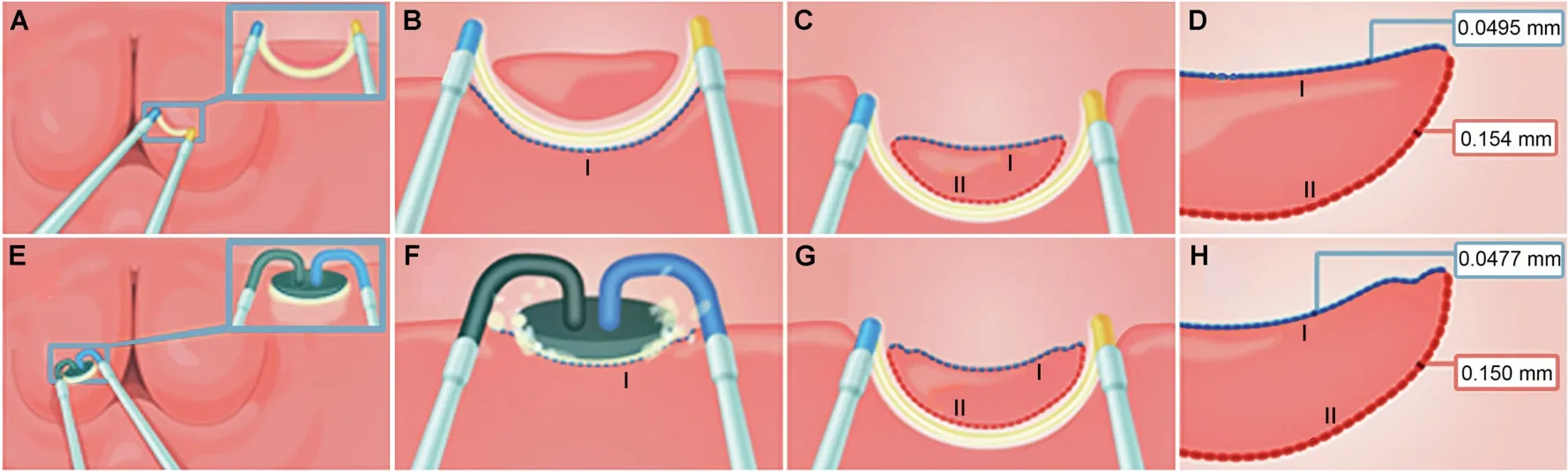
Figure 1 Schematic explanation of the two procedures.Resection (top; A—D): a mucosa-free layer of one lateral lobe was resected with the loop(A)forming a standardized resection area(side I;B);those specimens were not used for the study and were rinsed out;from the standardized resection area(side I)the study specimens were taken with the resection loop(C).Vaporization(bottom; E—H): the other lateral lobe was vaporized with the PlasmaButton on the mucosa-free area (side I; F); from this vaporization area study specimens were taken using the resection loop (G); the specimen surface (side I) represents the area,which would remain inside the patient.The specimens were collected for histopathological examination and measurement of necrosis depth on sides I and II.The numbers in the red and blue boxes represent the mean necrosis zone depths (D and H).
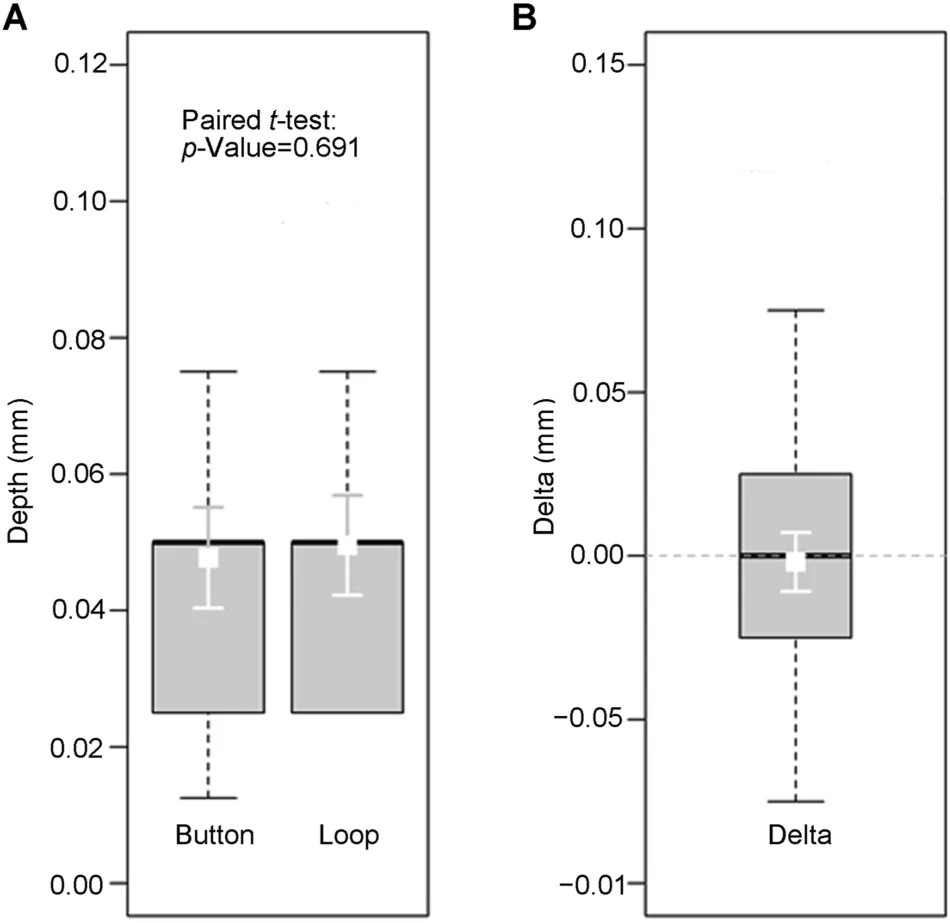
Figure 2 Necrotic zone depths at the concave side (side I).(A) Absolute depth values in both methods (Button and Loop,respectively; in millimeter).(B) Difference (Delta) of the necrotic zone depths at side I between PlasmaButton and resection loop(in millimeter).Boxes represent the interquartile range with the bold lines representing the medians and the whiskers the 10th/90th percentiles.The white squares and error bars in the boxes show the mean values ± 95% confidence intervals.The mean difference (PlasmaButton—resection loop; in millimeter)was with-0.0018 mm not significant(paired t-test:p=0.691).

Figure 3 Necrotic zone depths at the convex side(side II).(A)Absolute depth values in both methods (Button and Loop,respectively; in millimeter).(B) Difference (Delta) of the necrotic zone depths at side II between PlasmaButton and resection loop(in millimeter).Boxes represent the interquartile range with the bold lines representing the medians and the whiskers the 10th/90th percentiles.The white squares and error bars in the boxes show the mean values±95%confidence intervals.The mean difference (PlasmaButton—resection loop;in millimeter) was with -0.003 mm not significant (paired t-test: p=0.798).
The surface of the representative specimens was defined by its characteristic concave shape after resection or vaporization.This surface(side I)remained in the patient’s body and represented the relevant necrotic zone.Side I was marked with Tipp-Ex correction pen (Tipp-Ex GmbH & Co.KG, Eltville, Germany) before the specimens were fixated in 4% formalin for no longer than 24 h.
2.3.Histopathologic examination
Histopathological examinations were performed by two independent pathologists.A Brightfield Microscope with two times two oculars (Olympus BX40, Olympus Europe,Hamburg, Germany) was used for evaluation with four-,ten-and twenty-fold magnifications of the samples.Due to randomization of the specimens, the pathologists were blinded to the sampling of the specimen(resection loopvs.PlasmaButton).Prostate specimens were processed in paraffin blocks and section cuts.They were stained with hematoxylin and eosin.From each patient and each side 3—5 specimens were analyzed (260 specimens in total).Coagulation depth was measured with a calibrated ocular micrometer at the most prominent spot at the Tipp-Ex marked (concave) surface (side I) and additionally at the opposite (convex) side (side II, Fig.1D and H).Metachromasia of stroma and changed gland structure as characteristic tissue artifacts were used as evaluation criterion for necrosis depth.
2.4.Statistics
The required sample size was calculated based on the results of a pilot study[13].For a 95%confidence interval,the calculation revealed a minimum sample size (number of patients) of 53.The histopathological results were given in terms of necrosis depth(mean±standard deviation[SD])for the resection loop and for the PlasmaButton.Comparison between the resection loop and PlasmaButton procedures was determined by pairedt-test(Software package PASS 12,NCSS,LCC,Kaysville,UT,USA).The test was two-sided with a significance level of 0.05.Descriptive and interference statistics were determined using the statistics program R Version 3.2.4 (R project for statistical computing, Vienna,Austria).The final sample size of 55 allowed to detect mean differences as small as 0.4 mm with a power greater than 80%.
3.Results
The analyzed sample consisted of 55 male patients.The mean prostate volume was 70.42 (SD: 33.76) cm3.Prostatespecific antigen value was determined as 4.69 (SD:3.62) ng/mL.Prostate-specific medication was taken by 52.7%of the patients.Of these,20.0%took 5alpha-reductaseinhibitor (dutasteride or finasteride), 23.6% alpha1 inhibitor(tamsulosin) and 9.1% a combined medication consisting of 5alpha-reductase-inhibitor and alpha1-inhibitor.From the patients,29.1%reported an intake of blood-clotting inhibitor(acetylsalicylic acid)(Table 1).
At the relevant necrotic zone (side I), the histopathological analysis revealed mean necrosis depths of 0.0477 (SD: 0.0276) mm and 0.0495 (SD: 0.0274) mm for the PlasmaButton and for the resection loop,respectively.The distributions of necrotic zone depths at side I for both procedures are presented as Box-and-whisker-plots(Fig.2A) indicating that there was no statistically significant difference.The difference of means (Plasma-Button—resection loop)was with-0.0018 mm(very small)(Fig.2B), which was confirmed by a pairedt-test(p=0.691).
In addition, measurements were performed at the opposite side II.The mean necrosis depths at this side were 0.150 (SD: 0.086) mm for the PlasmaButton and 0.154 (SD: 0.084) mm for the resection loop (p=0.798).Data are presented as Box-and-whisker-plots in Fig.3A.The difference of means (PlasmaButton—resection loop) was-0.003 mm (Fig.3B).
4.Discussion
For the first time, we measured the absolute depth of the necrosis zone after application of plasma in the transurethral treatment of BPH.The strengths of the present study are i) the standardized and reproducible procedures of measurement and evaluation methods, ii) the direct comparison of two bipolar electrodes within the same patient,and iii) a sufficient number of samples permitting a statistically relevant statement.
There are plenty of publications investigating the quality of transurethral resection, which is mainly determined by the functional outcome and the degree of tissue damage.The quality of the resection results depends on a variety of factors such as the energy mode applied for transurethral treatment (monopolar versus bipolar resection or vaporization).The functional outcomes (i.e., micturition parameters) were described to be similar among the various methods [3,5,15], but the results on tissue damage presented in the literature are inconsistent.Some authors found deeper coagulation zones with bipolar resection compared to monopolar intervention [7,11,12]; others reported lower necrosis depths after bipolar treatments[8,9].Low carbonization and less severe tissue damage have been found to be important advantages of bipolar treatments [15,16].In general, many studies reported absolute values of necrosis depth after bipolar and monopolar treatment ranging clearly below or around 0.5 mm [8—11].
The diversity of study designs, examined tissue, and performed methods may explain, at least in parts, some discrepant results.The studies of Maddox et al.[6] and Akgl et al.[7]reported much higher necrosis depth values(mean±SD) after bipolar resection of 2.4±0.84 mm and 3.2±1.53 mm, respectively.Maddox et al.[6] performed their measurementsin vivoin human prostatic tissue from a total of 12 male subjects undergoing bipolar vaporization of the prostate using the Olympus high-frequency resection electrode PlasmaButton at standardized generator settings.No further specifications concerning the electrosurgical generator were given.The authors used a bipolar loop electrode for taking the deep resection specimen of the vaporized area and assessed necrosis depth by light microscopy.
In addition,differences in study species and tissue might contribute to the divergent results.Many authors used animal models and other tissues such as liver or kidney[8—10,12].Finally,the exact measurement conditions have an essential impact on the result.Of particular importance is the side of measurement (resected and vaporized surface).Maddox et al.[6] did not specify on which side necrosis depth was measured or if the given result of 2.4±0.84 mm might be a subsumption of resected and vaporized surface.The results of the present study showed considerable differences between the two sides of the specimens,which are discussed in detail below.Akgl et al.[7] did not use a button electrode for vaporization, which may limit the comparability to Maddox et al.’s [6] and our present results.
Data on clinical outcome and quality of life after different transurethral interventions are still a matter of debate, but many studies found better results for bipolar techniques [2,12,17—19].Singh et al.[5] reported that dysuria, a very common postoperative symptom, occurred less often after bipolar resection while efficacy was the same as in monopolar resection.Measurement of necrosis depth was not assessed in their study.Animal studies provided a potential indirect explanation for the reduced postoperative symptoms after bipolar resection: Huang et al.[10] compared the depth of necrotic zones after bipolar versus monopolar resection in a canine model.Directly after resection, necrotic zones were deeper after bipolar thanaftermonopolarintervention(mean±SD:0.237±0.020 mmvs.0.200±0.019 mm).However, the postoperative decrease of the coagulation zone depth was faster in the bipolar resection group (mean±SD:0.113±0.016 mmvs.0.129±0.017 mm after 1 week and 0.106±0.160 mmvs.0.116±0.025 mm after 2 weeks).The authors suggested that bipolar TURP caused less penetrative thermal damage than conventional monopolar TURP.A further indirect indicator for less severe tissue damage was shown in another canine model by Ko et al.[8].The authors compared the histopathological results and the thermal effects of monopolar and bipolar TURP.Lower values of necrotic zone depth were detected for the bipolar resections (0.07±0.08 mm for bipolar resection with a single loop device, 0.15±0.02 mm for bipolar resection with a double loop device, and 0.59±0.27 mm for monopolar device).In addition, the changes in tissue temperature at standardized locations in the prostatic tissue and in the capsular region close to the neurovascular bundle were measured.Temperature changes were significantly higher(mean±SD: 24.2±3.9°C) after monopolar treatment compared to bipolar treatment (mean±SD: 8.1±1.5°C;p<0.001).In both procedures, temperature changes were only observed within a distance of 6 mm to the temperature sensor.The significantly smaller change in temperature supports the assumption of less severe tissue damage after bipolar resection and may explain the observation of weaker postoperative symptoms.Moreover, the data suggest that less severe tissue damage leads to improved histopathological assessment.To the best of our knowledge, no study has proven this hypothesis.
Depth of the necrotic zone critically determines the degree of tissue damage and consequently, the clinical outcome of the patients.Therefore, exact values of necrotic zone depth may remarkably improve the assessment of intervention results.Published data vary widely,mainly due to huge differences between experimental settings and conditions of measurements.The present study is the first one applying a standardized procedure for measuring necrosis depth after bipolar intervention of the human prostatein vivo.Moreover, we directly compared the necrotic zone depth after bipolar resection and vaporization.Specimens were collected in a standardized way by preparing equal initial positions and resection procedures.Furthermore, histopathologic evaluation of the tissue was performed by pathologists blinded to the obtaining of the specimens.Consequently, the results on the depth of necrotic zones after bipolar resection and vaporization were highly consistent.No considerable differences were detected between these two procedures.This finding clearly confirms our assumption based on the physical equivalence of bipolar resection and vaporization.The values obtained in our study were 0.0495 mm and 0.0477 mm on average after bipolar resection and vaporization, respectively.These values were much lower than those published by Maddox et al.[6] and Akgl et al.[7].Direct comparability between their and our studies is not always possible due to differences in study design, examined tissue, or technical equipment.In addition, we compared the necrotic zones in the two sides I and II of the specimens and observed considerable differences between them.We suppose that necrotic zones of 2 mm and more—as published by Maddox et al.[6]and Akgl et al.[7]—are very likely due to clinically relevant postoperative disorders.
Of note, the results of our study showed considerable differences between the two sides of the specimens.On side I representing the resection side that remained in the patient’s body,the measured values were only one third of those on the opposite surface(side II).Such a big difference was not expected and was very surprising.A technical explanation is based on physical rules: according to Ohm’s law the current flows through the conductor with the lowest resistance [20].A lower resistance causes greater power dissipation and therefore,an increased energy input into the tissue.This can be observed when using the resection loop:the higher current flows through the convex upper side of the loop (side II) towards the shaft of the electrode than through the concave lower side of the loop(side I).Hence, the energy input on the convex side of the loop is greater than on the concave side.
The physical phenomena can explain the lower grade of adverse effects (e.g., postoperative dysuria) after bipolar treatment,that were described by Singh et al.[5],but they cannot explain the lower rate of peri- and post-operative bleeding observed by several authors [11,12,19].In addition, it has to be considered that in clinical practice the surgical treatment is finished by coagulating the resected surface.Therefore, an additional analysis of the bipolar and monopolar coagulation currents (instead of cutting currents) with their resulting tissue artefacts would be necessary to draw conclusions on clinical outcome and postoperative side effects after monopolar or bipolar transurethral BPH-treatment.
It should be emphasized that this study did not focus on the clinical outcome of the patients.The depth of the necrotic zone is an important but not the sole factor affecting the clinical outcome.Another essential factor is the completeness of adenoma removal, which can be achieved by enucleation.Further studies have to assess the influence of necrotic zone depth on clinical outcome.We think that knowledge of the exact depth of necrotic zones is an important prerequisite for assessing of which operative treatment is superior to any other.Our measurements showed the lowest necrotic zone depths compared to all other types of energy in transurethral BPH treatment.For comparison, with modern laser interventions(green light,holmium or thulium laser)necrosis depth ranged between 0.2 mm and 0.8 mm [21] and was more than five times higher than in the present study.
5.Conclusion
This is the first study presenting directly measured values of the absolute necrosis zone depth after application of plasma in the transurethral treatment of BPH.The small necrotic zones measured in bipolar resection and vaporization (0.0495 mm and 0.0477 mm, respectively) indicate that the probability of deeper tissue injury in any bipolar treatment using an impedance-controlled generator is negligibly small.To put it more clearly, with bipolar treatment, the necrotic zone depth is the lowest of all transurethral BPH treatments.Since BPH treatment is always a combination of cutting and coagulation, tissue effects after bipolar coagulation seem to be interesting as well and should be analyzed separately.In clinical practice,however, the absolute depths of necrotic zones are independent of the applied electrodes.
Author contributions
Study concept and design: Clara Breitling, Jrg Rassler.Conduction of operative interventions: Jrg Rassler.
Collection and preparation of tissue samples: Clara Breitling.
Histopathological evaluation: Hans Nenning.
Data analysis: Clara Breitling, Hans Nenning.
Drafting of manuscript: Jrg Rassler.
Critical revision of the manuscript: Clara Breitling, Hans Nenning, Jrg Rassler.
Conflicts of interest
The authors of this publication had research support from Olympus, Hamburg, Germany.The authors declare no conflict of interest.
Acknowledgement
We thank the pathologists from the Institute for Pathology“Am Elsapark”,Leipzig,for their support in histopathologic evaluation of the prostate specimens.Further, we gratefully appreciate the valuable study support of Dr.Susanne Stahlkopf and the language editing by Samuel Faran.
 Asian Journal of Urology2023年2期
Asian Journal of Urology2023年2期
- Asian Journal of Urology的其它文章
- Role of circulating tumor cell clusters in patients with metastatic hormone-sensitive prostate cancer receiving a gonadotropin-releasing hormone antagonist: A pilot study
- Tunica albuginea versus buccal mucosa graft urethroplasty for anterior urethral stricture:A prospective randomised pilot study
- Subadventitial resection of the ureter—new method for surgical corrections of the ureteropelvic junction and ureterovesical junction obstructions
- Percutaneous embolization by direct puncture for the treatment of high-flow priapism
- Antioxidant status in patients with bladder cancer regarding cancer stage and grade
- The role of quick Sepsis-related Organ Failure Assessment score as simple scoring system to predict Fournier gangrene mortality and the correlation with Fournier’s Gangrene Severity Index: Analysis of 69 patients
