CuI催化的A3-偶联反应合成邻羟基苯基丙炔胺类化合物的研究
樊陈莉 谢吉娜 惠文杰 陈浩宇 何心伟


摘要:丙炔胺類化合物因其具有多个反应位点而在有机合成中具有广泛的应用。本文发展了一种CuI催化的水杨醛、四氢吡咯和末端炔的A3-偶联反应合成邻羟基苯基丙炔胺衍生物的方法。水杨醛和芳基乙炔中无论是吸电子基团还是供电子基团均能较好的适用于该反应,且水杨醛中含有多个取代基时也能得到相应的目标产物,并利用1H,13C NMR和HRMS等手段对目标化合物的结构进行了表征。该合成方法具有操作简单、合成效率高和取代基容忍性好等优点,为邻羟基苯基丙炔胺类化合物的合成提供了一种新的合成思路。
关键词:A3-偶联反应;丙炔胺;水杨醛;苯乙炔
中图分类号:O626文献标志码: A文章编号:1001-2443(2023)02-0138-08
丙炔胺是一类具有广泛用途的含氮化合物,近年来在药理和药物化学领域的应用日益广泛[1]。优降宁[2]、雷沙吉兰[3]、司来吉兰[4]等丙炔胺衍生物被发现在治疗帕金森症和阿尔兹海默症等精神类疾病中具有很好的效果。此外,丙炔胺类化合物具有多个反应位点,早期通过金属催化和环加成反应等用于合成脂肪族或芳香族杂环化合物,如吡咯、咪唑、吡唑、喹啉等重要含氮杂环化合物[5-6]。近期,丙炔胺类化合物的多样性化学转化在天然产物和功能材料分子合成中也得到了广泛应用[7-8]。因此,其合成方法也备受关注。结构简单的丙炔胺化合物可以通过炔基卤化物或炔酸酯类化合物的氨基化反应得到[9-11]。此外,醛或酮的还原氨化也成为构建丙炔胺骨架常用且高效的合成方法[12]。过去十年,过渡金属催化的末端炔类化合物对亚胺的加成反应已成为合成丙炔胺的一种较流行的方法[13]。但是,这些方法大多存在反应条件苛刻、反应时间长或使用当量金属催化剂等缺陷,因此,需要发展简单、高效的合成丙炔胺类化合物的方法。
醛或酮与氨和炔的三组分偶联反应称之为A3-偶联反应,已成为近年来直接构建丙炔胺类化合物的通用方法[14-15],该方法通过使用催化量的金属催化剂(如Cu, Ag, Au, In等)在温和实验条件下实现丙炔胺类化合物的高效合成[16-20]。此外,手性催化剂调控的不对成A3-偶联反应[21]及金属催化的串联脱羧A3-偶联反应[22-23]被广泛应用于合成手性丙炔胺类化合物和非对称丙炔胺或3-氨基-1,4-烯炔化合物等。在绿色化学理念指导下,各种负载的铜催化剂被制备并用于无溶剂下的A3-偶联反应,这种催化剂具有较好的可回收性,但其催化剂回收过程的成本仍然很高[24-28]。因此,有必要寻找一种简单有效的合成丙炔胺衍生物的方法。本文以廉价易得的CuI为催化剂,利用水杨醛、四氢吡咯和末端炔的A3-偶联反应实现一种结构新颖的邻羟基苯基丙炔胺类化合物的克级规模合成,为丙炔胺类化合物在有机合成中的应用提供了依据。设计合成路线如图1所示。
1 实验部分
1.1 实验试剂和仪器
BRUKER-AV-500型核磁共振仪(500 MHz,CDCl3为溶剂,TMS为内标) ; X-4 数字显示显微熔点测定仪(北京泰克仪器有限公司,温度计未经校正)。
实验所用溶剂和试剂均为分析纯(可直接使用)。
1.2 丙炔胺衍生物的合成步骤
在装有磁性搅拌棒的25 mL圆底烧瓶中加入胺(6.5 mmol)、醛(5.0 mmol)、乙炔(6.5 mmol)、碘化亚铜(I) (20 mol%)和甲苯(10 mL)。将混合物脱气并回填氮气,然后在预热至80 °C的油浴中搅拌8小时(TLC监测)。反应完成后(薄层色谱法测定),将反应混合物冷却至室温,用CH2Cl2(10 mL)稀释,通过硅胶薄层过滤。用CH2Cl2洗涤滤饼,将组合滤液在真空中浓缩。采用硅胶闪柱色谱法对粗品进行纯化,得到相应的丙炔胺3a-3u。
1.3 产物表征数据
2-(3-Phenyl-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3a): White solid (85%, 1.18 g); mp = 80~81 °C,
1H NMR (500 MHz, CDCl3) δ 7.55~7.52 (m, 3H), 7.37~7.35 (m, 3H), 7.23 (t, J = 7.5 Hz, 1H), 6.87~6.84 (m, 2H), 5.29 (s, 1H), 2.92~2.87 (m, 2H), 2.83~2.79 (m, 2H), 1.91~1.85 (m, 4H);13C NMR (125 MHz, CDCl3) δ 157.6, 131.9, 129.3, 128.6, 128.4, 127.8, 122.5, 122.1, 118.9, 116.3, 89.0, 82.9, 57.0, 48.9, 23.8; HRMS (APCI) m/z: calcd for C19H19NO [M + H]+278.1539, found 278.1538.
2-(1-(Pyrrolidin-1-yl)-3-(p-tolyl)prop-2-yn-1-yl)phenol (3b): White solid (87%, 1.27 g); mp = 61~62 ℃,
1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 1H), 7.43 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 7.5 Hz, 1H), 7.17 (d, J = 8.0 Hz, 2H), 6.86~6.83 (m, 2H), 5.28 (s,1H), 2.88 (s, 2H), 2.81~2.79 (m, 2H), 2.37 (s, 3H), 1.87 (s, 4H);13C NMR (125 MHz, CDCl3) δ 157.5, 138.7, 131.8, 129.3, 129.1, 127.9, 122.2, 119.4, 119.0, 116.3, 89.2, 82.1, 56.9, 48.9, 23.8, 21.5; HRMS (APCI) m/z: calcd for C20H21NO [M + H]+292.1695, found 292.1694.
2-(3-(4-Chlorophenyl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3c): White solid (80%, 1.24 g); mp = 69~70 °C,1H NMR (500 MHz, CDCl3) δ 7.49 (d, J = 7.5 Hz,1H), 7.45 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 7.22 (t, J = 7.5 Hz,1H), 6.86~6.83 (m, 2H), 5.27 (s,1H), 2.87 (s, 2H), 2.81~2.79 (m, 2H), 1.88 (s, 4H);13C NMR (125 MHz, CDCl3) δ 157.5, 134.8, 133.8, 133.2, 129.6, 129.0, 128.8, 127.9, 122.0, 119.2, 116.6, 88.0, 84.1, 57.1, 49.2, 23.9; HRMS (APCI) m/z: calcd for C19H18ClNO [M + H]+312.1149, found 312.1147.
4-Methyl-2-(3-phenyl-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3d): Yellow oil (82%, 1.19 g);1H NMR (500 MHz, CDCl3) δ 7.54~7.52 (m, 2H), 7.37~7.35 (m, 3H), 7.31 (s, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.76 (d, J = 8.0 Hz, 1H), 5.23 (s, 1H), 2.89~2.85 (m, 2H), 2.82~2.77 (m, 2H), 2.28 (s, 3H), 1.88~1.85 (m, 4H);13C NMR (125 MHz, CDCl3) δ 155.1, 131.9, 129.7, 128.5, 128.4, 128.3, 128.0, 122.6, 121.8, 116.0, 88.8, 83.1, 57.0, 48.9, 23.8, 20.7; HRMS (APCI) m/z: calcd for C20H21NO [M + H]+292.1695, found 292.1696.
4-Methyl-2-(1-(pyrrolidin-1-yl)-3-(p-tolyl)prop-2-yn-1-yl)phenol (3e): Yellow solid (89%, 1.36 g); mp = 67~68 °C,1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 8.0 Hz, 2H), 7.31 (s, 1H), 7.17 (d, J = 8.0 Hz, 2H), 7.02 (d, J = 8.0 Hz, 1H), 6.75 (d, J = 8.0 Hz, 1H), 5.23 (s, 1H), 2.89~2.85 (m, 2H), 2.81~2.77 (m, 2H), 2.38 (s, 3H), 2.28 (s, 3H), 1.88~1.85 (m, 4H);13C NMR (125 MHz, CDCl3) δ 155.1, 138.7, 131.8, 129.7, 129.1, 128.3, 128.0, 121.9, 119.5, 116.0, 89.0, 82.3, 57.0, 48.9, 23.8, 21.5, 20.7; HRMS (APCI) m/z: calcd for C21H23NO [M + H]+306.1852, found 306.1850.
2-(3-(4-Chlorophenyl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)-4-methylphenol (3f): Yellow solid (86%, 1.40 g); mp = 79~80 °C,1H NMR (500 MHz, CDCl3) δ 7.46 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.03 (d, J = 8.0 Hz, 1H), 6.76 (d, J = 8.0 Hz, 1H), 5.20 (s, 1H), 2.84 (s, 2H), 2.79~2.77 (m, 2H), 2.28 (s, 3H), 1.87 (s, 4H);13C NMR (125 MHz, CDCl3) δ 155.0, 134.6, 133.1, 129.8, 128.7, 128.2, 128.1, 121.6, 121.0, 116.1, 87.7, 84.3, 57.1, 49.1, 23.8, 20.7; HRMS (APCI) m/z: calcd for C20H20ClNO [M + H]+326.1306, found 326.1307.
4-Chloro-2-(3-phenyl-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3g): Yellow solid (75%, 1.17 g); mp = 61~62 °C,1H NMR (500 MHz, CDCl3) δ 7.54~7.52 (m, 2H), 7.50 (d, J = 7.5 Hz, 1H), 7.38~7.35 (m, 3H), 7.17 (d, J = 8.5 Hz, 1H), 6.78 (d, J = 8.5 Hz, 1H), 5.24 (s, 1H), 2.88 (s, 2H), 2.80~2.76 (m, 2H), 1.89~1.86 (m, 4H);13C NMR (125 MHz, CDCl3) δ 156.3, 131.9, 129.1, 128.8, 128.4, 127.7, 123.6, 122.2, 117.5, 89.5, 82.0, 56.7, 48.9, 23.8; HRMS (APCI) m/z: calcd for C19H18ClNO [M + H]+312.1149, found 312.1146.
4-Chloro-2-(1-(pyrrolidin-1-yl)-3-(p-tolyl)prop-2-yn-1-yl)phenol (3h): White solid (88%, 1.43 g); mp = 75~76 °C,1H NMR (500 MHz, CDCl3) δ 7.50 (s, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.18~7.15 (m, 3H), 6.78 (d, J = 8.5 Hz, 1H), 5.24 (s, 1H), 2.88 (s, 2H), 2.79~2.78 (m, 2H), 2.38 (s, 3H), 1.87 (s, 4H);13C NMR (125 MHz, CDCl3) δ 156.2, 139.0, 131.8, 129.2, 129.1, 127.8, 123.7, 123.6, 119.1, 117.6, 89.7, 81.1, 56.6, 48.9, 23.8, 21.5; HRMS (APCI) m/z: calcd for C20H20ClNO [M + H]+326.1306, found 326.1303.
4-Chloro-2-(3-(4-chlorophenyl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3i): Yellow solid (80%, 1.38 g); mp = 94~95 °C,1H NMR (500 MHz, CDCl3) δ 7.46 (d, J = 8.5 Hz, 3H), 7.34 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.5 Hz, 1H), 6.78 (d, J = 8.5 Hz, 1H), 5.21 (s, 1H), 2.85 (s, 2H), 2.78~2.76 (m, 2H), 1.88 (s, 4H);13C NMR (125 MHz, CDCl3) δ 156.2, 134.9, 133.7, 133.2, 128.9, 128.8, 127.6, 123.6, 123.4, 120.6, 117.7, 88.4, 83.1, 56.7, 49.0, 23.8; HRMS (APCI) m/z: calcd for C19H17Cl2NO [M + H]+346.0760, found 346.0757.
2-Bromo-6-(1-(pyrrolidin-1-yl)-3-(p-tolyl)prop-2-yn-1-yl)phenol (3j): Yellow solid (82%, 1.51 g); mp = 86~87 °C,1H NMR (500 MHz, CDCl3) δ 7.49~7.45 (m, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 7.5 Hz, 2H), 6.72 (t, J = 7.5 Hz, 1H), 5.28 (s, 1H), 2.92~2.90 (m, 2H), 2.81~-2.80 (m, 2H), 2.37 (s, 3H), 1.88 (s, 4H);13C NMR (125 MHz, CDCl3) δ 154.6, 139.0, 132.7, 131.8, 129.2, 127.1, 123.3, 119.6, 119.1, 110.3, 89.7, 81.2, 57.1, 48.8, 23.8, 21.6; HRMS (APCI) m/z: calcd for C20H20BrNO [M + H]+370.0801, found 370.0799.
2-Bromo-4-chloro-6-(3-(4-methoxyphenyl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3k): Yellow solid (75%, 1.58 g); mp = 86~87 °C,1H NMR (500 MHz, CDCl3) δ 7.47~7.45 (m, 4H), 6.89 (d, J = 8.5 Hz, 2H), 5.24 (s, 1H), 3.83 (s, 3H), 2.91~2.90 (m, 2H), 2.80~2.78 (m, 2H), 1.90~1.88 (m, 4H);13C NMR (125 MHz, CDCl3) δ 160.1, 153.5, 133.4, 131.9, 127.1, 124.2, 123.5, 114.1, 113.8, 110.6, 90.0, 79.6, 56.9, 55.3, 48.8, 23.8; HRMS (APCI) m/z: calcd for C20H19BrClNO2[M + H]+422.0339, found 422.0333.
2,4-Di-tert-butyl-6-(1-(pyrrolidin-1-yl)-3-(p-tolyl)prop-2-yn-1-yl)phenol (3l): Yellow solid (70%, 1.41 g); mp = 74~75 °C,1H NMR (500 MHz, CDCl3) δ 7.51 (s, 1H), 7.43 (d, J = 6.5 Hz, 2H), 7.27 (s, 1H), 7.18 (d, J = 8.0 Hz, 2H), 5.26 (s, 1H), 2.88 (s, 2H), 2.80 (s, 2H), 2.38 (s, 3H), 1.87 (s, 4H), 1.45 (s, 9H), 1.33 (s, 9H);13C NMR (125 MHz, CDCl3) δ 154.0, 140.0, 138.5, 135.4, 131.7, 129.1, 123.3, 122.7, 121.4, 119.8, 89.0, 82.9, 57.4, 48.7, 34.9, 34.3, 31.6, 29.6, 24.0, 22.6, 21.5; HRMS (APCI) m/z: calcd for C28H37NO [M + H]+404.2947, found 404.2944.
4-Bromo-2-(3-(3-chlorophenyl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3m): Yellow solid (75%, 1.47 g); mp = 74~75 °C,1H NMR (500 MHz, CDCl3) δ 7.57 (s, 1H), 7.51 (s, 1H), 7.42 (d, J = 7.5 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.32~7.28 (m, 2H), 6.74 (d, J = 8.5 Hz, 1H), 5.22 (s, 1H), 2.85 (s, 2H), 2.79~2.75 (m, 2H), 1.90~1.87 (m, 4H);13C NMR (125 MHz, CDCl3) δ 156.7, 134.3, 132.2, 131.8, 130.4, 130.1, 129.7, 129.1, 123.8, 118.2, 110.8, 88.1, 83.3, 56.6, 49.0, 23.8; HRMS (APCI) m/z: calcd for C19H17BrClNO [M + H]+ 392.0233, found 392.0234.
4-Nitro-2-(3-phenyl-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3n): Red solid (68%, 1.09 g); mp = 72~73 °C,1H NMR (500 MHz, CDCl3) δ 8.56 (s, 1H), 8.13 (d, J = 9.0 Hz, 1H), 7.38 (d, J = 9.0 Hz, 1H), 7.32~7.29 (m, 2H), 7.26~7.21 (m, 3H), 4.25 (s, 2H), 3.44 (s, 4H), 2.02 (s, 4H);13C NMR (125 MHz, CDCl3) δ 156.3, 147.2, 143.0, 138.1, 128.6, 128.3, 126.6, 126.1, 119.4, 119.2, 117.1, 111.6, 111.5, 52.1, 33.2, 25.3; HRMS (APCI) m/z: calcd for C19H18N2O3[M + H]+323.1390, found 323.1386.
2-(1-(Pyrrolidin-1-yl)non-2-yn-1-yl)phenol (3o): Yellow oil (72%, 1.03 g);1H NMR (500 MHz, CDCl3) δ 7.47 (d, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 6.84~6.81 (m, 2H), 5.03 (s, 1H), 2.81~2.77 (m, 2H), 2.73~2.68 (m, 2H), 2.34 (t, J = 7.5 Hz, 2H), 1.85~1.82 (m, 4H), 1.62~1.56 (m, 2H), 1.49~1.43 (m, 2H), 1.36~1.32 (m, 4H), 0.91 (t, J = 7.5 Hz, 3H);13C NMR (125 MHz, CDCl3) δ 157.6, 129.1, 127.8, 122.7, 118.7, 116.0, 89.6, 73.3, 56.6, 48.6, 31.3, 28.8, 28.5, 23.8, 22.6, 18.7, 14.0; HRMS (APCI) m/z: calcd for C19H27NO [M + H]+286.2165, found 286.2163.
4-Bromo-2-(3-(cyclohex-1-en-1-yl)-1-(pyrrolidin-1-yl)prop-2-yn-1-yl)phenol (3p): Yellow solid (87%, 1.56 g); mp = 76~77 °C,1H NMR (500 MHz, CDCl3) δ 7.56 (s, 1H), 7.30 (d, J = 7.5 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 6.24 (s, 1H), 5.14 (s, 1H), 2.82 (s, 2H), 2.73 (s, 2H), 2.23 (s, 2H), 2.16 (s, 2H), 1.87 (s, 4H), 1.71 (s, 2H), 1.65 (s, 2H);13C NMR (125 MHz, CDCl3) δ 156.8, 135.9, 131.9, 130.6, 124.4, 119.9, 118.0, 110.7, 91.5, 78.9, 56.5, 48.7, 29.4, 25.6, 23.8, 22.2, 21.4; HRMS (APCI) m/z: calcd for C19H22BrNO [M + H]+360.0957, found 360.0953.
4-Bromo-2-(1-(pyrrolidin-1-yl)-3-(thiophen-3-yl)prop-2-yn-1-yl)phenol (3q): White solid (87%, 1.57 g); mp = 101~102 °C,1H NMR (500 MHz, CDCl3) δ 7.60 (s, 1H), 7.55 (s, 1H), 7.31 (s, 2H), 7.19 (s, 1H), 6.74 (d, J = 8.0 Hz, 1H), 5.21 (s, 1H), 2.86 (s, 2H), 2.78 (s, 2H), 1.87 (s, 4H), 1.71 (s, 2H);13C NMR (125 MHz, CDCl3) δ 156.8, 132.1, 130.6, 130.0, 129.6, 125.6, 124.1, 121.1, 118.1, 110.8, 84.6, 81.6, 56.7, 48.9, 23.8; HRMS (APCI) m/z: calcd for C17H16BrNOS [M + H]+364.0188, found 361.0189.
2-(3-Phenyl-1-(piperidin-1-yl)prop-2-yn-1-yl)phenol (3r): White solid (80%, 1.16 g); mp = 83~84 °C,
1H NMR (500 MHz, CDCl3) δ 7.56~7.54 (m, 3H), 7.38~7.35 (m, 3H), 7.22 (d, J = 8.0 Hz, 1H), 6.87~6.84 (m, 3H), 5.11 (s, 1H), 2.76~2.72 (m, 4H), 1.89 (br, 6H);13C NMR (125 MHz, CDCl3) δ 157.6, 131.9, 129.4, 128.6, 128.5, 128.4, 122.6, 121.3, 119.0, 116.4, 89.8, 82.3, 61.0, 25.9, 23.9; HRMS (APCI) m/z: calcd for C20H21NO [M + H]+292.1695, found 292.1693.
2-(1-Morpholino-3-phenylprop-2-yn-1-yl)phenol (3s): Yellow solid (68%, 0.99 g); mp = 98~99 °C,1H NMR (500 MHz, CDCl3) δ 7.57~7.54 (m, 3H), 7.38~7.35 (m, 3H), 7.27~7.23 (m, 2H), 6.91~6.87 (m, 2H), 5.12 (s, 1H), 3.81 (s, 4H), 2.81 (s, 4H);13C NMR (125 MHz, CDCl3) δ 156.9, 131.9, 129.8, 128.8, 128.1, 122.1, 120.5, 119.5, 118.7, 90.5, 81.4, 66.7, 60.6; HRMS (APCI) m/z: calcd for C19H19NO2[M + H]+294.1488, found 294.1485.
2-(1-(3,4-Dihydroisoquinolin-2(1H)-yl)-3-phenylprop-2-yn-1-yl)phenol (3t): White solid (84%, 1.42 g); mp = 112~113 °C,1H NMR (500 MHz, CDCl3) δ 10.3 (s, 1H), 7.45~7.43 (m, 2H), 7.32~7.28 (m, 4H), 7.23~7.19 (m, 3H), 7.16~7.11 (m, 2H), 6.86~6.83 (m, 2H), 4.95 (s, 1H), 4.18 (dd, J = 14.0 Hz, J = 14.0 Hz, 2H), 3.19~3.14 (s, 1H), 3.12~3.06 (s, 1H), 2.96~2.92 (s, 1H), 2.88~2.84 (s, 1H);13C NMR (125 MHz, CDCl3) δ 157.9, 134.3, 133.1, 131.8, 129.2, 129.0, 128.4, 128.3, 127.8, 127.4, 126.2, 122.6, 121.1, 119.3, 116.2, 87.6, 85.6, 58.4, 54.2, 45.2, 28.6; HRMS (APCI) m/z: calcd for C24H21NO [M + H]+340.1695, found 340.1692.
1-(1,3-Diphenylprop-2-yn-1-yl)pyrrolidine (3u): Yellow oil (80%, 1.04 g);1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 7.5 Hz, 2H), 7.51~7.50 (m, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.33~7.30 (m, 4H), 4.90 (s, 1H), 2.71~2.69 (m, 4H), 1.84~1.79 (m, 4H)13C NMR (125 MHz, CDCl3) δ 139.6, 131.8, 128.5, 128.2, 128.1, 128.0, 127.5, 123.2, 86.9, 86.7, 59.1, 50.3, 23.5; HRMS (APCI) m/z: calcd for C19H19N [M + H]+ 262.1596, found 262.1598.
2 結果与讨论
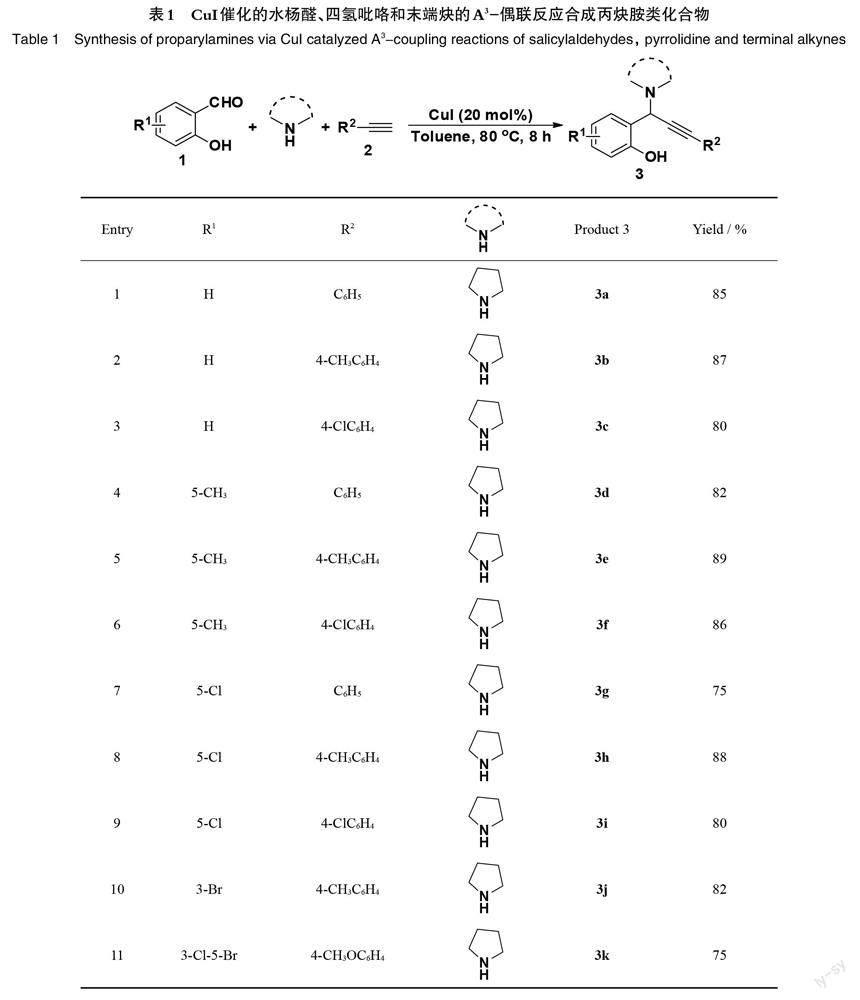
选择不同取代基的水杨醛、二级胺和末端炔为原料,以20 mol%的CuI为催化剂,甲苯作溶剂,80 oC的温度下反应8 h,合成得到了系列结构不同的丙炔胺衍生物,考察了底物取代基的电子效应和空间位阻效应对该反应的影响,具体结果见表1。
结果表明,各种取代基的水杨醛、苯乙炔等均能很好的适用于该反应,并以良好以上收率得到相应的丙炔胺类化合物。对苯乙炔而言,无论苯环上连有吸电子基(如—Cl),还是连有供电子基(如—CH3),对该反应的影响不大(表1, entries 1~3)。当使用活性较弱的烷基末端炔时,产率出现稍微下降(表1, entry 15);令人满意的是,当使用1-环己烯基乙炔和3-噻吩乙炔进行反应时,均能以优异的产率获得相应的产物3p和3q (表1, entries 16, 17)。对水杨醛而言,当苯环羟基对位连有较弱的吸电子基(如—Cl,—Br),或连有供电子基(如—CH3)时,对该反应没有明显影响,仍能以75%~89%的收率得到目标产物(表1, entries 4~13);而当羟基对位连有较强的吸电子基(如—NO2)时,由于取代基的电子效应,水杨醛活性降低,使得产物3n的产率出现明显降低(表1, entry 14)。值得一提的是,当水杨醛羟基邻位和对位连有较大的取代基时(如—Br, —tBu),该反应亦能较好的进行,并以70%和75%的产率得到产物3k和3l (表1, entries 11, 12)。进一步地,使用水杨醛作为底物时,在反应也能较好的进行,并以80%的产率得到相应的产物3u (表1, entry 21)。
随后考察了二级胺对该反应的影响,结果发现使用不同的二级胺时,产物的产率有所不同。使用活性相当的哌啶或活性较小的四氢异喹啉时,反应产率没有明显改变,仍能以良好以上收率得到目标产物3r和3t (表1, entries 18, 20)。当使用活性较弱的吗啉作为胺类化合物时,产物3s的产率明显降低(表1, entry 19)。
3 结论
本文发展了一种CuI催化三组分A3-偶联反应合成苯丙炔胺类化合物的方法,该方法以水杨醛、四氢吡咯和末端炔为底物合成得到一种具有多个反应位点的邻羟基苯基丙炔胺类化合物。该方法底物普适性广,取代基容忍性好,具有原料来源广泛,实验条件温和,反应选择性高、产率优异等优点,可以实现邻羟基苯基丙炔胺类化合物的克级规模合成,具有一定的实际应用价值和良好的应用前景。
参考文献:
[1] LAUDER K, TOSCANI A, SCALACCI N, et al. Synthesis and reactivity of propargylamines in organic chemistry[J]. Chemical Reviews,2017, 117(24): 14091?14200.
[2] LANGSTON J W, IRWIN I, LANGSTON E B, et al. Pargyline prevents MPTP-induced parkinsonism in primates[J]. Science, 1984, 225: 1480?1482.
[3] CHEN J J,SWOPE D M.Clinical pharmacology of rasagiline: A novel, second-generation propargylamine for the treatment of Parkinson disease [J]. Journal of Clinical Pharmacology, 2005, 45(8):878?894.
[4] BIRKS J, FLICKER L. Selegiline for Alzheimers disease [J]. Cochrane Database of Systematic Reviews,2003,1:CD00044210.
[5] YAMAMOTO Y, HAYASHI H, SAIGOKU T, et al.Domino coupling relay approach to polycyclic pyrrole-2-carboxylates[J]. Journal of the American Chemical Society,2005, 127(31):10804-10805.
[6] FENG H D, ERMOLAT'EV D S, SONG G H, et al. Synthesis of Oxazolidin-2-ones via a copper(I)-catalyzed tandem decarboxylative/carboxylative cyclization of a propiolic acid, a primary amine and an aldehyde[J]. Advanced Synthesis & Catalysis, 2012, 354(2-3):505-509.
[7] ERMOLAT'EV D S, BARIWAL J B, STEENACKERS H P L, et al. Concise and diversityoriented route toward polysubstituted 2-aminoimidazole alkaloids and their analogues[J]. Angewandte Chemie International Edition, 2010, 49(49):9465?9468.
[8] ZINDO F T, JOUBERT J, MALAN S F. Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond[J]. Future Medicinal Chemistry, 2015, 7(5):609?629.
[9] MAO F, LI J, WEI H, et al. Tacrine?propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimers disease[J]. Journal of Enzyme Inhibition and Medicinal Chemistry, 2015, 30(6):995?1001.
[10] KOPKA I E, FATAFTAH Z A, RATHKE M W. Preparation of a series of highly hindered secondary amines, including bis-(triethylcarbinyl)amine[J]. The Journal of Organic Chemistry, 1980, 45(23):4616?4622.
[11] HENNION G F, HANZEL R S. The alkylation of amines with tacetylenic chlorides. Preparation of sterically hindered amines[J]. Journal of the American Chemical Society, 1960, 82(18):4908?4912.
[12] LI C J. The development of catalytic nucleophilic additions of terminal alkynes in water[J]. Accounts of Chemical Research, 2010, 43(4):581?590.
[13] BLAY G, MONLEON A, PEDRO J. Recent Developments in asymmetric alkynylation of imines[J]. Current Organic Chemistry, 2009, 13(15):1498–1539.
[14] GHOSH S, BISWAS K. Metal-free multicomponent approach for the synthesis of propargylamine: A review[J]. RSC Advances, 2021, 11(4):2047-2065
[15] MANUJYOTHI R, ANEEJA T, ANILKUMAR G. Solvent-free synthesis of propargylamines: An overview[J]. RSC Advances, 2021, 11(32):19433–19449.
[16] PESHKOV V A, PERESHIVKO O P, VAN DER EYCKEN E V. A walk around the A3-coupling[J]. Chemical Society Reviews, 2012, 41(10):3790-3807
[17] UHLI G N, LI C J. Site-Specific Modification of amino acids and peptides by aldehyde–alkyne–amine coupling under ambient aqueous conditions [J]. Organic Letters, 2012, 14(12): 3000-3003.
[18] LI J, XU Y, HU X, et al. Easy access to 2,4-disubstituted cyclopentenones by a gold(III)-catalyzed A3-coupling/cyclization cascade[J]. Organic Letters, 2020, 22(24):9478-9483.
[19] CHEN X, CHE N T, ZHOU Y, et al. Efficient synthesis of propargylamines from terminal alkynes, dichloromethane and tertiary amines over silver catalysts[J]. Organic & Biomolecular Chemistry, 2014, 12(2):247-250.
[20] ZHANG Y, LI P, WANG M, et al. Indium-catalyzed highly efficient three-component coupling of aldehyde, alkyne, and amine via C?H bond activation[J]. The Journal of Organic Chemistry, 2009, 74(11):4364-4367.
[21] ROKADE B V, BARKER J, GUIRY P J. Development of and recent advances in asymmetric A3coupling[J]. Chemical Society Reviews, 2019, 48(18):4766-4790.
[22] XU X, FENG H, VAN DER EYCKEN E V. Microwave-assisted Cu(I)-catalyzed synthesis of unsymmetrical 1,4-diamino-2-butynes via cross-A3-coupling/decarboxylative A3-coupling[J]. The Journal of Organic Chemistry, 2021, 86(20):14036-14043.
[23] FENG H, ERMOLATEV D S, SONG G, et al. Regioselective Cu(I)-catalyzed tandem A3-coupling/decarboxylative coupling to 3-amino-1,4-enynes[J]. Organic Letters, 2012, 14(7):1942-1945.
[24] YAN S, PAN S, OSAKO T, et al. Solvent-free A3and KA2 coupling reactions with mol ppm level loadings of a polymer-supported copper(II)?bipyridine complex for green synthesis of propargylamines[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10):9097?9102.
[25] KATKAR S V, JAYARAM R V. Cu?Ni bimetallic reusable catalyst for the synthesis of propargylamines via multicomponent coupling reaction under solvent-free conditions[J]. RSC Advances, 2014, 4(89):47958?47964.
[26] LI P, REGATI S, HUANG H C, et al. A sulfonate-based Cu(I) metal?organic framework as a highly efficient and reusable catalyst for the synthesis of propargylamines under solvent-free conditions[J]. Chinese Chemical Letters, 2015, 26(1):6?10.
[27] KAUR P, KUMAR B, KUMAR V, et al. Chitosan-supported copper as an efficient and recyclable heterogeneous catalyst for A3/decarboxylative A3-coupling reaction[J]. Tetrahedron Letters, 2018, 59(21):1986?1991.
[28] PERUMGANI P C, KEESARA S, PARVATHANENI S, et al. Polystyrene supported N-phenylpiperazine?Cu(II) complex: An efficient and reusable catalyst for KA2-coupling reactions under solvent-free conditions[J]. New Journal of Chemistry, 2016, 40(6):5113?5120.
CuI-catalyzed A3-Coupling Reactions for the Synthesis of o-Hydroxyphenyl Propargylamines
FAN Chen-li XIE Ji-na HUI Wen-jie CHEN Hao-yu HE Xin-wei
(1. Department of Material Engineering, Wuhu Institute of Technology, Wuhu 241003, China; 2. College of Chemistry and Materials Science, Anhui Normal University, Wuhu 241002, China)
Abstract: Propargylamine compounds have been widely used in organic synthesis because of their multiple reaction sites, and thus have attracted extensive attention. In this paper, a CuI-catalyzed A3-coupling reactions of salicyladehydes, pyrrolidine and terminal alkynes for the synthesis of propargylamines has been developed. Salicyladehydes and aromatic alkynes bearing with electron-withdrawing or electron-donating groups were well tolerated. In particular, disubtituted salicyladehydes were investigated to showcase the prospective utility of this protocol. Moreover, all products were characterized by1H,13C NMR and HRMS. The results showed that the method has the advantages of wide scope of substrates, good tolerance of substituents, and without separation of intermediates by using multi-component one-pot reaction, achieving step economy and application in organic synthesis.
Key words: A3-coupling reactions; propargylamines; salicylaldehydes; terminal alkynes
(责任编辑:王海燕)

