Efficacy of ripasudil in reducing intraocular pressure and medication score for ocular hypertension with inflammation and corticosteroid
Ryoji Yanai, Sho-Hei Uchi, Masaaki Kobayashi, Tomohiko Nagai, Shinichiro Teranishi,Makiko Wakuta, Kazuhiro Kimura
Department of Ophthalmology, Yamaguchi University Graduate School of Medicine, Yamaguchi 755-8505, Japan
Abstract● AIM: To investigate the efficacy of ripasudil, a Rho kinase inhibitor, in reducing intraocular pressure (IOP) and medication scores of anti-glaucoma drugs in patients with ocular hypertension with inflammation and corticosteroid.
● KEYWORDS: intraocular pressure; medication score;uveitis; glaucoma; Rho kinase inhibitor
INTRODUCTION
A Rho-associated protein kinase (ROCK) inhibitor,ripasudil hydrochloride hydrate (Ripasudil, Kowa Pharmaceuticals Ltd., Nagoya, Japan), is an eye drop used for the treatment of glaucoma or ocular hypertension (OHT)[1-2].Given that ripasudil is a second-line drug in Japan, it tends to be prescribed to patients who have previously used glaucoma eye drops, such as prostaglandin analogs, alpha-2 agonists,and beta-blockers.Recently, ripasudil has shown a significant intraocular pressure (IOP)-reducing effect in cases of OHT with inflammation and corticosteroid[3-7].
OHT with inflammation and corticosteroids is one of the most severe complications of uveitis and one of the major complications related to treatment with corticosteroids for ocular inflammatory diseases.The prevalence of OHT in patients with ocular inflammation has been reported to range from 8.8% to 41.8%[8].Moreover, a recent report revealed that the progression from OHT to secondary glaucoma is relatively swift, and the aggressive presentation of secondary glaucoma requires surgical management in most cases[9-10].Medical management of OHT with inflammation is challenging because there are limitations to the use of alpha-2 agonists and betablockers when managing glaucoma patients with arrhythmia.Similarly, prostaglandin analogs for the treatment of OHT with inflammation must be used with caution as these have been shown to increase outflow resistance by decreasing the outflow facility of the trabecular meshwork (TM)[11].
This study investigated the efficacy of ripasudil in reducing ⅠOP and medication scores of anti-glaucoma drugs in patients with OHT with inflammation and corticosteroid.We also evaluated whether ripasudil contributes to the anti-inflammatory effect of corticosteroids (topical and systemic) and immunomodulatory therapy (ⅠMT) for ocular inflammation.Furthermore, we found that herpetic simplex iridocyclitis and cytomegalovirus iritis included the high-risk types of uveitis for glaucoma surgery described above; we did not think all kinds of infectious were resistant to the ROCK inhibitor’s ⅠOP-lowering effect.
SUBJECTS AND METHODS
Ethical ApprovalThe Ethics Committee approved this retrospective study and the Institutional Review Board of Yamaguchi University Hospital (Management No.H30-164).This study was performed in compliance with the provisions of the Declaration of Helsinki.The patients consented to the publication of their cases in writing.
PatientsThe study included 11 patients (15 eyes) diagnosed with OHT with inflammation and corticosteroid, all of whom were prescribed ripasudil eye drops (Kowa Pharmaceuticals,Nagoya, Japan) from May 2016 to April 2019 and followed up at Yamaguchi University Hospital for at least 2y.One drop of ripasudil was administered twice daily.The exclusion criteria were as follows: 1) lack of follow-up within 2y after initiating treatment; 2) glaucoma surgery at any time prior to initiating treatment; 3) non-glaucoma-related eye surgery (e.g.,cataract surgery, pars plana vitrectomy) more than 6mo prior to initiating treatment; 4) prescription of ripasudil eye drops by another clinic.
Clinical EvaluationⅠn this study, OHT was defined as an ⅠOP of ≥21 mm Hg induced by ocular inflammation and the use of corticosteroid to treat ocular inflammation; steroid glaucoma,irrespective of a glaucomatous optic disc change.This study collected data on age, sex, etiology, follow-up period, bestcorrected visual acuity (BCVA), location of uveitis, the status of anterior chamber angle and lens, visual field mean deviation(MD) measured by the Humphrey Field Analyzer (program SITA standard 30-2; Carl Zeiss Meditec, Dublin, CA, USA),glaucomatous optic disc change, previous medical treatments for uveitis and glaucoma, and ophthalmic surgeries.The intraocular inflammatory disease was evaluated along with the guidelines of the Standardization of Uveitis Nomenclature Working Group[12].Causes of ocular inflammation, including uveitis, were diagnosed from ophthalmic findings and medical history and classified based on the latest recommendations from the International Uveitis Study Group[13].
Intraocular Pressure MeasurementsIOP was measured three times using a non-contact tonometer (NT-500; Tomey,Kamagori, Japan) prior to enrollment and at each follow-up visit, and the mean of the three measurements was used for analysis.Central corneal thickness (CCT) was measured using non-contact specular microscopy (CEM-530; Nidek Co., Ltd.,Tokyo, Japan).The CCT was measured 3 times for each eye,and the average was noted.

Table 1 Demographic characteristics of 11 patients (15 eyes)
Medication ScoresThe medication scores for each patient were determined using the category previously reported:1 point for a single type of eye drop, 2 points for a fixed combination of eye drops, and 2 points for an oral medication with acetazolamide[14].
Statistical AnalysisQuantitative data are presented as mean±standard deviation (SD).Pre-treatment and posttreatment data were compared using a one-way analysis of variance and Kruskal-Wallis multiple-comparison tests.Data from the medication and glaucoma surgery groups were compared using the Chi-square test or Mann-WhitneyUtest.Statistical analyses were performed using Prism version 8 for MAC (GraphPad Software, San Diego, CA, USA), and statistical significance was set atP<0.05.
RESULTS
Patients’Demographics and DiagnosesThe detailed demographic information is provided in Table 1.
All 15 studied eyes showed the open-angle of the anterior chamber, and the status of the lens was phakic in 13 eyes(87%) and pseudophakic in two eyes (13%).Ten studies’eyes(66.7%) have already been treated with glaucoma eye drops,and five studies’eyes (33.3%) included ripasudil as the firstline drug for OHT.Non-infectious uveitis [13 eyes (87%)]was more common than infectious uveitis [2 eyes (13%)].Infectious uveitis was of viral etiology in the two cases(cytomegalovirus iritis and herpetic simplex iridocyclitis).Of the 15 eyes, four (26%) eyes showed anterior uveitis, and 11(74%) eyes showed pan-uveitis.
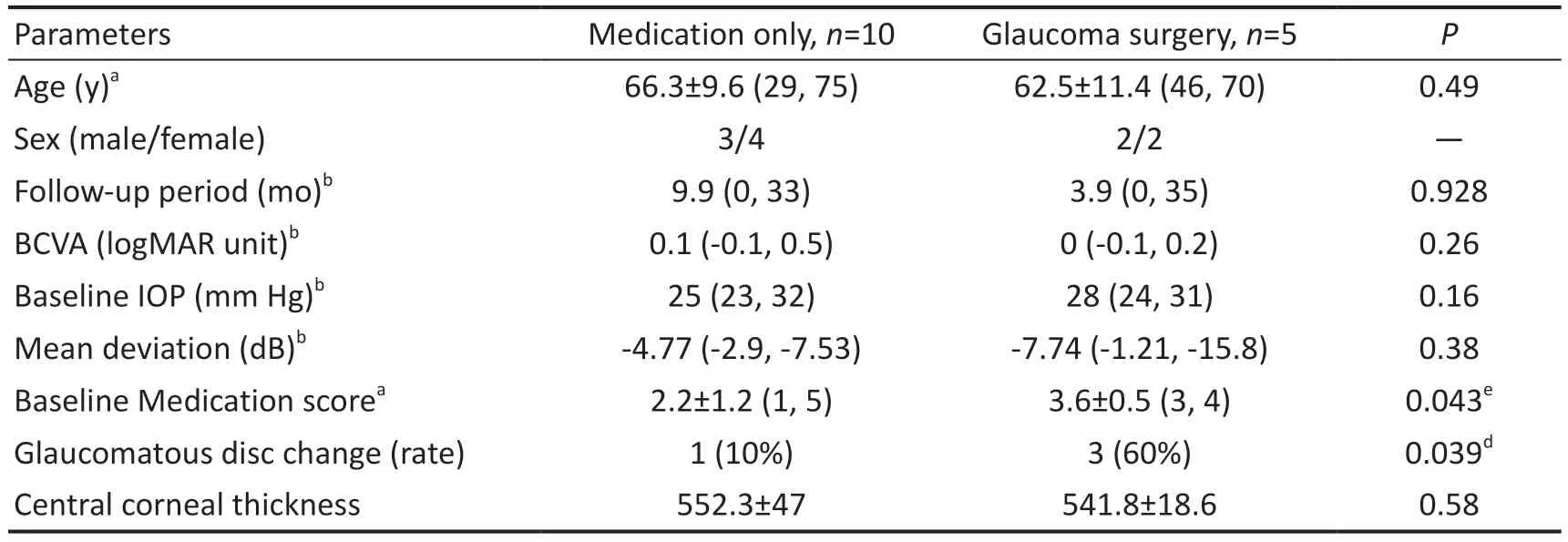
Table 2 Demographic characteristics
None of the studied eyes showed any ocular inflammation at the baseline evaluation, such as anterior chamber cells, flare,and vitreal haze.All these kinds of inflammation were absent.At that point, topical steroids were provided for seven eyes(46.7%), systemic steroids for three eyes (20%), and IMTs were provided for three eyes (20%) for control of ocular inflammation (Figure 1).
Intraocular PressureAfter ripasudil administration, the mean ⅠOP decreased significantly (26.4±2.9 to 13.7±3.3 mm Hg at 3mo) and remained in the low-teens for 2y (Figure 2A).
However, five eyes failed to respond to ripasudil treatment and required glaucoma surgery: three eyes in the first 3mo, one eye at 16mo, and one at 24mo after initiating treatment (Figure 2B).To clarify the characteristics of OHT requiring glaucoma surgery after ripasudil treatment (glaucoma surgery group) or not(medication group), we compared age, sex, follow-up period,BCVA, baseline IOP, MD, glaucomatous optic disc change,CCT and causes of uveitis (Table 2 and Figure 3).
Medication ScoreWe found that both baseline medication scores and glaucomatous optic disc change rates were significantly higher in the glaucoma surgery group than in the medication group.Finally, we evaluated the effect of ripasudil on glaucoma therapy for OHT.The number of medications for glaucoma (medication score) significantly reduced after 12mo of ripasudil treatment initiation (Figure 4).
Furthermore, anti-glaucoma medication was discontinued after 24mo in 80% of glaucoma surgery-free eyes (8 of 10 eyes).These results suggest that ripasudil therapy exerts an IOPlowering effect and maintains normal IOP after reducing or discontinuing anti-glaucoma medication.

Figure 1 Effects of ripasudil on anti-inflammatory therapy for uveitis control Data are shown excluding the five eyes that required glaucoma surgery.IMT: Immunomodulatory therapy.

Figure 2 Time course of intraocular pressure (IOP) after administration of ripasudil in all patients A: Mean IOP±standard deviation is shown without dropouts who required glaucoma surgery; B: Rate of glaucoma surgery-free patients with uveitic glaucoma after ripasudil treatment.IOP changes from the baseline were analyzed using a one-way analysis of variance and Kruskal-Wallis multiple-comparison tests.cP<0.0001.
DISCUSSION
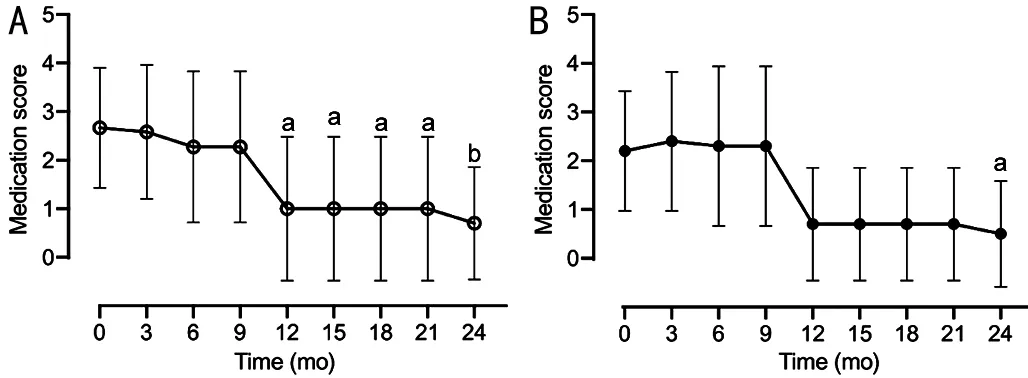
Figure 4 Time-dependent changes in medication score over 2y of follow-up after administration of ripasudil Mean medication scores±standard deviation are shown as maximum amount of data without dropouts or missing data for all eyes (A) or eyes with medication group excluding the five eyes that required glaucoma surgery (B).Changes of medication scores from the baseline was analyzed using one-way analysis of variance and Kruskal-Wallis multiple-comparison tests.aP<0.05, bP<0.01.
The present study showed that administration of ripasudil in patients with OHT with ocular inflammation significantly decreased IOP and maintained a low-teen 2-year period.Ripasudil significantly reduced the medication score of OHT patients after 12mo or more of ripasudil treatment.These findings suggest that ripasudil has an IOP-reducing effect and aids in recovery from OHT with ocular inflammation.Previous studies have substantiated the IOP-lowering effect of ripasudil[3-4]for ocular inflammatory diseases, including uveitis.To the best of our knowledge, this study is the first to report a ripasudil decrease in the medication score of OHT with inflammation.
Our findings are in accordance with previous studies suggesting a significant IOP-reducing effect of ripasudil in cases of OHT with inflammation[3-6].Futakuchiet al[6]highlighted the ⅠOP-lowering and anti-inflammatory effects of ripasudil in patients with uveitic glaucoma.The major route of aqueous humor outflow in primates is the conventional pathway consisting of the TM and Schlemm’s canal[15].In addition to healthy eyes, those with glaucoma also generate outflow resistance of the aqueous humor in the juxtacanalicular region[16].The structural characterizations of the cytoskeleton,adhesive interactions, Schlemm’s canal epithelial cell permeability, and TM cell secretion contributed to aqueous humor outflow[17-18].ROCK mediates the effects of RhoA on the actin cytoskeleton[19], with ROCK activation promoting the assembly of actin stress fiber, formation of focal adhesion proteins, and contraction in fibroblasts[20].Thus, ripasudil or ROCK inhibitors might enhance the paracellular outflow of aqueous humor by inhibiting contraction in TM fibroblasts,actin stress fiber assembly, and focal adhesion formation.
Recently, Yasudaet al[3]reported that ripasudil effectively decreased IOP in patients with OHT associated with inflammation and corticosteroid use, acting synergistically with uveitis treatment to reduce ocular inflammation.Our findings, which indicate the possible contributory anti-inflammatory effect of ripasudil in treating OHT with inflammation, may help explain the previous study’s findings.Yamadaet al[7]reported that the use of ripasudil is associated with the reorganization of the bloodaqueous barrier in anterior uveitis in humans andin vitro.
On the other hand, the effect of ripasudil for the ⅠOP reducing in OHT with ocular inflammation has some limitation.There were five eyes that required glaucoma surgery in this study: three eyes required surgery at 3-month intervals, one eye each at 15-month and 24-month intervals (Table 2).By comparing five eyes requiring glaucoma surgery with 10 eyes requiring ripasudil therapy, we identified that the risk factors for glaucoma surgery were both baseline medication scores and glaucomatous optic disc changes at the time of ripasudil administration.These risk factors will serve as targets for the treatment regimens of OHT with inflammation currently under development.
The present study has some limitations, including the small number of patients, the non-identified infectious or noninfectious uveitis, the single-center study design, and the absence of randomization.We also did not investigate the antiinflammatory effect of ripasudil in this study.The mechanism of action of ripasudil differs between eyes with infectious and non-infectious uveitis.Unfortunately, direct evidence of ROCK inhibitor in ocular hypertension with inflammation was not determined.However, several ROCK inhibitors did show suppression of infectious inflammation in macrophages[21],pneumonia[22], and myocarditis[23].These reports suggest that ROCK inhibitor has an efficacy in uveitic glaucoma to suppress some kind of infectious inflammation; therefore,we think that it is connected to IOP lowering effect.Further research with a larger study sample is required to clarify the differences in the efficacy and safety of ripasudil in eyes with infectious and non-infectious uveitis.
In conclusion, the ROCK inhibitor, ripasudil, reduces IOP in patients with uveitic glaucoma (UG).Our study demonstrated that ripasudil significantly reduced IOP and the medication score of glaucoma patients with UG.UG patients with higher medication scores and glaucomatous optic disc changes may require glaucoma surgery, regardless of the addition of ripasudil.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Authors’contributions:Yanai R and Uchi SH conceived and designed the study.Yanai R, Uchi SH, Kobayashi M,Nagai T, and Teranishi S contributed to the data acquisition.Wakuta M and Kimura K provided critical advice for revising the manuscript.All authors contributed to data interpretation and approved the final version for publication.Yanai R is the voucher of the current study, has full access to all data,and takes responsibility for the integrity of the datasets and accuracy of the analysis in the study.All authors reviewed the manuscript.
Conflicts of Interest:Yanai R, None; Uchi SH, None;Kobayashi M, None; Nagai T, None; Teranishi S, None;Wakuta M, None; Kimura K, None.
 International Journal of Ophthalmology2023年6期
International Journal of Ophthalmology2023年6期
- International Journal of Ophthalmology的其它文章
- Preliminary proteomic analysis of human tears in lacrimal adenoid cystic carcinoma and pleomorphic adenoma
- Assessment of the effects of intrastromal injection of adipose-derived stem cells in keratoconus patients
- Evaluation of optic nerve head vessels density changes after phacoemulsification cataract surgery using optical coherence tomography angiography
- Stability of neodymium:YAG laser posterior capsulotomy in eyes with capsular tension rings
- Comparison of the efficacy and safety of ultrasonic cycloplasty vs valve implantation and anti-VEGF for the treatment of fundus disease-related neovascular glaucoma
- Volumetric fluid analysis of fixed monthly anti-VEGF treatment in patients with neovascular age-related macular degeneration
