Using eye drops in compliance may improve the symptoms and quality of life in patients with seasonal allergic conjunctivitis: analysis of a two-season web survey
Asako Kodama, Dogru Murat, Hiroshi Fujishima, Kazumi Fukagawa
1Department of Ophthalmology, Eiju General Hospital, Tokyo 110-8645, Japan
2Department of Ophthalmology, Tsurumi University School of Dental Medicine, Kanagawa 230-0063, Japan
3Ryogoku Eye Clinic, Tokyo 130-0062, Japan
Dear Editor,
Allergic conjunctivitis is the most common ocular surface allergic disease, affecting more than 30% of the population[1-2].In the immediate phase of the allergic reactions(type Ⅰ) in patients with seasonal allergic conjunctivitis (SAC),the binding of antigen with the reaginic antibody leads to the formation and release of chemical mediators, such as histamines, in the local anaphylactic reaction[3].Stimulation of histamine H1 receptors is associated with effects such as prostaglandin production, inflammation, and pain.Ⅰn addition,the H1 receptor is the only histamine receptor associated with itching[4].Antihistamines act as an inverse agonist on the histamine receptor, thus primarily blocking the acute phase of allergic reaction.Mast cell stabilizers prevent the release of inflammatory mediators associated with the late phase of the allergic reaction[4-5].Topical eye drops that work as antihistamines and mast cell stabilizers have become the preferred treatment because of their dual action.In Japan, most antihistaminic eye drops have dual action and are used as the first choice of treatment for SAC.Regarding their use, the frequency of instillations is usually conveyed to the patient as four times a day, and the timing of instillations is described as“at morning, noon, evening and before bedtime”.According to a previous investigation, it was reported that about 30% of the SAC patients used eye drops over five times a day when the symptoms worsened[6].Though many studies were conducted to investigate the actual use of the eye drops and the treatment satisfaction[6], no studies compared the symptoms and quality of life (QOL) between the two groups: the instillation compliant group and the non-compliant group.We conducted a questionnaire survey in 2019 and 2020 to investigate the use of eye drops and the QOL and have reported by year that using eye drops in compliance improved the QOL[7-8].We summarize here the findings of a two-season web survey to provide further insights into the influence of instillation compliance on the symptoms and QOL.
Ethical ApprovalThis study was performed in accordance with the Helsinki Declaration.Approval was obtained from the Institutional Review Board at Kitamachi Clinic (Tokyo, Japan;IRB No.H31-002, H32-001)[7-8].The clinical trial registration ID number is UMIN 000036118 and 000039757.Informed consent was provided by each subject.
During Japan’s cedar pollen season in 2019 and 2020, we performed questionnaire surveys on the web (March 12-22,2019, and March 12-16, 2020).In the 2019 season, patients diagnosed with SAC and prescribed antihistaminic eye dropsq.i.d.were enrolled in this study.Epinastine hydrochloride is a strong H1 receptor antagonist and mast cell stabilizer.In November 2019, epinastine hydrochloride eye drop(ALESION®LX 0.1%, Santen, Japan) was released in Japan,as the firstb.i.d.antihistaminic eye drop.As this drug has less frequency of instillations per day than theq.i.d.form of the previously existing eye drops in the market, we thought that more patients might use the eye drop in compliance.In the 2020 season, patients diagnosed with SAC and prescribed antihistaminic eye dropsq.i.d.orb.i.d.were recruited for this study.Patients under 20 years of age, as were those with severe allergic conjunctivitis, such as vernal keratoconjunctivitis and atopic keratoconjunctivitis, were excluded from the study.We asked the patients to categorize their symptoms during the cedar pollen season into three periods: “when the symptoms were mild”, “when they were symptomatic during the season”, and “when the symptoms were more severe”.The instillation frequency and timing of the eye drops for each period were also observed.The severity of itching as the most typical subjective symptom for each period and the QOL were determined by a questionnaire.The symptoms and QOL had scores from 0 to 4, and the larger the score, the more severe the subjective symptoms and QOL were.We calculated the average score of itching for each period.About the QOL,we considered the following four aspects: 1) difficulty with studying, work, or housework; 2) fatigue; 3) malaise; 4)irritability.We calculated the average score for each aspect of QOL and the average of the total score of four aspects of QOL.Satisfaction with the current treatment was also evaluated.We asked the patients about the medication instruction by the doctors or pharmacists for the instillation frequency and timing of the eye drops.We defined the patients who regularly instilled eye dropsq.i.d.orb.i.d.with or without itching in each period as the instillation compliant group (compliant group).We defined the other patients as the instillation noncompliant group (non-compliant group).The probability (P)values for the itching scores were determined by the Mann-WhitneyU-test.For the QOL scores,Pvalues were determined using the Studentt-test for the 2019 season and the Welch’st-test for the 2020 season.The Fisher’s exact test was used to compare the ratio of the compliant group in the 2020 season.A level ofP<0.05 was accepted as statistically significant.All data are presented as means±standard deviation in the figures.The final number of responses was 1008 (389 men and 619 women) in the 2019 season and 1226 (410 men and 816 women) in the 2020 season.Figure 1[7-8]show the ratio of the compliant and non-compliant groups.The compliant group accounted for 4.0% of all the patients in the 2019 season and 5.1% in 2020.There was a trend that the ratio of the compliant group was very low throughout the two-season survey.Most of the SAC patients adjusted the instillation frequency of the eye drops according to their symptoms and instilled them only after they felt itching.The antihistaminic eye drops weaken the action of histamines by competing with the histamine receptor competitively.To suppress the histamine binding to the receptor and signal transduction, it is important to maintain the concentration of the antihistamines in the conjunctiva above a certain level by using the eye drops at the correct instillation frequency and timing.The itching score of the compliant and non-compliant groups is shown in Figure 2.In the 2019 season, the itching score was significantly lower in the compliant group only when the symptoms were more severe than in the non-compliant group (P=0.0047).In the 2020 season, though the itching score was lower in the compliant group when the symptoms were more severe, there were no significant differences between the two groups.Figure 3 show the QOL score of the compliant and non-compliant groups.In the 2019 season, the QOL score and the total QOL score were significantly lower in the compliant group than in the non-compliant group.In the 2020 season, the QOL score,except for the difficulty with studying, work, or housework,and the total QOL score were significantly lower in the compliant group than in the non-compliant group.Regarding the itching score, there was a significant difference between the two groups only when the symptoms were more severe in the 2019 season.The behavior of the patients in the non-compliant group,i.e., using eye drops only after they felt itching appears to lead to uncontrollable symptoms when the symptoms were severe.Also, such behavior might have led to the worse QOL scores.Because the onset of the symptoms can be affected by the amount of pollen scattered, it may be difficult for the patients to predict when the symptoms will occur.Therefore,not to worsen the QOL, we recommend using the eye drops regularly with or without itching.In the 2020 season, unlike the 2019 season, there were no significant differences between the two groups in the QOL score of “difficulty with studying,work, or housework”and the itching scores.We considered the following factors as to why no significant differences were observed between the two groups.The compliant group had a significantly higher itching score than the non-compliant group when no eye drops were used (Figure 2B).In the 2020 season, the bias from the patients with strong symptoms in the compliant group might have affected the results.Totally 43.9% of all the patients in the 2019 season and 33.5% in 2020 used steroid eye drops[7-8].The steroid eye drops/ointment may have influenced the results.Further study that excludes patients who use steroid eye drops/ointment concomitantly is required.Regarding the satisfaction of the eye drops, there was no significant difference between the compliant and noncompliant groups (P=0.7090)[7].Several factors might have masked the difference in trends between the two groups.These factors include the patients’high expectations for the immediate effects of eye drops or the feeling of a burden by instilling the eye drops four times a day.Concerning the instillation instructions, significantly more patients in the compliant group were instructed correctly about the instillation frequency and timing compared to the non-compliant group throughout the two-season survey[7-8].Surprisingly, about 30%of the patients were instructed to instill eye drops when they got itching.The correct understanding of eye drop instillation,obtained by proper instruction by the doctors and pharmacists,leads to instillation compliance.Our findings suggest that the ratio of the compliant group was significantly higher in theb.i.d.group (11.7%) than in theq.i.d.eye drop group (3.9%;P=0.0001, Figure 1C, 1D).There were significantly more patients satisfied with the eye drop in theb.i.d.group than in theq.i.d.eye drop group (P=0.0049)[8].The reasons for the high instillation compliance and satisfaction in theb.i.d.eye drop group may be the reduction in the instillation frequency(4 to 2), allowing the patients to use the eye drops correctly.In addition, the doubled concentration of the eye drop might have led to a longer duration of effect.
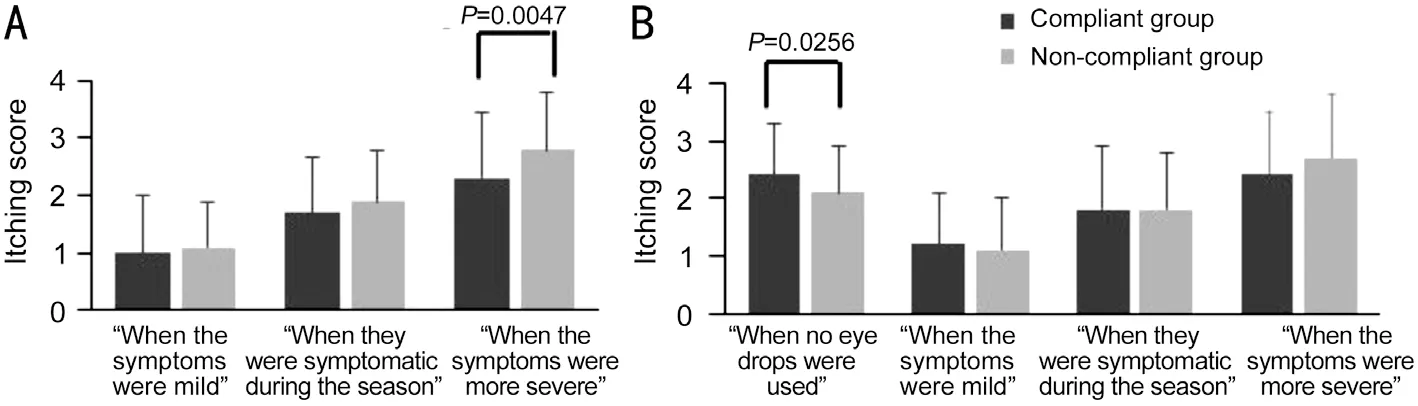
Figure 2 The itching score of the compliant and non-compliant groups A: 2019 season; B: 2020 season.In the 2019 season, the itching score was significantly lower in the compliant group only when the symptoms were more severe than in the non-compliant group (P=0.0047, Mann-Whitney U-test).Data are shown as means±standard deviation.

Figure 3 The quality of life (QOL) score of the compliant and non-compliant groups A: 2019 season; B: 2020 season.In the 2019 season, the QOL score and the total QOL score were significantly lower in the compliant group than in the non-compliant group (Student t-test).In the 2020 season, the QOL score, except for the difficulty with studying, work, or housework, and the total QOL score were significantly lower in the compliant group than in the non-compliant group (Welch’s t-test).Data are shown as means±standard deviation.
The SAC patients are known to be hypersensitive to histamine and prone to itching even with low exposure to antigens.Previous reports suggested that once an allergic reaction develops, mild asymptomatic inflammation persists, and allergic symptoms are more likely to occur in the end[9].It is essential to use the antihistaminic eye drops in compliance even when the symptoms are relieved to control the occurrence of itching.
The amount of pollen scattered varies from year to year.Therefore, by analyzing a two-season web survey, we could more precisely investigate the influence of instillation compliance on the symptoms and QOL.In conclusion, we found that very few patients complied with the use of eye drops.We showed that using antihistaminic eye drops in compliance may improve the symptoms and QOL.It was also speculated that theb.i.d.eye drop, which is easy to use in compliance, improved the patient’s symptoms and QOL.
ACKNOWLEDGEMENTS
Conflicts of Interest:Kodama A, None; Murat D, None;Fujishima H, None; Fukagawa K, None.
 International Journal of Ophthalmology2023年6期
International Journal of Ophthalmology2023年6期
- International Journal of Ophthalmology的其它文章
- Preliminary proteomic analysis of human tears in lacrimal adenoid cystic carcinoma and pleomorphic adenoma
- Assessment of the effects of intrastromal injection of adipose-derived stem cells in keratoconus patients
- Evaluation of optic nerve head vessels density changes after phacoemulsification cataract surgery using optical coherence tomography angiography
- Stability of neodymium:YAG laser posterior capsulotomy in eyes with capsular tension rings
- Comparison of the efficacy and safety of ultrasonic cycloplasty vs valve implantation and anti-VEGF for the treatment of fundus disease-related neovascular glaucoma
- Volumetric fluid analysis of fixed monthly anti-VEGF treatment in patients with neovascular age-related macular degeneration
