Atrial fibrillation and coronary artery disease: An integrative review focusing on therapeutic implications of this relationship
Akash Batta,Juniali Hatwal,Akshey Batta,Samman Verma,Yash Paul Sharma
Akash Batta,Department of Cardiology,Dayanand Medical College and Hospital,Ludhiana 141001,Punjab,India
Juniali Hatwal,Department of Internal Medicine,Post Graduate Institute of Medical Education & Research,Chandigarh 160012,India
Akshey Batta,Department of Medicine and Surgery,Sohana Multi Super Specialty Hospital,Mohali 160062,Punjab,India
Samman Verma,Yash Paul Sharma,Department of Cardiology,Post Graduate Institute of Medical Education & Research,Chandigarh 160012,India
Abstract The incidence of both atrial fibrillation (AF) and coronary artery disease (CAD) increases with advancing age.They share common risk factors and very often coexist.Evidence points to an intricate relationship between atrial tissue excitability and neuronal remodeling with ischemia at the microcirculatory level.In this review,we delineated this complex relationship,identified a common theme between the two,and discussed how the knowledge of this relationship translates into a positive and meaningful impact in patient management.Recent research indicates a high prevalence of CAD among AF patients undergoing coronary angiography.Further,the incidence of AF is much higher in those suffering from CAD compared to age-matched adults without CAD underlying this reciprocal relationship.CAD adversely affects AF by promoting progression via re-entry and increasing excitability of atrial tissue as a result of ischemia and electrical inhomogeneity.AF in turn accelerates atherosclerosis via endothelial dysfunctional and inflammation and together with enhanced thrombogenicity and hypercoagulability contribute to micro and macrothrombi throughout cardiovascular system.In a nutshell,the two form a vicious cycle wherein one disease promotes the other.Most AF recommendations focuses on rate/rhythm control and prevention of thromboembolism.Very few studies have discussed the importance of unmasking coexistent CAD and how the treatment of underlying ischemia will impact the burden of AF in these patients.Inflammation and endothelial dysfunction remain central to both disease processes and form a handsome therapeutic target in the management of the two diseases.The relationship between AF and CAD is complex and much more than mere coincidence.The two diseases share common risk factor and pathophysiology.Hence,it is impractical to treat them in isolation.Accordingly,we share the implications of managing underlying ischemia and inflammation to positively impact and improve quality of life among AF patients.
Key Words: Atrial fibrillation; Coronary artery disease; Antithrombotic therapy; Ischemia; Early rhythm control; Endothelial dysfunction
INTRODUCTION
Cardiovascular diseases including coronary artery disease (CAD) and atrial fibrillation (AF) along with other cardiovascular diseases remain the leading cause of morbidity and mortality worldwide[1].The prevalence of both CAD and AF increases with advancing age and they often coexist[2-5].However,this relationship is not a mere coincidence and recent evidence points to the intricate relationship between the two.Dedicated studies have demonstrated a high prevalence of CAD among nonvalvular AF patients,with majority of AF (> 50%) patients having underlying CAD as identified by invasive or computed tomography coronary angiography[5-7].This is significantly higher than the prevalence of CAD in the general population,which is estimated to be about 12%-14%.Furthermore,there is abundant evidence that AF is an independent risk factor for CAD and incident acute coronary syndromes[4,8,9].The interrelationship of the two is further highlighted by the fact that the people with coexistent AF and CAD have a more severe CAD and higher SYNTAX scores compared to those without AF[8,10].Also,the morbidity and mortality is significantly higher when CAD is associated with paroxysmal or persistent AF with increased odds for developing heart failure,ventricular arrythmias,and major adverse cardio-cerebro vascular events (MACCEs)[4,8-10].However,despite the obvious association,this relationship is also influenced by a number of confounding risk factors such as diabetes,hypertension,age,and obesity which are common to both CAD and AF.Hence,this leads to confusion in establishing causality and reverse causality solely based on the results from registries and observational studies.
A recent mendelian randomization study by Yanetal[4] shed important light on this relationship and concluded beyond doubt that CAD is an independent risk factor for AF after removing all bias.Furthermore,they made an argument that treatment and prevention of AF is crucial to prevent MACCE among CAD patients[4].Hence,the two might be more closely related than thought,and logically the therapeutic strategies are expected to be similar as well.
PATHOPHYSIOLOGICAL BASIS OF THE RELATIONSHIP
The basic pathology in CAD is the formation and progression of atherosclerotic plaques in coronary arteries leading to narrowing and resultant myocardial ischemia.Indeed,this process is identical in other vascular beds involved by atherosclerotic process and manifests as variable clinical presentation depending on the vasculature involved.In the heart,the same can manifest as acute coronary syndrome (ACS) or chronic coronary syndrome (CCS) depending upon the progression and stability of the atherosclerotic plaques.The major pathophysiological pathways involved in initiation and progression of AF include re-entry and focal ectopic activity.The re-entry is in turn promoted by the short refractoriness,slowed conduction,and atrial remodeling as a result of atrial dilatation.The enhanced automaticity of the atrial tissue stems from the enhanced early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs)[9,11].
Common risk factors
The two diseases share identical risk factors,which trigger varied pathophysiological responses culminating in either or both of the two diseases.Diabetes mellitus (DM),hypertension,advancing age,dyslipidemia,obesity,smoking,and decreased physical activity are the major risk factors for CAD as per abundant evidence in the scientific literature.Indeed,these very same risk factors remain the most commonly implicated factors responsible for the initiation and progression of AF[8,9,11,12].The interplay of the various risk factors and their role in the pathogenesis of AF and CAD have been demonstrated in Figure 1.

Figure 1 Interplay of various common risk factors in the pathogenesis of coronary artery disease and atrial fibrillation.
DM particularly in the setting of poorly controlled blood sugars is a major risk factor for initiation of AF.Research has shown a consistent positive correlation between increasing hemoglobin A1C and AF burden.The most popular hypothesis for this association is the structural alteration and fibrosis in atrial myocardium as a result of inflammation,endothelial dysfunction,formation of reactive oxygen intermediates,and deposition of advanced glycation end products[13,14].All of these to a large extent result in microvascular dysfunction and atrial tissue hypoxemia.Prolonged action potential duration secondary to ischemia pave the way for EADs and DADs.Often there is coexistent autonomic neuropathy and dysfunction in DM,which together with altered calcium (Ca2+) andInahomeostasis contributes to the progression of AF[15].
Hypertension remains the most common risk factor for both AF and CAD.It increases the risk of development of AF by more than 30% for any given age[16].Left ventricular hypertrophy and subsequent diastolic dysfunction in longstanding hypertension contributes to atrial dilatation and dysfunction.Furthermore,alterations in the renin angiotensin aldosterone system along with the increased expression of pro-inflammatory cytokines induce atrial fibrosis[17,18].The net result of these pathogenic mechanisms is the development of focal aberrant ectopic firing due to altered Ca2+homeostasis and the development of re-entry circuits along clinical or subclinical fibrosis in atrial myocardium[19].
Obesity is often associated with other cardiovascular comorbidities,which contribute to enhanced overall MACCE risk.In addition,many advocate that obesity is an inflammatory disease process characterized by increased expression of various pro-inflammatory cytokines and resultant endothelial dysfunction.This often correlates with increased left atrial volume and fibrosis,which have a pathogenic role in the development of AF[20].According to certain estimates,the risk of AF increases in a linear fashion with increasing body mass index (BMI) and roughly a 1 kg/m2increase in BMI confers a 4% increased risk of developing AF[9,21].
Positive feedback cycle
Besides sharing common risk factors,CAD and AF by themselves have a direct relationship with each other.A positive feedback mechanism between the two,culminates in a vicious cycle resulting in increased the burden of the two diseases.CAD comprising both macrovascular and microvascular disease leads to ischemia of the atrial tissue which precedes local inflammation and culminates in fibrosis and prolonged conduction times,all of which trigger the three principle mechanisms involved in the pathogenesis of AF which are focal ectopy,re-entry and neural alteration.In addition,there is heterogeneity in the electrical conduction,altered Ca2+and sodium currents,and autonomic system dysregulation,all of which promote the progression and persistence of AF[4,8,9,11,12,22,23] (Figure 2).
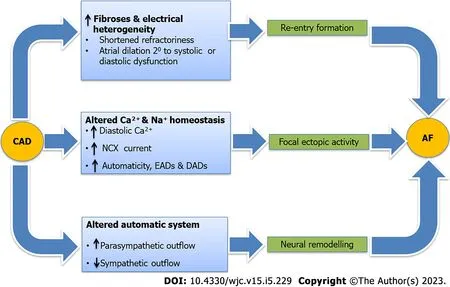
Figure 2 Pathogenic mechanism that predisposes an individual suffering from coronary artery disease to develop atrial fibrillation.
On the other end,AF by itself can induce the two key pathogenic mechanisms involved in CAD,namely endothelial dysfunction and inflammation.Decreased release of nitric oxide coupled with increased expression of von Willebrand factor is the key pathogenic process in endothelial dysfunction[9,11,17].AF also triggers systemic and myocardial inflammation by virtue of enhancing expression of protease-activated receptors and inflammatory cytokines,which not only initiate atherosclerotic process but also contribute to plaque instability and resultant ACS.Besides this,the beat-beat variability resulting in inefficient contractility and reduced cardiac output also contribute to reduced coronary blood flow and resultant ischemia independent of atherosclerotic CAD.Coagulation system activation coupled with enhanced platelet activity due to increased expression of p-selectin and cluster of differentiation 63 (CD63) on endothelial cells leads to micro and macro thrombi,which not only increases the risk of cerebrovascular accident but also ACS[24-26] (Figure 3).

Figure 3 Pathogenic mechanisms that predispose an individual suffering from atrial fibrillation to develop coronary artery disease.
The two more often than not coexist and together they confer worse outcomes than when the two occur in isolation.When AF complicates pre-existing CCS or ACS,it leads on to higher MACCE events including stroke,heart failure,and cardiogenic shock and also doubles overall cardiovascular mortality[8,9,11,27].Further,it complicates clinical decision making and predisposes an individual to not only increased thrombotic events but also to major bleeding events secondary to aggressive antithrombotic therapy,which is often indicated.As a result,there is a need for a comprehensive assessment and management of the two diseases in conjunction and not as separate disease entities.The two diseases more often than not are linked in their etiopathogenesis and warrant kindred treatment to break the links which propagate the two diseases.
Differences in pathogenesis of AF in CAD patients compared to those without CAD
The vast majority of patients suffering from AF (> 85%) have underlying CAD or cardiovascular risk factors including hypertension,diabetes,obesity,and dyslipidemia.In less than 15% of all AF patients,none of these risk factors are present[28,29].Most of them are relatively younger and commonly labeled as lone AF.Familial AF also contributes to a fraction of lone AF patients with well-defined chromosomal abnormalities most notably 10q22-q24[9,29].The basic difference in the pathophysiology of AF in individuals having underlying cardiovascular risk factors and CAD predominantly is that they have structural alterations in the atrial tissue,which predispose them to develop electrical remodeling or directly lead to re-entry and ectopy culminating in AF.On the other hand,AF occurring in younger individuals without any risk factors is often attributable to the electrical remodeling as a result of abnormal Ca2+homeostasis,dysregulated ryanodine receptors,altered action potential durations,and reduced atrial refractory period.All of these culminate in changes in ion channel function,enhanced automaticity and atrial ectopy,which is a precursor to AF[28-30].Whatever may be the initial insult,the pathways soon converge and either of the structural or the electrical remodeling ultimately aggravates the other resulting in a vicious cycle of AF initiation and progression (Figure 4).Another important difference is in the clinical presentation and outcomes.Patients with underlying cardiovascular risk factors and CAD have less symptoms related to AF,but rather commonly present with complications related to AF including heart failure or stroke.By contrast,the risk of thromboembolism is relatively lesser in the lone AF/familial AF patients,who very often present to outpatient clinics with symptoms related to AF such as recurrent palpitations or dyspnea[28,30].Given the aggressive disease course with accelerated atherosclerosis and thromboembolism in those with underlying cardiovascular risk factors,there is a need for the aggressive control of risk factors and institution of effective antithrombotic therapies to prevent complications.
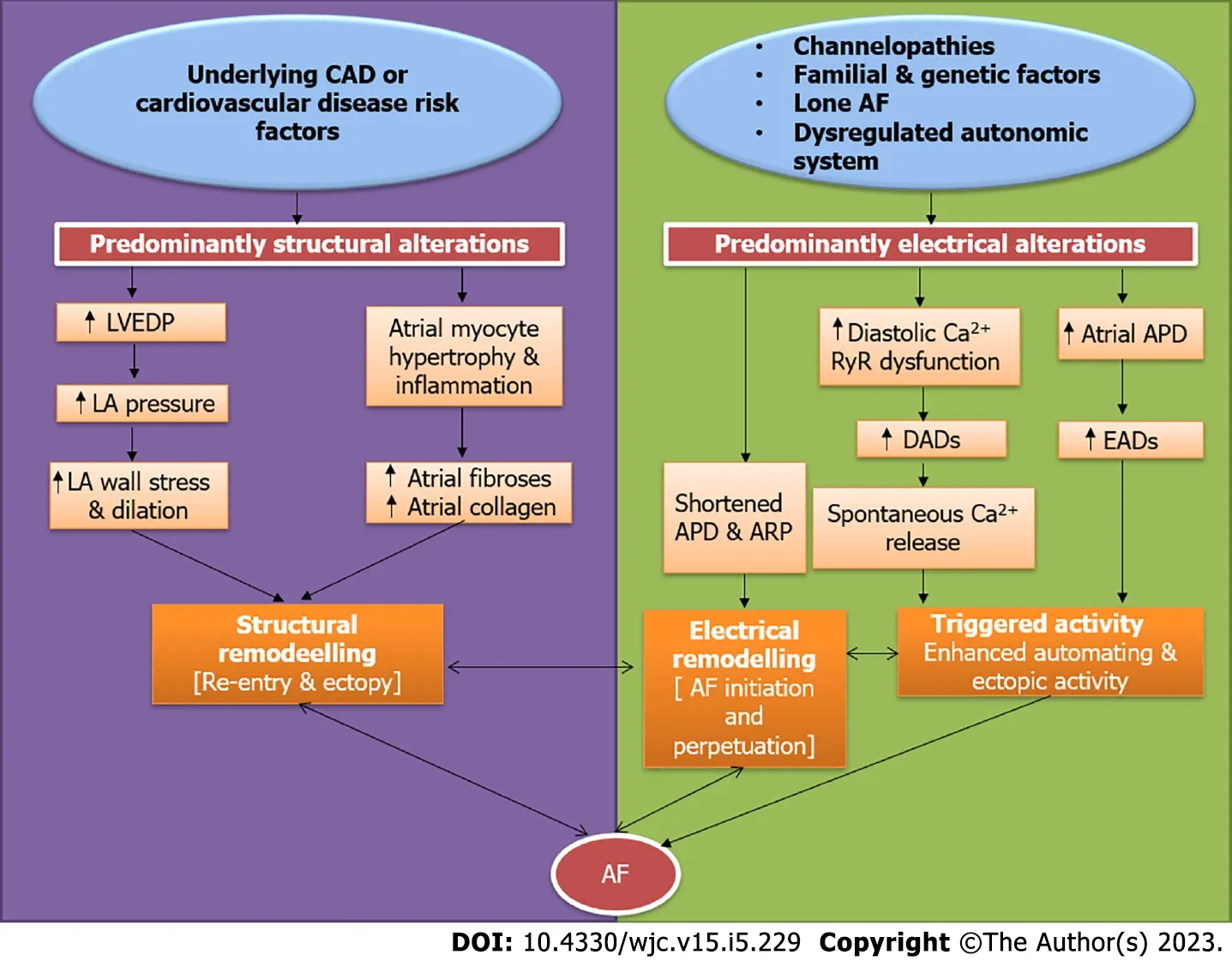
Figure 4 Key differences in the pathogenesis of atrial fibrillation in patients with underlying coronary artery disease or risk factors compared to those without underlying cardiovascular risk factors.
IMPLICATION ON CHA2DS2VASC SCORE AND STROKE RISK
A consistent pool of evidence points towards the increased burden of AF in those having coexistent CAD compared to those without CAD[8,11,27].Furthermore,this increased duration and burden of AF translates into increased MACCE events in patients with coexistent CAD compared to those without CAD.Therefore,it makes sense that people with AF and coexistent CAD need better risk factor modification,pharmacological therapy for CAD,and more aggressive antithrombotic therapy to prevent adverse outcomes[6,31].
Among the components of the CHA2DS2VASc score,the symbol ‘V’ stands for vascular disease.The widely accepted determinants of this vascular disease as per the guidelines are prior myocardial infarction,peripheral artery disease,or the presence of an aortic plaque.Most of the current guidelines and online medical calculators thus do not account for CAD as a determinant of ‘V’ while calculating the CHA2DS2VASc score[32,33].Therefore,this gives an impression that underlying CAD status (excluding past myocardial infarction) has no bearing on stroke risk as determined by the CHA2DS2VASc score.
However,in a recent,large,prospective study by Steensigetal[6],underlying CAD not only was very frequent among AF patients,but more importantly CAD was strongly associated with elevated thromboembolic risk beyond the usual components of the CHA2DS2VASc score[6].Hence,the study made a strong case for inclusion of significant angiographically proven CAD in the ‘V’ component of the CHA2DS2VASc score to more comprehensively account for the thromboembolic risk in a given individual with AF.Indeed,this made a turning point in the approach to managing AF patients and the same was reflected in the European Society of Cardiology 2020 AF guidelines.For the first time,angiographically proven CAD was included as a determinant of in the ‘V’ in the CHA2DS2VASc score[34].Since then,the inclusion of significant CAD has gained acceptance among practicing cardiologists as evidenced by a recent survey by the European Heart Rhythm Association,wherein 79% of the respondents were aware of the inclusion of significant CAD and employed the same in their practice[35].
A recent study by Shietal[36] shed new insights into the stroke risk in AF patients.They concluded that among AF patients with coexistent CAD,the stroke risk was not dependent on AF but on the atherosclerotic risk factors and the presence of CAD.They made a strong case for aggressive risk factor modification in particularly underlying CAD for stroke reduction in AF patients[36].
CLINICAL IMPACT OF UNDERLYING CAD ON AF
Recent evidence supports the close association of AF and CAD.Not only does underlying CAD increase the odds of developing AF but it also has significant therapeutic and clinical implications.Consistent literature points towards increased MACCE in AF patients,who have underlying CAD compared to those without underlying CAD.Furthermore,complexity in administering appropriate antithrombotic regimen is a challenge and it predisposes an individual to increased minor and major bleeding events.Hence,given the significant therapeutic and prognostic implications of CAD on AF,a more holistic and balanced approach is needed while managing the two diseases,as it is impractical to treat either one in isolation.Table 1 highlights the prominent studies over the last three decades,which have analyzed the clinical impact of underlying CAD in AF patients[6,36-44].Consistently,underlying CAD in AF patients has been shown to correlate positively with worse overall outcomes.
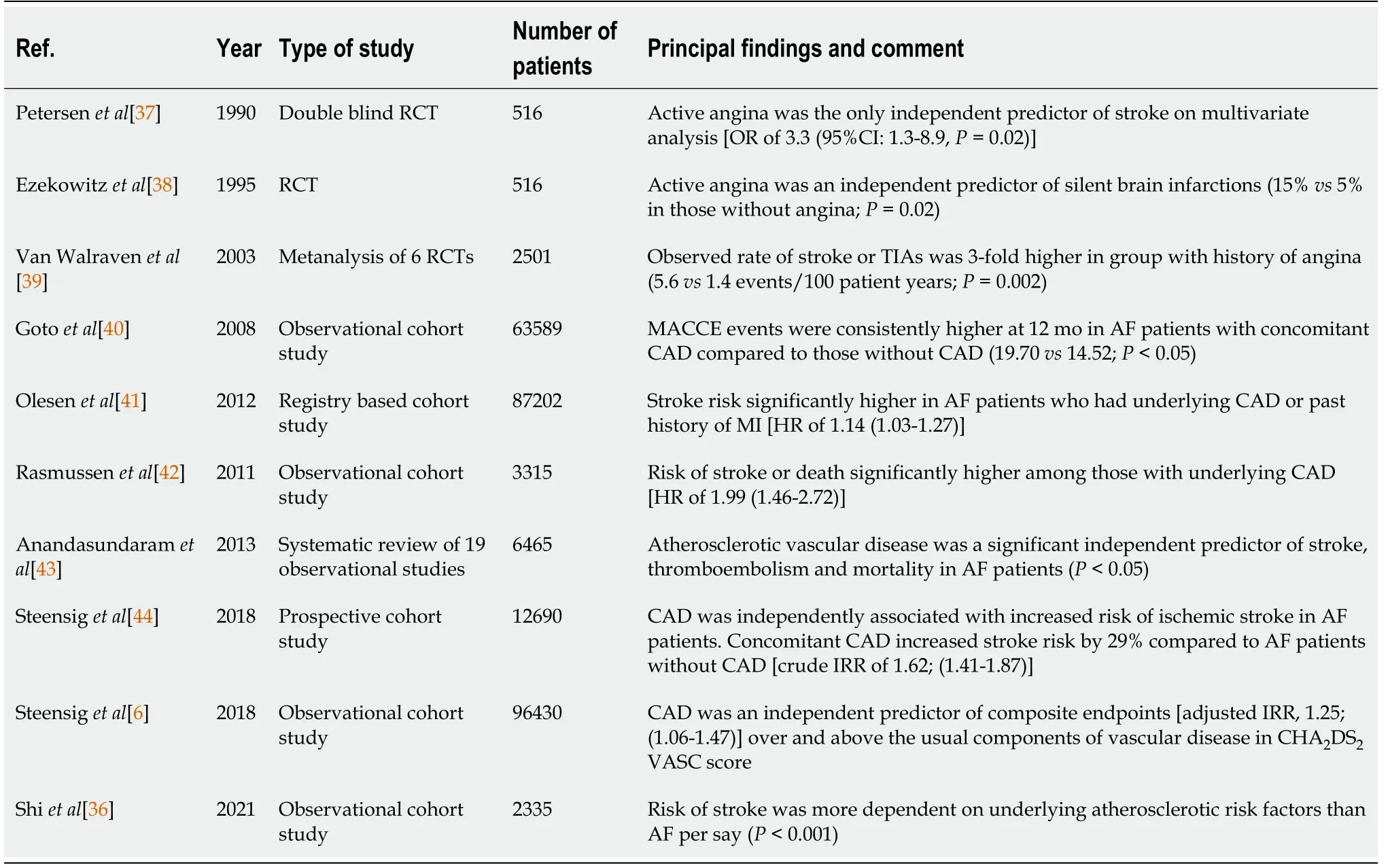
Table 1 Prominent studies over the last three decades highlighting the clinical impact of underlying coronary artery disease in patients suffering from atrial fibrillation
“COMBINED APPROACH” TO REDUCING THE BURDEN OF THE TWO DISEASES
A recent article by Fanaroffetal[27] and the accompanying editorial nicely summarize the impact of coexistent AF and CAD on various clinical endpoints[27,45].The main theme of the paper was the heightened thrombogenicity when the two occur together and authors concluded that the downstream risk of recurrent ACS and percutaneous coronary intervention (PCI) was extremely high in these group of patients compared to when they occurred in isolation.While they focused on only one and a very important aspect of this relationship,others have gone beyond the antithrombotic therapy.Another very relevant aspect is the enhanced atherogenicity throughout the systemic vasculature,which has been well documented in literature and elaborated upon in this review.The coexistence of the two sets in motion a vicious cycle that culminates in accelerated atherosclerosis and its various clinical manifestations[9,28,30].Besides the increased thrombogenicity and atherogenicity conferred as a result of the coexistence of the two diseases,there is a direct relationship of one with the other disease.Such that,one disease can directly lead to the other and vice versa (Figures 2 and 3).Hence,targeting and breaking the common links between the two makes sense and should be considered in any individual suffering from either of the two diseases (Figure 5).
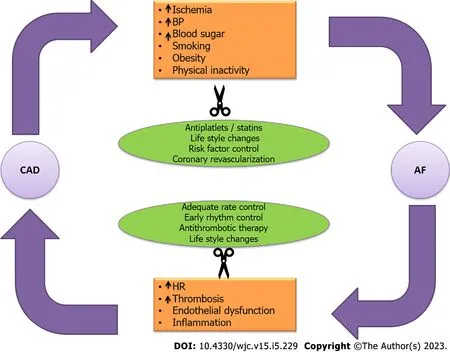
Figure 5 Vicious cycle between coronary artery disease and atrial fibrillation that culminates in a positive feedback as a result of the connections as highlighted in the orange boxes.
THERAPEUTIC STRATEGIES FOR DISRUPTING THE VICIOUS CYCLE
Similar to all cardiovascular diseases,the prevention starts with risk factor control and modification right at the primary care level.Controlling the most commonly implicated risk factors including physical activity,obesity,dietary modifications with reduced intake of sweetened foods and salt,smoking cessation,blood pressure control,and management of dyslipidemia and blood sugars when altered leads to a reduction of both CAD and AF[9,11].The optimal control of these risk factors markedly reduces ones odds of developing CAD and AF by inhibiting common initiating pathways and weakening the links between them two (Figure 5).
When one of the two diseases is diagnosed in a given individual,every attempt should be made to unmask the other disease as very often the two are associated.Coexistent CAD has been reported in more than half of AF patients in various studies[5-7,46].Thus,diagnosing the concomitant CAD by invasive or noninvasive seems logical.This translates into optimal management of not only the masked CAD and in reducing the burden of AF but also predicts an individual’s thromboembolic risk and guides optimal antithrombotic regimen[6,27].Similarly,in those with CAD and other risk factors,the occurrence of MACCE events including stroke and heart failure should be followed by active surveillance for paroxysmal or persistent AF by appropriate rhythm monitoring tools.Unmasking paroxysmal AF guides institution of oral anticoagulants which leads to significant reduction of not only thromboembolic risk but also myocardial infarction among CAD patients.
Besides primary prevention,in those with established CAD and/or AF,the key to improve outcomes is simultaneous and optimal control of both the disease.Accordingly,given the intricate relationship between the two,it is impractical to treat the two in isolation.Most therapies that reduce the burden of either of these diseases,invariably also modifies and reduces the burden of the other disease.For example,statins that are used in CAD,have been shown to reduce the incidence and burden of AF[47].Also,therapies aimed at reducing the burden of CAD including PCI or bypass graft surgery,have shown to significantly reduce the burden of concomitant AF and improve morbidity and mortality.Likewise,therapies such as rate control in AF patients using beta blockers or calcium channel blockers also significantly reduce coronary ischemia and myocardial oxygen uptake[48].
SPECIFIC THERAPIES
Antithrombotic agents
Antithrombotic agents encompass both antiplatelet and oral anticoagulants.Both CAD and AF are characterized by heightened thrombogenicity in the blood and resultant ischemic events.This is logarithmical increase in this thrombogenicity when the two coexist.To further complicate clinical decision making,there is an increased risk of bleeding as well attributable to these antithrombotic drugs.Balancing ischemic and bleeding risk in a given patient remains the top priority and necessitates adherence to clinical practicing guidelines.
The choice of antithrombotic agents in coexistent CAD and AF depends upon the clinical status of the underlying CAD.In patients suffering from CCS and AF,the consensus is towards the use of oral anticoagulants alone,preferably using the newer oral anticoagulants (NOACs).Recent research has shown that NOACs alone fair comparably to the combination of NOACs and aspirin but with the advantage of significantly lower risk of bleeding.
The decision making in AF patients with ACS and those undergoing PCI and stenting is however complex.In patients with ACS the recommendations are combining a P2Y12antiplatelet agent with an oral anticoagulant (preferably NOAC over vitamin K analogues) for at least 6-12 mo after ACS and then continuing only oral anticoagulant beyond 1 year.In those undergoing PCI,the guidelines recommend triple antithrombotic therapy including aspirin,P2Y12agent and a NOAC for the 1stmonth following PCI,followed by dual therapy with a P2Y12agent and NOAC for 6-12 mo and continuing only a NOAC in most patients beyond the 1styear.However,despite the evidence and clear guidelines only a minority of AF and CAD patients receive optimal antithrombotic therapy largely attributable to the gaps in knowledge,fear of bleeding or physician preference rendering these patients at high risk of recurrent ischemic events[27,49,50].
Statins
Statins have emerged as one of the most important and first-line therapy for prevention and treatment of CAD.Besides its lipid-lowering effects,it has pleiotropic effects on the form of reduction in inflammation in the atherosclerotic plaques and improving plaque stability.Recent studies have shown that the early initiation of statin therapy in ACS patients help in reducing the incidence of atrial and ventricular arrythmias[47,51].These beneficial actions are in part attributable to the improved autonomic control and improved myocardial stability.A large recent metanalysis has shown that prior statin use markedly reduced the incidence of new-onset AF after admission for ACS[47].Hence,early statin use and adequate lipid control are essential for reducing AF burden among CAD patients.
Rate controlling and antianginal agents
Tachycardia in AF predisposes patients with underlying CAD to recurrent myocardial ischemia due to increased myocardial oxygen consumption and reduced diastolic coronary perfusion at higher heart rates.This not only translates into worse symptoms but roughly doubles the risk of ACS in this population.Hence,rate control is the initial and most crucial step in managing people with both CAD and AF.Beta blockers and nondihydropyridine calcium channel blockers are the preferred agents in this regard and the resting target heart rate is less than 110/min[9,48].Ivabradine is ineffective in controlling heart rates in AF patients and on the contrary may even aggravate AF as was seen in the SIGNIFY trial and a recent meta-analysis[52,53].Hence,it should be avoided in AF.Digoxin,although an effective drug in controlling the heart rates in AF patients especially those with left ventricular dysfunction,is best avoided in patients with CAD for the fear of predisposition to arrythmias and worsening myocardial ischemia due to increased myocardial oxygen consumption[48].
Among the choice of antianginal agents in patients symptomatic despite adequate rate control,ranolazine is preferred among the second-line drugs,as it prevents the automaticity in atrial tissue by suppressing diastolic depolarization and atrial tissue excitability in addition to suppressing the early and delayed after depolarizations.All of this results in the increased initiation and progression of AF.Moreover ranolazine use is tied to better rhythm control in AF patients in a recent meta-analysis[54,55].Trimetazidine is a second-line antianginal used especially in those with underlying left ventricular dysfunction.It largely has a neutral effect on underlying AF and can be used as an add-on therapy in those with ischemic cardiomyopathy and AF.Limited data have suggested that favorable effects on Pwave duration and dispersion may help reduce the incidence of AF in these subgroup of patients[56].The use of nitrate and nicorandil in AF should be avoided as these have been tied with increased incidence and aggravation of underlying AF in CAD patients[48].
Early rhythm control strategy
Early rhythm control strategy preferably with catheter ablation has been increasingly realized as an effective means of reducing the overall MACCE events in patients suffering from AF[57-59].The benefit is most in those with high comorbidly burden and in those with a recent diagnosis of AF.There has been a clear trend in the superiority of rhythm control compared to rate control in recent years largely attributable to the incremental benefit of early rhythm over rate control alone in terms of improved overall symptoms and quality of life scores and reduced heart failure hospitalizations,stroke,dementia,and overall cardiovascular death[57,58].This has reflected in increasing recommendations for catheter ablations in multiple subsets of patients including those with underlying CAD.Since AF is common in patients with underlying CAD and high comorbidity burden,all attempts should be made to diagnose it early and accordingly if symptoms are not controlled despite initial medical therapy and rate control,catheter ablation should be considered.
Targeting endothelial dysfunction
Robust evidence points towards the central role of endothelial dysfunction in CAD initiation and progression.Further,endothelial dysfunction now is increasingly realized as an important mediator in AF pathogenesis as well[60].Often it coexists with other cardiovascular comorbidity such as diabetes,hypertension,dyslipidemia,and obesity.Decreased expression of nitric oxide,inflammation,increased oxidate stress,increased apoptosis,and vascular remodeling all contribute to endothelial dysfunction at the cellular level.Endothelial dysfunction as diagnosed by flow-mediated vasodilation often correlates with increased systemic vascular complications and poor outcomes[61].Endothelial dysfunction is a dynamic thing and is reversible to large extent with appropriate intervention.At present,the only therapy to improve endothelial dysfunction includes aggressive risk factor modification including smoking cessation,appropriate blood pressure and blood glucose control,weight reduction,and exercise.Pharmacological therapies including antithrombotic therapies and statins also have shown incremental benefit in addition to lifestyle intervention.Other pharmacological agents including calcium channel blockers,angiotensin inhibitors,antioxidant agents,betablockers,phosphodiesterase inhibitors,nicorandil,ivabradine,and l-arginine have also shown some benefit in small studies but it is yet early stages to comment on the role of these agents in improving endothelial dysfunction in clinical practice[9,60,61].
Therapies targeting inflammation
Inflammation is implicated in pathogenesis of both CAD and AF.The testimony of the same lies in the fact that many antiinflammatory drugs have shown incremental benefit in reducing the incidence and burden of either of the two diseases.While evidence is more robust for its positive impact in CAD,data are emerging on its role in AF patients[62,63].Two large randomized studies have already shown the positive impact of colchicine and canakinumab in reducing MACCE events in CAD patients attributable to decreased inflammation[64,65].On the other hand emerging evidence shows that therapies targeting inflammation indeed prevent the occurrence or decrease the recurrences of AF in CAD patient.Recent studies have shown that colchicine or corticosteroids administration after catheter ablation can help reduce recurrence of AF[63].
Effect of diabetes and antidiabetics drugs on AF and CAD
Diabetes remains one of the largest independent risk factors for development of atherosclerosis.Approximately one-third of all patients suffering from diabetes have concomitant CAD,which remains the leading cause of morbidity and mortality in the diabetic population[66].Recent evidence points to the excess prevalence of AF in diabetic population,independent of other cardiovascular risk factors[67,68].Furthermore,patients with concomitant AF and diabetes have worse clinical outcomes including excess stroke,dementia,and heart failure compared to AF in the absence of diabetes[67,69].Diabetes confers enhanced systemic vascular atherogenicity and thrombogenicity,which in part is driven by endothelial dysfunction and inflammation,a pathogenic process very similar to both AF and CAD.The major contributors to this pathogenesis include the direct glucose and free fatty acid toxicity at the cellular levels,which results in excess of reactive oxygen species,advanced glycation end-products,upregulation of the polyol,hexosamine,and protein kinase C pathways.This results in dysregulated cellular metabolism and mitochondrial function,which are essential for normal endothelial function and its antiinflammatory and antithrombotic properties[67,68].As such,this relationship is very relevant while managing patients with AF and/or CAD.Naturally,there is a desire to use antidiabetic drugs,which help improve the burden of these diseases.Table 2 illustrates the prominent effects of various classes of antidiabetic drugs on AF and CAD.Expectedly,the antidiabetic drugs,which improve the clinical endpoints of either CAD or AF,are expected to confer a beneficial effect on the other disease as well.Overall,antidiabetic drugs that have consistently shown incremental benefit in reducing burden of either AF and CAD include sodium-glucose cotransporter-2 inhibitors,dipeptidyl peptidase-4 inhibitors,and metformin.Accordingly,we believe that these agents should preferentially be used during institution of antidiabetic therapy in these patients ahead of agents,which have neutral or harmful effects on either of the two diseases (sulfonylureas,thiazolidinediones)[68,70].
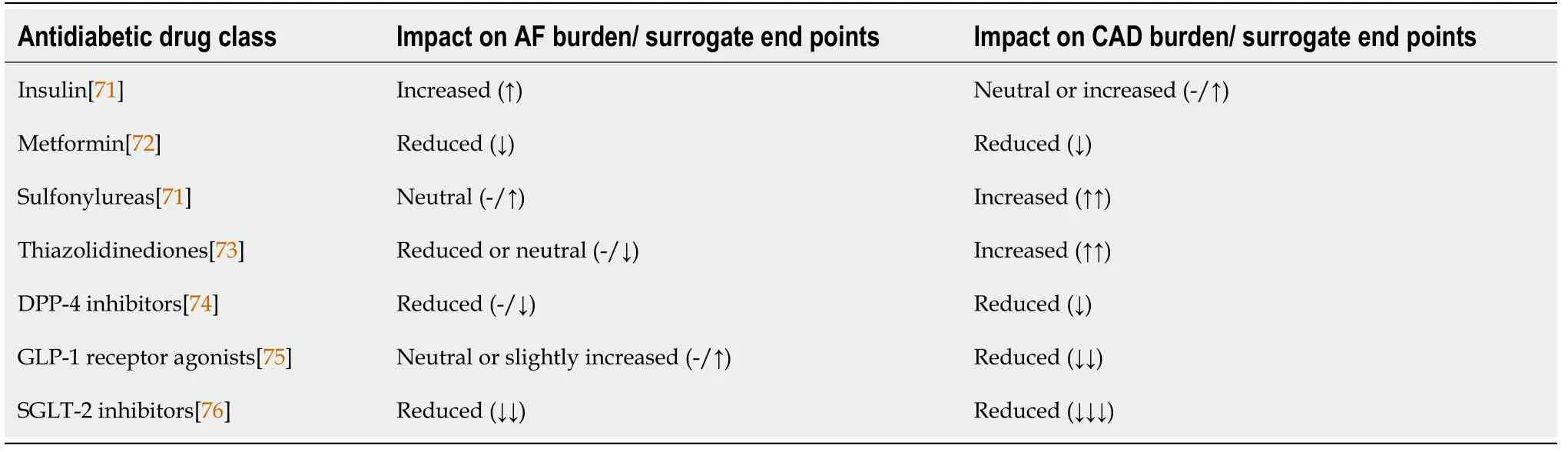
Table 2 Impact of various antidiabetic agents on the burden of atrial fibrillation and coronary artery disease and their surrogate endpoints
CONCLUSION
The relationship between AF and CAD is complex and the two are intricately related at the pathophysiological level.The two diseases share common risk factors and pathogenesis and often culminate in a vicious cycle.Hence,it is impractical to treat them in isolation.The worsening of one is invariably accompanied by accelerated progression of the other disease as well.Accordingly,we share the implications of this relationship in diagnoses and management of the two diseases.In this review,we discuss the key strategies to break the cycle and highlight the recent,evidence-based therapeutic options to break the common links between the two and reduce morbidity and mortality.
FOOTNOTES
Author contributions:Batta A and Sharma YP contributed to the conception and design; Batta A,Hatwal J,and Sharma YP contributed to the analysis and interpretation; Batta A,Batta Ak,and Verma S contributed to the data collection; Batta A and Hatwal J contributed to writing the article; Batta A and Sharma YP contributed to critical revision of the article; Batta A and Sharma YP contributed to final approval of the article; Batta Ak contributed to the statistical analyses; Batta A and Sharma YP will serve as guarantors for the overall accuracy of the manuscript.
Conflict-of-interest statement:All authors have no conflicts of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Akash Batta 0000-0002-7606-5826; Juniali Hatwal 0000-0001-5433-0433; Akshey Batta 0000-0001-6281-8041; Samman Verma 0000-0003-1536-2429; Yash Paul Sharma 0000-0002-1938-3377.
Corresponding Author's Membership in Professional Societies:American College of Cardiology,3445007; European society of Cardiology,1036629.
S-Editor:Li L
L-Editor:Filipodia
P-Editor:Yu HG
 World Journal of Cardiology2023年5期
World Journal of Cardiology2023年5期
- World Journal of Cardiology的其它文章
- Acute heart failure as an adverse event of tumor necrosis factor inhibitor therapy in inflammatory bowel disease: A review of the literature
- Current knowledge and contemporary management of non-A non-B aortic dissections
- Importance of concomitant functional mitral regurgitation on survival in severe aortic stenosis patients undergoing aortic valve replacement
- Impact of erythropoietin therapy on cardiorenal syndrome: A systematic review with meta-analysis
- Extracorporeal veno-venous ultrafiltration in congestive heart failure: What’s the state of the art? A mini-review
- Pharmacoepidemiologic study of association between apparent treatment resistant hypertension,cardiovascular disease and interaction effect by sex and age
