Preliminary proteomic analysis of human tears in lacrimal adenoid cystic carcinoma and pleomorphic adenoma
Han Yue, Feng-Xi Meng, Rui Zhang, Jiang Qian
1Department of Ophthalmology, Eye & ENT Hospital of Fudan University, Shanghai 200031, China
2Shanghai Key Laboratory of Visual Impairment and Restoration, Shanghai 200031, China
Abstract● AIM: To detect proteomic differences in tears between adenoid cystic carcinoma (ACC) and pleomorphic adenoma(PA).
● KEYWORDS: adenoid cystic carcinoma; pleomorphic adenoma; tear; proteome
INTRODUCTION
Lacrimal gland lesions are very common in orbital diseases, with an estimated incidence of one per million population per year[1].Diseases of the lacrimal gland can be divided into epithelial (20%-45%) and nonepithelial (55%-80%) lesions[1].Among the former, adenoid cystic carcinoma(ACC) and pleomorphic adenoma (PA) are the most common malignant and benign epithelial lesions.Compared with PA,ACC has a more acute onset, with pain and numbness, and imaging shows more bone invasions and a tendency to extend to the orbital apex[1].Thus, their treatments and outcomes are much different.PA is widely suggested to be excised intact without any incisional biopsy in case of a recurrence, which may undergo a malignant transformation at an incidence of 10% by 20y[1-2].For ACC, complete tumor excision and postoperative external beam radiation therapy (EBRT) are usually recommended; even so, the chances of surviving 10y after diagnosis are only 20%-30% due to distant metastases[3].Therefore, early diagnosis and treatment might lead to better prognosis[3].However, although both tumors have their own distinct features and completely different outcomes, and both share some similar clinical and imaging features, especially when they are in the early stages, such as swelling of the eyelid, proptosis in the medial inferior direction, reduced eye motility, round-to-ovoid supratemporal orbital mass in computed tomography (CT) scan, and some similar magnetic resonance imaging (MRI) features, which sometimes can cause confusion in diagnosis.Therefore, how to assess and distinguish these diseases at an early stage without premature incisional biopsy is of great importance.
Tears, which overlie the surface of the eye, are complex extracellular fluids containing many types of molecules,including proteins/peptides, lipids, small-molecule metabolites,and electrolytes.They are secreted from the main and accessory lacrimal glands, meibomian glands, goblet cells,and ocular surface epithelial cells[4-5].Thus, these components can both quantitatively and qualitatively reflect the unhealthy changes of the underlying tissues and are useful for the evaluation of many ocular diseases.As an easily accessible source for noninvasive investigation, tears have been widely used as a source for discovering biomarkers of diseases such as dye eye disease[6-7], herpes simplex keratitis[8], keratoconus[9],glaucoma[10], cataract[11], high myopia[12], diabetic retinopathy[13],meibomian gland dysfunction[14], thyroid eye disease[15-16], and lacrimal gland diseases[17].However, tear analysis of lacrimal gland diseases is still limited, especially for tumors such as ACC and PA.A related study tested leptin concentrations in tears and found it was greater in patients with ACC than in those with PA[17].Thus, in this study, we used a more extensive screening tool, label-free analysis using liquid chromatographytandem mass spectrometry (LC-MS/MS) together with parallel reaction monitoring (PRM), for validation to detect some proteomics differences in tears between ACC and PA and to try to further identify specific biomarkers for early and easy diagnosis in the future.
SUBJECTS AND METHODS
Ethical ApprovalInstitutional Review Board approval was given for this study, which adhered to the tenets of the Declaration of Helsinki.Written informed consent was obtained from each study participant.
PatientsFour patients with ACC and fvie with PA from the Eye and ENT Hospital of Fudan University were enrolled for analysis.PA recurrence with malignant transformation diagnosed by an ocular pathologist after resection and ACC with distant metastasis were excluded, and none of the ACC patients had previously received any radiotherapy or chemotherapy.Patients with other ocular diseases, such as dry eyes, keratitis,conjunctivitis, blepharitis, and lacrimal duct obstruction,were also excluded.As a normal control, 2 cases with orbital fracture and 2 with orbital schwannoma were also studied.
Tear CollectionAll study participants had tears collected in the afternoon in a quiet room with weak light.Tear samples from the lower fornices of the eyes were collected in capillary tubes and then expelled into Eppendorf tubes.Tears from lesional eyes in the ACC and PA groups were collected, and for the normal group, tears from normal eyes were gathered to eliminate the potential effects of the primary disease.The volume of each sample was usually 10 to 20 µL.Anesthesia was not used during the process.All the specimens were transported immediately and stored at -80℃ until processed.
Label-free Analysis of the Tear ProteomeThe three pooled tear specimens for label-free analysis were subjected to highabundance protein depletion.The most abundant proteins,such as albumin, immunoglobulin G (IgG), antitrypsin, andⅠgA, were removed using a Removal System Affinity Column(Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions.The proteins in the samples were precipitated with isopropanol and extracted using the SDT pyrolysis method [4% (w/v) sodium dodecyl sulfate (SDS),100 mmol/L Tris/HCl pH 7.6, 0.1 mol/L DTT].The samples were then quantified with the BCA Protein Assay Kit (Bio-Rad,USA).Approximately 300 μg of protein from each sample was digested by the Filer-aided proteome preparation method.The peptide fragments of each group were divided into 3 fractions using offline high-pH reversed-phase separation and freezedried after desalination on C18 cartridges.Reconstituted in 40 µL of 0.1% (v/v) formic acid, the peptide was estimated by ultraviolet (UV) light spectral density at 280 nm.
Each fraction was injected for LC-MS/MS analysis separately.The peptide mixture was loaded onto a reversed-phase trap column (Thermo Scientific Acclaim PepMap100, 100 μm ×2 cm,nanoViper C18) connecting the analytical column (Thermo Scientific Easy Column, 10 cm long, 75 μm inner diameter,3 μm resin, C18-A2) in buffer A (0.1% formic acid) and separated with a linear gradient of buffer B (84% acetonitrile)at a flow rate of 300 nL/min.
LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific) with Easy nLC (Thermo Fisher Scientific) for 120min.The mass spectrometer was operated in the positive ion mode.The survey scan for higher energy collisional dissociation (HCD) fragmentation was 300-1800 m/z.The automatic gain control (AGC) target was set to 1×106, the maximum injection time to 50ms, and the dynamic exclusion duration to 60s.Survey scans were acquired at a resolution of 70 000 at m/z 200, the resolution for HCD spectra was set to 17 500 at m/z 200, and the isolation width was 2 m/z.The normalized collision energy was 30 eV,and the underfill ratio was defined as 0.1%.The MS data were analyzed using MaxQuant software version 1.5.3.17, and labelfree quantitation (LFQ) was used for quantitative analysis.
Bioinformatics AnalysisGene Ontology (GO) annotation was performed using Blast2Go software.The process can be briefly divided into 4 steps: blast, mapping, annotation, and annotation augmentation.The sequences of differentially expressed proteins were blasted using Kyoto Encyclopedia of Genes and Genomes (KEGG) automatic annotation server software to retrieve their KOs and were subsequently mapped to pathways in the KEGG.To further explore the impact of differentially expressed proteins on different biological processes and discover internal relationships between differentially expressedproteins, enrichment analysis was performed.GO annotation and KEGG pathway enrichment analyses were performed based on Fisher’s exact test.Pathways with adjustedPvalues<0.05 were considered significant.

Table 2 Statistics of the quantitative results of proteins in each group
Targeted Protein Verification and Quantification byParallel Reaction MonitoringTo verify differential proteins obtained from global quantitative proteomics, we used targeted PRM to quantify selected proteins in the three groups.Each sample was analyzed separately by LC-PRM/MS.Briefly,peptides were prepared according to the label-free protocol,and a PRTC stable isotope peptide (20 fmol) was spiked in each sample (1.5 µg) as an internal reference standard.Peptide samples were separated using an HPLC system.Buffer A was 0.1% formic acid, and buffer B was 84% acetonitrile.Samples were added onto the chromatographic column balanced with 95% buffer A at a flow rate of 300 nL/min.The following gradient steps were performed: a linear ramp from 5%-10%buffer B in 2min; increase to 30% buffer B in 45min; 10min later increase to 100% buffer B and equilibrate in 60min.PRM analysis was performed on a Q Exactive HF mass spectrometer(Thermo Scientific).The mass spectrometer was operated in positive ion mode, and the analysis time was 1h.After the full MS1 scan, full MS2 scans were followed by 20 PRM.The scan was acquired with a resolution of 30 000 (at 200 m/z), ACG target values of 3.0×106, and 120ms maximum ion injection times.The targeted peptides were isolated with a 1.6Th window.Ion activation/dissociation was performed at a normalized collision energy of 27.The raw data of the three groups were analyzed using Skyline 3.5.0 (MacCoss Lab, University of Washington),where signal intensities for each peptide sequence for each protein were quantified relative to each sample and normalized to the standard reference.
RESULTS
Patient DataThe demographics and clinical characteristics of the patients are listed in Table 1.Of the 9 patients with PA and ACC, 3 were female, and 8 tumors were located in the right eye.The mean age was 43±14.2y, with a range from 24 to 67y.In the normal group, one was female, and the average age was 54±10y, with a range from 41 to 65y.
Statistics of Quantitative Protein Results by Label-free AnalysisLabel-free quantitative proteomics sequencing technology is the mass spectrometry analysis of protein enzymolysis peptides by liquid chromatography and mass spectrometry.By comparing the signal intensity of the corresponding peptide segments in different samples, the relative quantification of the protein corresponding to the peptide segment can be carried out.The advantages of labelfree lie in that proteins do not need expensive isotope labels for labeling, the total amount of samples required is small, and the coverage of low-abundance peptides is high.Thus, it is highly preferable for biomarker research, especially for the microsamples like tears.
By label-free analysis, we identified a total of 1059 proteins in tear samples.The proteins whose expression levels changed more than 2-fold (increase >2-fold or decrease <0.5-fold)and withPvalues <0.05 between groups were included in the subsequent bioinformatics analysis.A Venn diagram demonstrating proteome coverage in different groups is shown in Figure 1, and statistics of the quantitative results of proteins in each group are listed in Table 2.

Figure 1 Venn diagram demonstrating proteome coverage in different groups ACC: Adenoid cystic carcinoma; PA: Pleomorphic adenoma; N: Normal.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Pathway AnnotationTo better understand the biological functions of the differentially expressed proteins,both GO annotations and KEGG pathway analyses were performed.Based on the GO annotation between ACC and PA (Figure 2), in terms of the number of correlated proteins,the largest group within the biological process category was in response to nutrient levels.Enzyme regulator activity and serine-type endopeptidase inhibitor activity were predominant in the category of molecular function.The cellular components of these proteins mainly included blood microparticles and extracellular matrix.Based on the KEGG pathway annotation (Figure 3), the different proteins between ACC and PA mainly participated in complement and coagulation cascades, amoebiasis, African trypanosomiasis and cholesterol metabolism.
Selection and Verification of the Protein CandidatesTo reduce the scope of verification, we first listed all the differentially expressed proteins between the ACC and PA groups in the top 4 pathways withPvalues <0.05 based on the KEGG pathway annotation.Then, we chose the proteins with expression quantity changes greater than 10-fold (increase >10-fold or decrease <0.1-fold).Finally, we deleted the proteins whose expression levels were also close to those of the normal group.Thus, we chose 8 proteins for further verification,which are listed in Table 3.For confirmation purposes, the expression levels of eight proteins identified in the quantitative study were validated by PRM.PRM is a targeted proteomics technology based on high-resolution and high-precision mass spectrometry, which can selectively detect target proteins and peptide segments, thus achieving absolute quantification of target proteins/peptides.By PRM, all these candidates showed obvious quantitative differences, with at least a 3-fold increase in the ACC group compared with the PA group, and 5 proteins had more than a 10-fold increase, including B4E0R1 (integrin β), P01023 (α-2-macroglobulin), V9HVY1 (epididymal secretory sperm binding protein Li 78p), A0A024R1U4(RAB5C, member RAS oncogene family), and P01031(complement C5).Details are shown in Tables 3 and 4.

Figure 2 Top 20 GO categories assigned to the differentially expressed correlated proteins between the ACC and PA groups The correlated proteins are categorized based on GO annotation and the proportion of each category is displayed in the categories of BP,MF, and CC.GO: Gene Ontology; ACC: Adenoid cystic carcinoma;PA: Pleomorphic adenoma; BP: Biological process; MF: Molecular function; CC: Cellular component.

Figure 3 Top 20 KEGG pathways assigned to the differentially expressed correlated proteins between the ACC and PA groups The enriched KEGG pathways with a P value <0.05 between the two groups.KEGG: Kyoto Encyclopedia of Genes and Genomes; ACC:Adenoid cystic carcinoma; PA: Pleomorphic adenoma.
DISCUSSION
As the most common malignant and benign epithelial lesions of the lacrimal gland, ACC and PA have many similarities as well as differences in clinical features, imaging, surgical procedures and prognosis.PA with incomplete surgical resection carries a high risk of malignant transformation into various histologies of carcinoma ex PA (Ca-ex-PA) including adenocarcinoma, myoepithelial carcinoma, ACC, epithelialmyoepithelial carcinoma, squamous cell carcinoma, clear cell carcinoma and so on[18].Therefore, how to assess and distinguish these diseases at an early stage without premature incisional biopsy is of great importance.
Studies have identified differentiable markers to help in diagnosis.Using immunohistochemical methods, Zhang[18]found PA has a lower positivity of C-myc and Ki-67, while Ca-ex-PA had a higher positivity of these two biomarkers.Alsemariet al[19]tested the cell death protein 1 (PD-1) and DNA mismatch repair (MMR) expressions in PA and ACC,and found PD-L1 immunostaining was absent in 3/3 ACC and 5/6 PA specimens, and 1/3 ACC showed a pathogenic mutation in the MLH1 gene and 1/6 PA had a mutation in MSH6.Mendozaet al[20]found that Ki-67 and MYB were highly positive in ACC samples, while pleiomorphic adenoma gene 1 (PLAG1) was strongly positive in all PA samples by immunohistochemical staining.Another study using reverse transcription-polymerase chain reaction (RT-PCR) tested melanoma antigen gene (MAGE) expression and found it could serve as a biological marker in the differential diagnosis between PA and ACC, as it was strongly and widely expressed in ACC[21].For tear samples, Kimet al[17]used enzyme-linked immune-sorbent assay (ELISA) to test the leptin concentration in tears and found that it was greater in patients with ACC than in those with PA.Beuermanet al[22]used isobaric tags for relative and absolute quantification (iTRAQ) together with ELISA to quantitatively compare the tear proteomic profiles among malignant, benign and inflammatory lacrimal gland tumors and found that secreted proteins [LCN-1, LYZ, LFT,prolactin induced protein (PIP) and PRR4,etc.] were markedly reduced and matrix metalloprotein-9 (MMP-9) was elevated in malignant tumors compared with the two other groups.However, extensive tear analysis of lacrimal gland diseases is still limited, especially for tumors such as ACC and PA.
Ⅰn our study, we screened and verified eight proteins in tears that were quantitatively significantly different between ACC and PA.Among these candidates with relatively high protein ratios, integrin, α-2-macroglobulin, and RAB5C are more often reported in tumor-related studies based on a literature review and may serve as potential biomarkers for ACC (or PA), though more verification is needed in the future.1) Ⅰntegrin β (>23-fold) and integrin α (>8-fold) were obviously increased in ACC in our study.Integrins are a family of transmembrane receptors generally consisting of noncovalently linked α and β subunits.Integrins have a variety of functions and have recently been proven to be involved in many processes associated with tumor cell adhesion to the extracellular matrix, including migration,invasion, and metastasis[23].For example, integrin-β1, known as CD29, is found in many cancer types, may induce resistance to radiotherapies, chemotherapies, and targeted therapies and correlates with poor prognosis in several types of cancer[23].2) The expression of α-2-macroglobulin (α2M), which is a glycoprotein in blood that serves as a pan-proteinase inhibitor capable of inhibiting a large variety of proteinases, was also high in tears of the ACC group compared with PA (>10-fold)in our study.Ⅰts functions include inhibition of different types of nonspecific proteases and transport of cytokines, growth factors, and hormones and a pronounced immune-suppressive activity[24].One study showed that it has radioprotective effects and may contribute to antioxidant, antifibrosis, and antiinflammatory functions, maintenance of homeostasis, and enhancement of DNA repair and cell recovery processes[25].And the level of α2M was significantly changed in serum in several cancers[26-29], which may be a potential indicator of prognosis and diagnosis.3) In our study, RAB5C showed the highest increased expression (>30-fold) in ACC tears compared with PA tears.RAB5 molecules are members of the Rab family of small monomeric GTPases and participate in many biological processes, such as vesicle trafficking, cellular autophagy, mTOR signaling, cell migration, invasion, and motility[30].A study has shown that RAB5C is overexpressed in B acute lymphoblastic leukemia (B-ALL) and is important for B-ALL cell growth[30].It is also reported to have functions in regulating epidermal growth factor receptor (EGFR) and may be related to radio-resistance in rectal adenocarcinoma[31]and melanoma[32].However, all these potential candidates have been seldom reported in lacrimal diseases; thus, more samples and more specific experiments are needed to verify and determine their functional roles in these diseases.
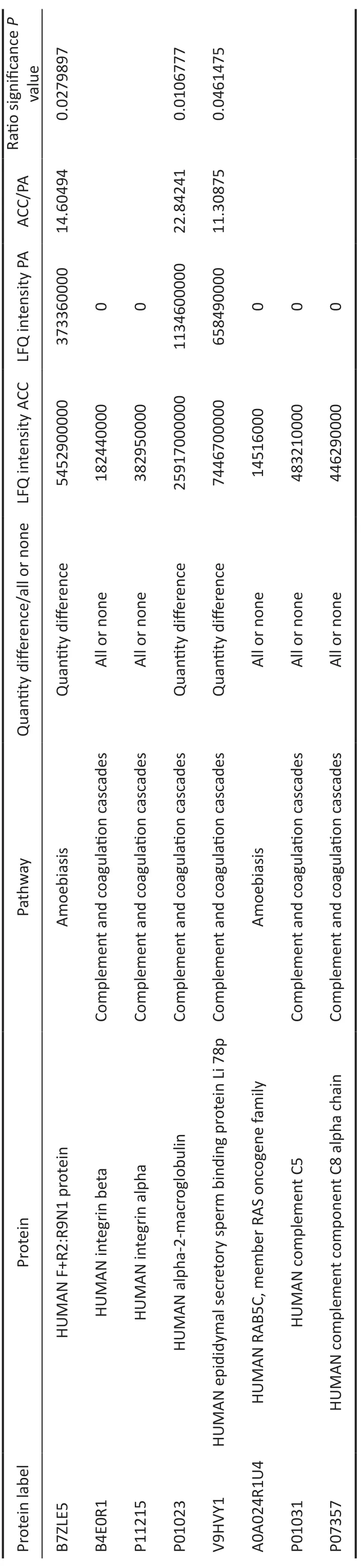
Table 3 List of the selected protein candidates for verification

Table 4 PRM verification results of the protein candidates
To screen and verify proteomics differences in tears between ACC and PA, we performed label-free quantitative mass spectrometry (LC-MS/MS) together with PRM.These combined tools are very effective and efficient, especially for samples such as tears.Recently, some new proteomics techniques have emerged in body fluid biopsy, including ultrasensitive ELISA[33], meso scale discovery (SMD)[34],Luminex liquid chip[35], and cytometric bead array[36].However,ITRAQ and label-free methods are becoming the most popular methods for quantitative proteomics.Both are very sensitive and extensive and thus are quite suitable to detect tear proteins[37-39].Compared with ITRAQ, label-free methods have some advantages.First, a label-free method needs less sample volume since there is no need for labeling.Second, it is able to detect very-low-abundance proteins, which may be ignored by ITRAQ.Third, its sample size is not limited, while ITRAQ can only detect 8 samples simultaneously.Finally, labelfree methods can also differentiate the none-or-all proteins in different samples, while ITRAQ can only illustrate quantity differences between samples, which may miss some unique proteins.Thus, in our study, we chose a label-free method to screen the different proteins.For verification, we chose PRM,which is a highly specific and selective method for targeted quantification and has become an alternative method of protein candidate verification[40].Compared with the protein detection gold standard Western blot, this assay can detect many proteins simultaneously using a very small amount of sample; thus, it is especially suitable when there are many candidates of interest to be tested in limited samples.
There are some limitations of this study: 1) Although several potential key biomarkers were identified between PA and ACC,the sample size of our study is still limited and the functions of these biomarkers were not certain.More studies are planned to verify the candidates in tear samples, blood serum and tumor tissues with a larger sample size, and to determine the underlying functions and roles of the markers in the future.2) Although ACC and PA are the most common malignant and benign epithelial lesions of the lacrimal gland, there are much more kinds of lacrimal gland diseases that also need careful identification.Thus, other future research directions for lacrimal gland diseases using tears can be extended to some systemic diseases, such as IgG4-related disease, Sjögren’s syndrome, and anti-neutrophil cytoplasmic antibody (ANCA)-associated small vessel vasculitis (Wegener’s granulomatosis),and to differential malignant tumors, such as ACC, lymphoma,adenocarcinoma, and mucoepidermoid carcinoma.
Our study has the following strengths: 1) We used very extensive proteomics test tools, label-free analysis and parallel reaction, to screen and validate the tear proteome of ACC and PA.These combined tools with high throughput are very effective and efficient, especially feasible for samples such as tears.2) We have detected several potential key biomarkers between PA and ACC, which have played crucial roles in tumor invasion, immune-suppressive activity, prognosis, radioresistance, and so on.Hopefully, the data from tears could provide more clues for the diagnosis, and treatment of these diseases shortly soon.
In summary, we used label-free analysis together with PRM to screen and verify some proteomics differences in tears between ACC and PA and identified some specific biomarker candidates for future exploration.
ACKNOWLEDGEMENTS
Authors’contributions:Manuscript writing, data collection and analysis: Yue H, Meng FX; data sources: Zhang R, Qian J;design and supervision: Qian J.
Conflicts of Interest:Yue H, None; Meng FX, None; Zhang R, None; Qian J, None.
 International Journal of Ophthalmology2023年6期
International Journal of Ophthalmology2023年6期
- International Journal of Ophthalmology的其它文章
- Assessment of the effects of intrastromal injection of adipose-derived stem cells in keratoconus patients
- Evaluation of optic nerve head vessels density changes after phacoemulsification cataract surgery using optical coherence tomography angiography
- Stability of neodymium:YAG laser posterior capsulotomy in eyes with capsular tension rings
- Comparison of the efficacy and safety of ultrasonic cycloplasty vs valve implantation and anti-VEGF for the treatment of fundus disease-related neovascular glaucoma
- Volumetric fluid analysis of fixed monthly anti-VEGF treatment in patients with neovascular age-related macular degeneration
- Time in range as a useful marker for evaluating retinal functional changes in diabetic retinopathy patients
