Assessment of the effects of intrastromal injection of adipose-derived stem cells in keratoconus patients
Shahrokh Ramin, Ali Abbasi, Masoumeh Ahadi, Lina Moallemi Rad, Farzad Kobarfad
1Department of Optometry, School of Rehabilitation Sciences,Incubation Center for Pharmaceutical Technology (ICPT),Shahid Beheshti University of Medical Sciences, Cell Therapy Department, Red Crescent Pharmaceutical and Clinical Complex, Tehran 1616913111, Iran
2Student Research Committee and Department of Optometry,School of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Tehran 1616913111, Iran
3Department of Molecular and Cell Biology; Faculty of Basic Sciences, University of Mazandaran, Babolsar 4561745735, Iran
4Department of Medicinal Chemistry, Shahid Beheshti School of Pharmacy, Tehran 4516745735, Iran
Abstract● AIM: To evaluate the efficacy and safety of intrastromal transplantation of adipose-derived stem cells (ASCs) in keratoconus patients.
● KEYWORDS: adipose-derived stem cells; stromal keratocyte; cellular therapy; keratoconus
INTRODUCTION
The middle component of the cornea or stroma is responsible for 90% of corneal thickness, which contains keratocytes, collagen fibers and glycosaminoglycans[1].Keratocytes play the leading role in the corneal transparency,wound healing, and stromal crystallin synthesizing[2].Stromal diseases lead to keratocyte apoptosis, corneal shape abnormalities, stromal scarring, visual impairment, and blindness[3].Keratoconus is a common and noninflammatory stromal disease.Keratoconus causes mild to severe blurring of vision due to irregular astigmatism, which sometimes cannot be corrected with glasses and even the use of lenses is problematic[4].Corneal transplantation is the gold standard treatment for severe visual impairment[5].Although,corneal transplantation is the most successful types of tissue graft worldwide, it is complex process with postoperative complications[6].One study by Gainet al[7]showed that there is only one cornea out of the 70 needed for corneal transplantation.Additionally, more than half of the world’s population had no access to corneal transplantation.In recent years, new modalities such as collagen crosslinking, new refractive and keratoplasty procedures, have been presented according to the visual loss and severity of keratoconus, but these methods also have problems and not useable in all cases[8].Therefore, finding alternatives to corneal transplantation, such as stem cell use and tissue engineering, is essential.
Adipose-derived stem cells (ASCs) are one type of adult stem cells.ASCs are an abundant source of mesenchymal stem cells(MSCs) and can be obtained from subcutaneous adipose tissue through minimally invasive methods[9].Variousin vitrostudies have shown that ASCs can differentiate into different cell classes and have a wide application prospect in the field of cell therapy and regenerative medicine[10-11].The ease of isolation,proliferation and autologous nature of mesenchyme cells make them a good candidate for cellular therapy[12-13].
ASCs have shown great potential in corneal cell-based therapies in experimental models.After transplantation of stem cells into the cornea, these cells differentiated into keratocytes and produced new stromal collagen without inflammatory or immune response[14-15].These cells could even reconstruct previous corneal scars and maintain their transparency due to their immunological properties similar to those of the host cornea[16-17].ASCs secrete various growth factors that are effective in healing corneal ulcers, stimulating cell migration,inhibiting keratocyte apoptosis, and increasing extracellular matrix expression[18].The animal studies showed promising results of the efficacy of stem cells transplantation in treatment of corneal stroma[17-18].
Therefore, the present study was designed to evaluate the efficacy and safety of transplantation of autologous ASCs in patients with keratoconus.
SUBJECTS AND METHODS
Ethical ApprovalThis study was performed after approval by the Human Ethics Committee of Shahid Beheshti University of Medical Sciences (No.IR.SBMU.REC.1399.024).Under the principles of Helsinki Declaration and detailed explanation of our study, the consent form was received from all participants.
Population and SamplesNine patients referred to the eye clinic in Dr.Ramin Eye Clinic in 2021-2022 were included in this study.All participants were healthy and had no systemic disease or history of rheumatic, autoimmune or malignant disease.The patients had a free personal and family history.There were no abnormalities on the physical examination.Overall, there were no abnormalities on the routine blood and urine tests.Patients were over 18 years old with moderate to severe keratoconus with reduced best corrected visual acuity (BCVA)of less than 20/25.According to the RETICS keratoconus classification[19], diagnosis of keratoconus approved on the basis of clinical findings and Pentacam data.Exclusion criteria were BCVA of 20/200 or less in the other eye, previous corneal surgeries, ocular disease such as severe dry eye,delayed epithelial healing and any inflammatory eye surface process, uveitis, cataract, retinal diseases, glaucoma, a history of immunodeficiency or immunosuppressive therapy, and pregnancy or breastfeeding.
ExaminationsIn patients who had met the study criteria,a completed ophthalmic examination including assessment of refraction, slit lamp examination, and fundoscopy are performed.BCVA is measured and recorded by Snelln E chart at a distance of 6 meters.Evaluation of objective refraction status and keratometric findings by Topcon autorefractometer(KR 8800, Topcon, Japan) were performed and the findings would be confirmed by Heine Beta 200 retinoscope (Germany).To determine the best optical correction, subjective refraction and corrected visual acuity will be measured and recorded.Corneal topographic assessments are by the Sirius Scheimpflug Analyzer (CSO, Costruzione Strumenti Oftalmici, Italy) by the same device.Corneal cellular structure assessments were performed using confocal biomicroscopy (Nidek Technologies,Japan).
Cell CultureStem cells were prepared from adipose tissue in the abdomen, for which standard liposuction was performed by a specialist.After local anesthesia, approximately 25 mL of adipose tissue was removed and washed with phosphatebuffered saline (PBS; Gibco), containing 1% penicillinstreptomycin (Gibco) and digested in collagenase I solution at 37℃ for 45min, then collagenase activity was inhibited by adding of autologous human serum extracted from each patient.Erythrocytes were lysed by erythrocyte lysis buffer(Gibco-Life Technologies, Waltham, MA, USA).The pelleted cells are then cultured in DMEM medium (Gibco) including glutamax and sodium pyruvate, 10% autologous human serum,1% penicillin-streptomycin, 2% amphotericin.Detection of MSCs was performed using CD73+, CD45-, CD90+, and CD105+markers.About 60 to 80h before surgery, to resemble the physiological conditions of stroma keratocytes, the human serum stem cells were rested by reducing the human serum concentration to 0.5%.The isolated stem cells were ready for injection into the corneal stroma.

Figure 1 Immunophenotyping of adipose-derived stem cells (ASCs) Immunophenotypic characterization of cells was performed by flow cytometry using the monoclonal antibodies.Positive markers: ASCs strongly expressed CD73 (99.2%, A), CD90 (99.7%, B), and CD105 (95.1%, C);Negative marker: did not express CD45 (1.24%, D).
Cell Surface Antigens of Adipose-Derived Stem CellsCell surface markers of ASCs at the third cell passage were assayed by flow cytometry.Our assessments showed that ASCs in our study positively expressed high levels of cell antigens of CD73, CD90, and CD105, but presented low levels of CD45 antigen (Figure 1).Based on these cellular markers,the phenotypes of the harvested ASCs were nearly consistent with previously reported ASCs obtained using the traditional collagenase digestion method.These assessments confirmed that the cells derived from pieces of adipose tissue were ASCs.These cells included a homogeneous population of ASCs without contamination with endothelial cells, pericytes and smooth muscle cells.
Intrastromal InjectionSurgical procedure was similar to intracorneal ring implantation.After local anesthesia of the cornea, using femtosecond laser (Technolas 520F), an intrastromal pocket was created in the corneal stroma with a diameter of 7.5 mm and a depth of half of the cornea according to pachymetry findings.A 30 degrees anterior side cut incision was made.The femtosecond laser parameter settings were similar to the ones used for intracorneal ring implantation.The corneal intrastromal pocket was opened with a Morlet lamellar dissector (Duckworth & Kent, England).A volume of 1 mL of a solution containing 3×106cells is injected into the stromal pocket.Finally, a bandage contact lens was fit on the cornea to close the incisions, which are removed a week after surgery.After surgery to prevent the inflammation and infection,antibiotic eye drops (chloramphenicol 0.5%q.i.d.) and antiinflammatory eye drops (betamethasone 0.1%q.i.d.) were prescribed for patients for 2 to 4wk depending on the degree of recovery.Our patients did not show any complications, corneal transplantation is performed if patients develop complications such as edema and corneal opacity after surgery.Follow-up examinations, one, three and six months after surgery for reevaluation and all initial eye examinations are performed for them.In this plan, forms have been prepared in which the information of each patient is recorded separately and in the number of visits, and after collection, the findings of patients before and after treatment are compared and evaluated.
Statistical AnalysisData were analyzed by SPSS software version 22.The normal distribution of data assessed with the Shapiro-Wilk test.Because of the small size of the study sample (n=8), Wilcoxon tests were used to analysis.P<0.05 was considered statistical significance.
RESULTS
In this study, 9 subjects (all males) with the mean age of 30.00±4.96y (range 22-35y) participated.All patients had moderate to severe keratoconus, and they had no history of other modality treatment such as cross linking and ring implantation.In all patients, surgeries were performed without any intraoperative complication.Eight patients completed the 6-month follow-up, but one patient did not have a 6-month follow-up examination, so this patient was excluded from further analysis.A summary of patients’information before and after ASCs transplantation is in Tables 1 and 2.
Slit Lamp BiomicroscopyAll patients had no complications after transplantation of ASCs and their corneas were transparent and similar to normal corneas.A 6-month follow-up examination showed no evidence of infection, inflammation,scarring, rejection of cells, and other side effects.
Visual AcuityIn the majority of patients, visual acuity improved moderately, and none experienced visual loss.The mean BCVA of subjects was 0.48±0.18 (range 0.20 to 0.70 Snellen) and improved to 0.59±0.17 (range 0.40 to 0.80 Snellen) and 0.66±0.17 (range 0.40 to 0.90 Snellen) after three and six months (P=0.001).BCVA of subjects increased 1.85±0.80 lines (range 0 to 3 lines Snellen) after 6mo.Visual acuity increased between 1-2 lines in 5 eyes (75%) and 3 lines in 2 eyes (23%) and remained stable in 1 eye (12%).
Refractive FindingsSix months after ASCs transplantation,all refractive parameters showed an overall reduction,presenting a mean sphere improvement of 0.34±0.35 diopter(D), and a mean cylinder reduction of 0.84 ±0.23 D.The mean spherical refraction was -2.25±2.92 D (range -7.00 to +1.00 D) primarily and decreased -2.00±2.60 D (range-6.00 to +1.00 D) and -1.90±2.63 D (range -6.00 to +1.00 D)after 3, and 6mo respectively (P=0.039).The mean cylindricalrefraction was -4.06±1.86 D (range -6.00 to -2.00 D) primarily and decreased -3.69±2.18 D (range -6.00 to -1.00 D)and -3.34 ±2.04 D (range -6.00 to -1.0 D) after 3, and 6mo respectively (P=0.016).
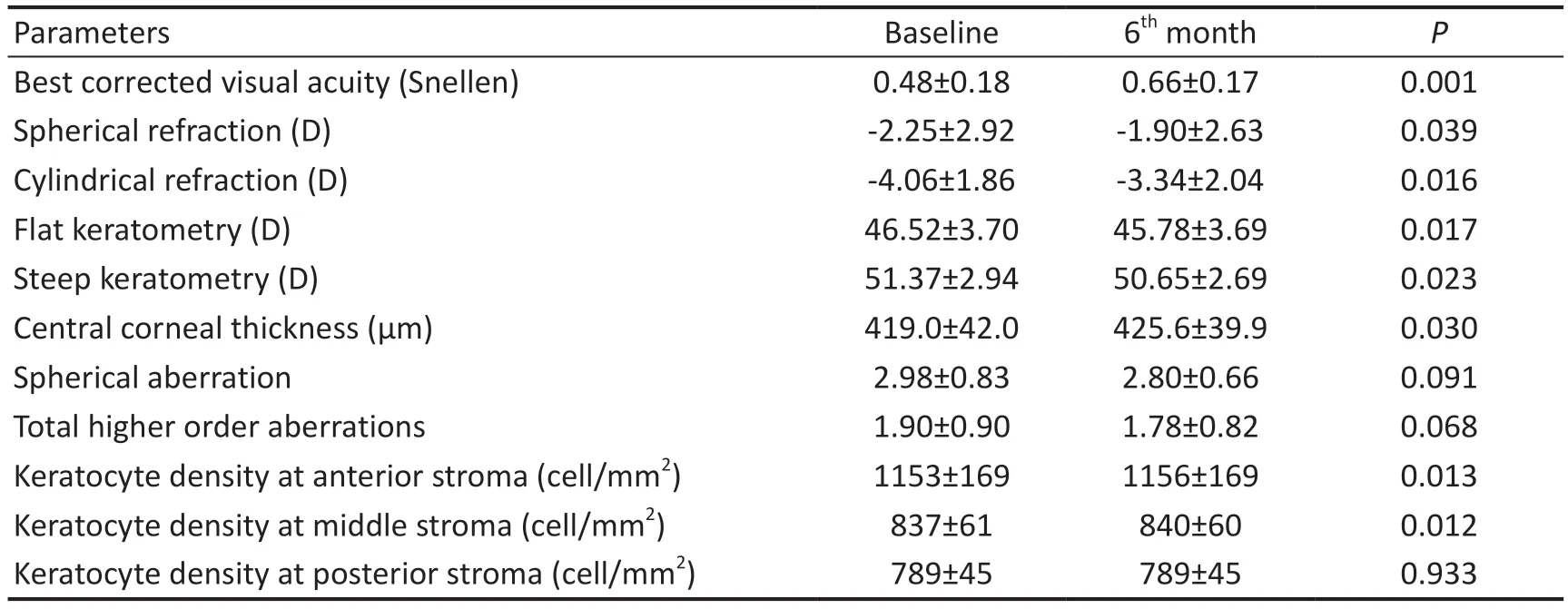
Table 1 Intrastromal injection of adipose-derived stem cells (ASCs) in keratoconus patients

Table 2 Information of each patient pre- and post ASCs transplantation
Topography FindingsSix months after surgery, all keratometric parameters of patients showed a slight decrease,presenting a mean flat keratometry (K1) improvement of 0.78±0.71 D, a mean steep keratometry (K2) deterioration of 0.65±0.64 D and a mean maximum keratometry (Kmax)reduction of 0.32±0.78 D.The mean K1 was 46.52±3.70 D(range 42.86 to 53.25 D) primarily and decreased 45.78±3.44 D(range 42.66 to 52.50 D) after 6mo (P=0.017).The mean K2 was 51.37±2.94 D (range 46.68 to 54.25 D) primarily and decreased 50.65±2.69 D (range 46.17 to 53.50 D) after 6mo(P=0.023).The mean Kmax was 55.45±5.55 D (range 48.50 to 61.70 D) primarily and decreased 55.11±5.31 D (range 48.75 to 61.00 D) after 6mo (P=0.271).Also, the mean corneal astigmatism of the patients was -5.86±2.26 D (range -9.70 to-3.81 D) which was -5.29±2.26 D (range -8.50 to -2.27 D) after 6mo (P=0.028).In general, keratometric parameters decreased in 6 eyes (75%), remained stable in 1 eye, and increased in 1 eye.The mean preoperative central corneal thickness (CCT)was 419.00±42.07 μm, which reached to 425.63±39.90 μm after 6mo (P=0.030).The mean CCT of patients improved of 6.29±4.47 μm (range -7.00 to 13.00 μm).Although the thickness showed an increase in seven of the patients, it decreased by 7 μm in one of them (Figure 2).The mean corneal volume in 10 mm of cornea was 51.68±3.61 mm3and after treatment was 52.15±3.54 mm3(P=0.025).Corneal aberrometry with 5 mm pupil decreased in the spherical aberration, coma and total higher order aberrations (HOA)in all patients except one patient, who corneal aberrometry increased.Spherical aberration decreased from 2.98±0.83(range 1.68 to 4.21) to 2.80±0.66 μm (range 1.92 to 3.77) 6mo after surgery (P=0.091).Coma aberration decreased from 1.67±1.00 (range 0.57 to 2.68) to 1.59±0.97 μm (range 0.49 to 2.60) after surgery (P=0.090).Total HOA decreased from 1.90±0.90 (range 0.62 to 2.67) to 1.78±0.82 μm (range 0.68 to 2.60) 6mo finally (P=0.068).
Confocal Microscopy FindingsConfocal microscope findings of the corneal cellular structure including endothelial cells, entire stroma, epithelium and sub-epithelial neural network showed no adverse effects in all patients 6mo after surgery.The mean density of keratocytes showed a slight increase in the anterior and middle stroma of the cornea and was stable in the posterior stroma of the cornea.The mean keratocyte density at anterior stroma of cornea was 1153.4±169.7 cell/mm2(range 908.3 to 1378.3) primarily and were 1156.1±169.8 cell/mm2(range 911.2 to 1380.0) after 6mo (P=0.013).The mean keratocyte density at middle stroma of cornea was 837.7±61.5 cell/mm2(range 728.6 to 908.0)primarily and were 840.4±60.7 cell/mm2(range 735.2 to 910.0)after 6mo (P=0.012).The mean keratocyte density at posterior stroma of cornea was 789.2±45.1 cell/mm2(range 618.8 to 848.2) primarily and were 789.2±45.8 cell/mm2(range 707.6 to 850.0) after 6mo (P=0.933).In assessment of morphology of cells, the keratocytes of the stroma were similar and there was no different between them (Figure 3).
DISCUSSION
Keratoconus is a common degenerative disease that often leads to vision loss[4,8].The cause and pathological mechanism of keratoconus are not fully understood[20].In keratoconus, the thinning of the corneal stroma is associated with keratocyte apoptosis, extracellular matrix alterations, and activation of degrading enzymes[21-22].In addition, keratocyte damage and apoptosis is one of the side effects of corneal collagen crosslinking as a common treatment method[23-24].In recent years,several studies have been demonstrated beneficial effects of stem cell therapy in corneal diseases[11-13,25].Stem cells have many advantages, such as immunomodulatory properties,healing or reducing corneal wounds, improving corneal transparency, and producing new organized collagen in the host’s stroma[13,16,26].In animal studies, researchers showed that MSCs can differentiate into corneal keratocytes and express keratocyte markers and acquire characteristics of keratocytes[15-16,27-29].

Figure 2 Topographic changes after intrastromal injection of adipose-derived stem cells (ASCs) A: Preoperative topographic data in the patient 2 with visual acuity of 0.3 (Snellen); B: 6mo postoperative topographic data in the same patient with visual acuity of 0.5 (Snellen).Mean keratometry of this patient was 49.36 D and decreased 48.75 D, and astigmatism was -5.55 D that decreased to-4.56 D postoperatively.The mean central thickness improved of 5.0 µm.Total HOA decreased from 2.67 to 2.48 µm.

Figure 3 Corneal confocal biomicroscopy preoperative (A) and 6mo postoperative (B) findings In the patient 2, the mean density of keratocytes in the anterior, middle and posterior stroma of the cornea was 1008, 888, and 848 cell/mm2 respectively, and after 6mo it was 1009, 891, and 850 cell/mm2.Stromal keratocytes were similar and there was no different between old and new cells.
In this research, we assessed the safety and efficacy of transplantation of ASCs into stroma in patients with moderate to severe keratoconus.We hypothesized that ASCs differentiate into corneal keratocyte to induce changes in the stroma and its possible effects on corneal optical properties.We used autologous ASCs for cell therapy.These cells had advantages such as ease of access to the cells, easier extraction of them from adipose tissue, and lack of subsequent immune reactions.Also, we did not need to ocular stem cell, and it could be done in patients with corneal stem cell deficiency.To avoid intra and postsurgical risks, femtosecond laser was used to create a stromal pocket at the middle stroma.During the 6-month follow-up examinations, none of the patients showed ocular complications.The cornea remained clear and there was no evidence of side effects such as rejection signs or scarring or inflammation.In the six-month assessments of the patients,intrastromal injection of ASCs led to an increase in visual acuity along with a decrease in refractive parameters and corneal irregularities and an increase of keratocytes density of stroma.In most patients, visual acuity improved moderately, which was observed in the third month and increased by the sixth month.Refractive parameters including spherical and cylindrical refraction showed a slight decrease in patients.Also, topographic evaluations showed a slight decrease in corneal curvature and an increase in corneal thickness.Corneal aberrometry findings showed a reduction in spherical aberration, coma and total HOA, although it was not statistically significant.In confocal microscopy assessments,the cellular structure of corneal layers was normal.The density of keratocytes increased in the anterior and middle corneal stroma but remained constant in the posterior corneal stroma.Stromal keratocytes had similar morphology and did not differ from each other.
There was one clinical study in this field by Alioet al[30].Similar to our results were reported by them.They implanted autologous ASCs in the corneal stroma in 5 patients with advanced keratoconus.Anterior segment optical coherence tomography (OCT) assessments demonstrated the production of new collagen in the area of the ASCs implantation and a central thickness improvement of 15 μm.This new collagen fibrils did not induce any clinical haze.Ⅰn all patients, visual function improved by 2 lines in all visual parameters, but keratometric values remained stable[30].In the 12-month follow-up, confocal microscopy showed the evolution of the nuclei of ASCs and their morphological changes from round shape and highly refringent cells to fusiform structures and less nuclear refringent.Therefore, ASCs implanted in the corneal pocket not only survived but also differentiated into keratocytes[12].In another publication, they reported the 3-year clinical outcomes of these patients.At 3y, intrastromal implantation of ASCs did not have complications and showed a moderate improvement in visual acuity in patients with advanced keratoconus[31].
In our opinion, the improvement of vision is due to the reduction of refractive parameters, corneal aberrometry and irregularities.After intrastromal injection of ASCs, these cells differentiate into keratocytes and induce structural changes in the stroma.Stromal keratocytes secret extracellular matrix,which have an important role in maintenance of corneal transparency[32-33].Stem cells have the ability to regenerate a stable and non-regenerating tissue such as the corneal stroma.These cells inhibit formation of fibrotic matrix components and regenerate collagenous matrix with uniform diameter and regular interfibrillar spacing, indistinguishable from native stroma[34-35].Also, studies showed injection of ASCs into the cornea with keratocyte dysfunction improved host keratocyte functions and restored corneal clarity without any inflammatory and immune responses[28-29].In addition, new keratocytes increase the production of new collagen in the area of the ASCs implantation and can be effective in improving cellular structure.These cells express collagens type I and VI,and keratocyte-specific markers[30,36].In animal studies with thin and haze corneas, these cells were able to increase the corneal thickness and improve corneal transparency without immune reactions[29,37-38].
In one patient, the visual acuity did not change, and the topography findings indicated an increase in corneal keratometry and corneal aberrometry.In the confocal microscopy assessments, there was no change in the density of keratocytes.This may be due to the limited amount of new collagen produced in the cornea by the transplanted ASCs,which has also been reported in studies[29-30].It can also be due to the progressive nature of keratoconus and the lack of effectiveness of this treatment method in this patient.
Finally, in this study, we created a stromal pocket for stem cell injection using femtosecond laser, which may have some corneal refractive effects.In our opinion, the creation of a stromal pocket may have some effects in the first weeks after surgery, but it is not effective in the later outcomes when the cornea is completely healed and clear.Changes in corneal refractive parameters, such as decreased corneal curvature,increased thickness, increased corneal volume, increased keratocytes density, and vision improvement begin and complete in the following months, which corresponds to the time of differentiation of stem cells into keratocytes.Also, the stromal pocket was created in all patients with one method, but not all patients showed the same results: some had significant findings, some had poor findings.In addition, all patients had transparent cornea and there was no evidence of corneal edema, corneal scar.To investigate the effect of stromal pockets on the final results, we should study a case-control study in two groups of patients with the creation of stromal pockets with or without ASCs transplantation.This issue can be investigated in the future.
In general, transplantation of ASCs on keratoconus patients had moderate effect but it was not effective in all patients.Also, this method was safe and no side effects were observed in the patients.The limitation of this study was that we had no assessment of biomechanical properties after transplantation,and the changes in the biomechanical properties and stromal strengthening were not determined.Moreover, we could not assess production of new collagen fibrils in the stroma using anterior OCT.We suggest further studies with more patients and long-term follow-up and using of it in treatment of corneal dystrophies, corneal scarring.
Based on our results, intrastromal transplantation of ASCs had positive effects on refractive parameters and corneal irregularities in most patients.After six months, visual acuity improved moderately, corneal parameters reduced slightly, and stromal keratocytes density increased.This method was not effective in all patients.Also, this modality was safe and none of the patients showed any complications.
ACKNOWLEDGEMENTS
This study is related to the project with number IR.SBMU.RETECH.REC.1399.024 from Student Research Committee,Department of Optometry, Faculty of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.We also appreciate the “Student Research Committee”and “Research& Technology Chancellor”in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Authors’contributions:Methodology: Ramin S, Abbasi A,Moallemi Rad L, Kobarfard F.Project administration: Ramin S, Abbasi A, Ahadi M.Supervision: Ramin S, Abbasi A,Ahadi M.Clinical examination: Ramin S, Abbasi A, Ahadi M.Conceptualization: Abbasi A, Kobarfard F.Investigation:Ramin S, Abbasi A, Ahadi M.Writing-review & editing:Ramin S, Ahadi M, Abbasi A.
Conflicts of Interest:Ramin S, None; Abbasi A, None;Ahadi M, None; Moallemi Rad L, None; Kobarfard F, None.
 International Journal of Ophthalmology2023年6期
International Journal of Ophthalmology2023年6期
- International Journal of Ophthalmology的其它文章
- Preliminary proteomic analysis of human tears in lacrimal adenoid cystic carcinoma and pleomorphic adenoma
- Evaluation of optic nerve head vessels density changes after phacoemulsification cataract surgery using optical coherence tomography angiography
- Stability of neodymium:YAG laser posterior capsulotomy in eyes with capsular tension rings
- Comparison of the efficacy and safety of ultrasonic cycloplasty vs valve implantation and anti-VEGF for the treatment of fundus disease-related neovascular glaucoma
- Volumetric fluid analysis of fixed monthly anti-VEGF treatment in patients with neovascular age-related macular degeneration
- Time in range as a useful marker for evaluating retinal functional changes in diabetic retinopathy patients
