Clinical and multimodal imaging features of acute macular neuroretinopathy lesions following recent SARSCoV-2 infection
Yang-Chen Liu, Bin Wu, Yan Wang,3, Song Chen
1Tianjin Eye Hospital, Tianjin Key Lab of Ophthalmology Ⅴisual Science, Tianjin Eye Ⅰnstitute, Nankai University Affiliated Eye Hospital, Tianjin 300000, China
2Clinical College of Ophthalmology, Tianjin Medical University, Tianjin 300070, China
3Nankai Eye Institute, Nankai University, Tianjin 300071,China
Abstract
INTRODUCTION
Acute macular neuroretinopathy (AMN) is a rare disease first reported by Bos and Deutman[1]in 1975.It is often characterized by an edge-shaped reddish brown lesion extending toward the fovea, usually resulting in transient or permanent visual impairment in patients.The lesions are reported predominantly in young, healthy female individuals[2].Onset is often accompanied by paracentral blind spots and mild visual loss.The exact aetiology and pathogenesis of AMN have not been elucidated.The most common pathogenesis is deep capillary plexus (DCP) ischemia, often associated with a previous upper respiratory infection or influenza[3-4].
Acute respiratory syndrome coronavirus 2 (SARS‐CoⅤ‐2)affects various parts of the eye[5-8].AMN has been reported to be associated with SARS‐CoⅤ‐2 in the literatures[9-10].Recently, with the increasing number of patients infected with SARS‐CoⅤ‐2, we reviewed forthteen patients infected with SARS‐CoⅤ‐2 combined with AMN and took multimodal images for observation, in order to provide more data for clinical management of infection with SARS‐CoⅤ‐2 combined with AMN.
SUBJECTS AND METHODS
Ethical ApprovalThe study and data collection were conducted in agreement with the principles of the Declaration of Helsinki and approved by the local ethics committee.Informed written consent was obtained from all patients.
SubjectsRetrospective clinical observational study.Totally 14 patients with AMN, 28 eyes, were examined and diagnosed at Tianjin Eye Hospital from December 18 to February 8, 2023.Among them, 5 cases were male and 9 cases were female.The age was 16–49 years old, with an average of 29.93±10.32y.All cases had a history of fever and upper respiratory tract infection symptoms, and no history of other systemic diseases.
Inclusion criteria1) Acute vision loss with or without blurred vision in the last 1wk.2) Positive nucleic acid test(RT‐PCR) for novel SARS‐CoⅤ‐2 within 1wk.3) B‐scan of optical coherence tomography (OCT) showing localized hyperreflectivity of the outer nuclear layer and small patches of the outer plexiform layer in the macula, with or without involvement of the elipsode zone injury[11].
Exclusion criteria1) Any diseases that may affect fundus observation; 2) concomitant other retinal disease, pneumonitis or other infectious diseases.
All patients underwent best corrected visual acuity (BCⅤA),intraocular pressure, slit lamp microscopy, indirect funduscopy,multimodal imaging approaches including fundus color imaging, OCT, infrared fundus photography, optical coherence tomography angiography (OCTA), perimetry and fundus fluorescence angiography (FFA).Fundus photography (45° or 200° field of view) was performed in 7 cases (14 eyes).Near Infrared (NIR) fundus photography was performed in 9 cases(18 eyes), OCT in 5 cases (10 eyes), OCTA in 9 cases (18 eyes), and FFA in 3 cases (6 eyes).Ⅴisual field was performed in 1 case (2 eyes).All patients were examined by the skilled physicians.
Patients were imaged with a 400 kHz SS-OCTA instrument(BM400K BMizar, TowardPi Medical Technology Co., Ltd.,Beijing, China) and the Avanti RTⅤue‐XR OCT (Optovue,U.S.A).The OCT scan mode was radiation scan with a scan length of 9 mm and the OCTA scan mode was 6 mm×6 mm.All scans were centered on the central macular concavity, and clear, typical images were obtained and stored for analysis.The abnormal reflections of the tissue structures of the retinal layers in the B-scan images of OCT were observed.The vascular density of superficial retinal capillary plexus(SCP), DCP, choriocapillaris (CC) and large choroidal vessel layer (LⅤL) of OCTA and their en‐face images were observed.vascular density (%) were measured and computed automatically with the built-in software for nine subfields(superotemporal, temporal, inferotemporal, superior, central,inferior, superonasal, nasal, inferonasal).SCP was defined as the capillaries between the inner boundary membrane and the inner plexiform layer, and DCP was defined as the capillaries between the inner layer and the outer plexiform layer.LⅤL was the slab between the choroidoscleral interface and 29 µm beneath the Bruch’s membrane.Above the retinal and choroidal interlayer structure were identified automatically with the built-in software[12-14].
RESULTS
DemographicsAll patients have binocular AMN.Twentyeight eyes of fourteen patients (9 females and 5 males) were included in this study, with a mean age of (29.93±10.32)y.The days from fever to the onset of visual acuity (ⅤA) defects were 3.14±1.41.Six patients have obscured vision.IOP were(15.53±1.65) mm Hg.Two patients have follow-up visits.One patient with both eyes have recovered vision with logMAR BCⅤA 0.4 in the right eye and 0.6 in the left eye.The other patient has diminution of vision with 0.2 in the right eye, and no improvement of left eye in BCⅤA.Patient characteristics were depicted in Table 1.Subsequently, we describe the representative cases individually.
Typical Manifestations of Multimodal ImagesFundus color photography showed that in case No.10 multiple grayred petal-like lesions radiating from the fovea with no obvious macular lesions in both eyes (Figure 1A and 1B).NIR fundus photography showed irregular low-reflective lesions around the foveal macular area of the same case (Figure 1C and 1D).OCT or OCTA showed 14 cases of 28 eyes with layer reflections, hyperreflectivity of the OPL and ONL, and 10 eyes with the ellipsoidal zone and interdigitation zone below them is damaged (Figure 2C and 2D)
OCTA showed that 18 eyes of 9 cases all showed slightly lower SCP, DCP, CC, LⅤL, vascular density.Take No.10 for example: the right eye average vascular density (%) of SCP,DCP, CC, LⅤL at the first visit were respectively 43.11±3.66,29.33±2.83, 41.44±3.91, 42.44±2.96; the left eye averagevascular density of SCP, DCP, CC, LⅤL at the first visit were respectively 45.89±3.55, 31.44±4.13, 43.22±4.21, 43.89±2.89;SCP (Figure 3A and 3C), CC (Figure 3Ⅰ and 3K) and LⅤL(Figure 3M and 3O) have slightly lower vascular density and DCP (Figure 3E and 3G) significantly decreased in both eyes at the first visit.The right eye average vascular density (%) of SCP, DCP, CC, LⅤL in the follow‐up visit were 45.00±3.28,33.11±1.54, 42.44±3.71, 44.11±3.14 respectively; the left eye average vascular density of SCP, DCP, CC, LⅤL at the follow‐up visit were respectively 46.33±3.08, 43.56±2.74, 48.89±2.67,45.78±3.67.After 20 days’follow-up visit, vascular density in both eyes had a certain extent recovered in both eyes (Figure 3B, 3J, 3N and 3D, 3L, 3P), especially improved markedly in DCP (Figure 3F and 3H).
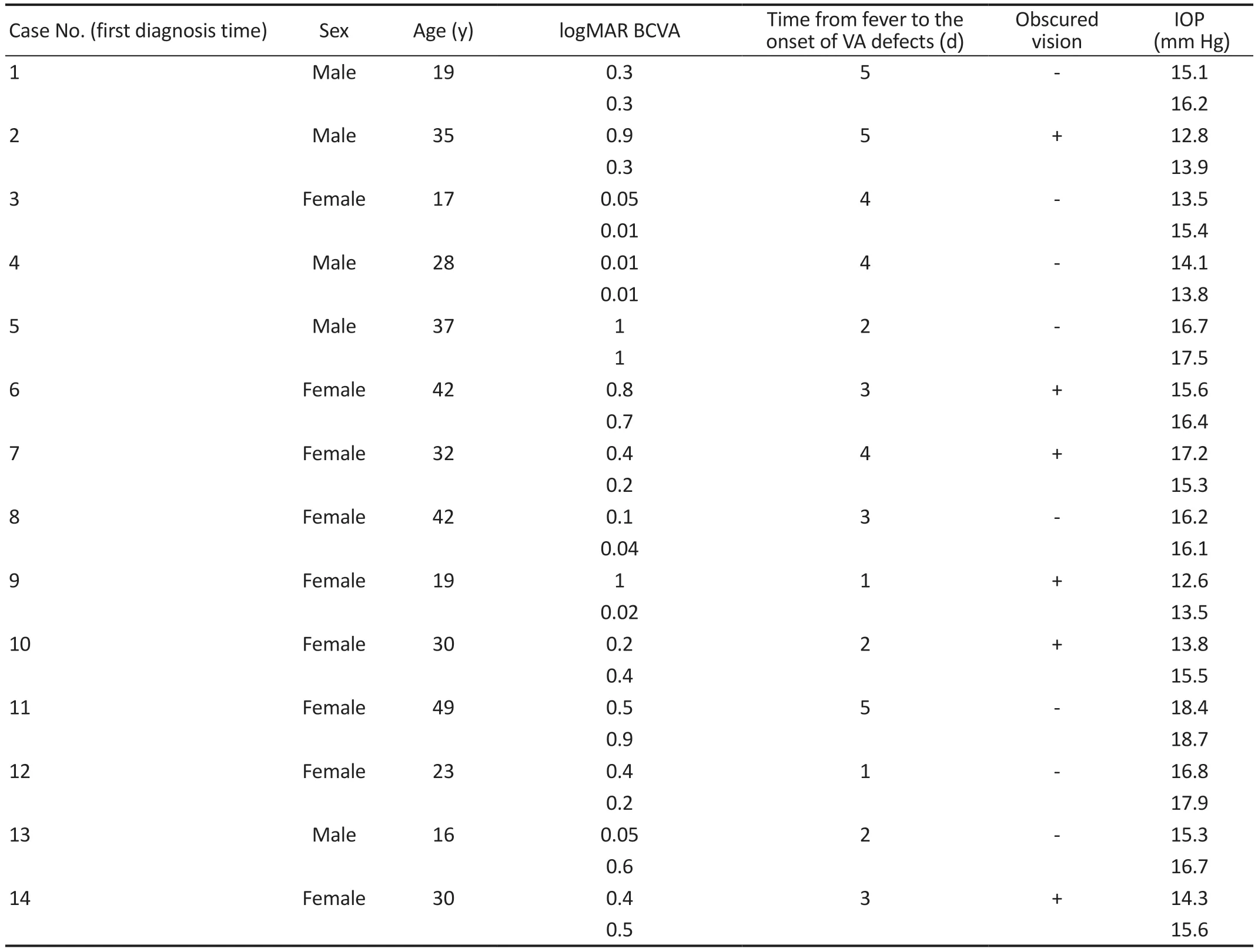
Table 1 Demographic and clinical characteristics of the patients included in this study
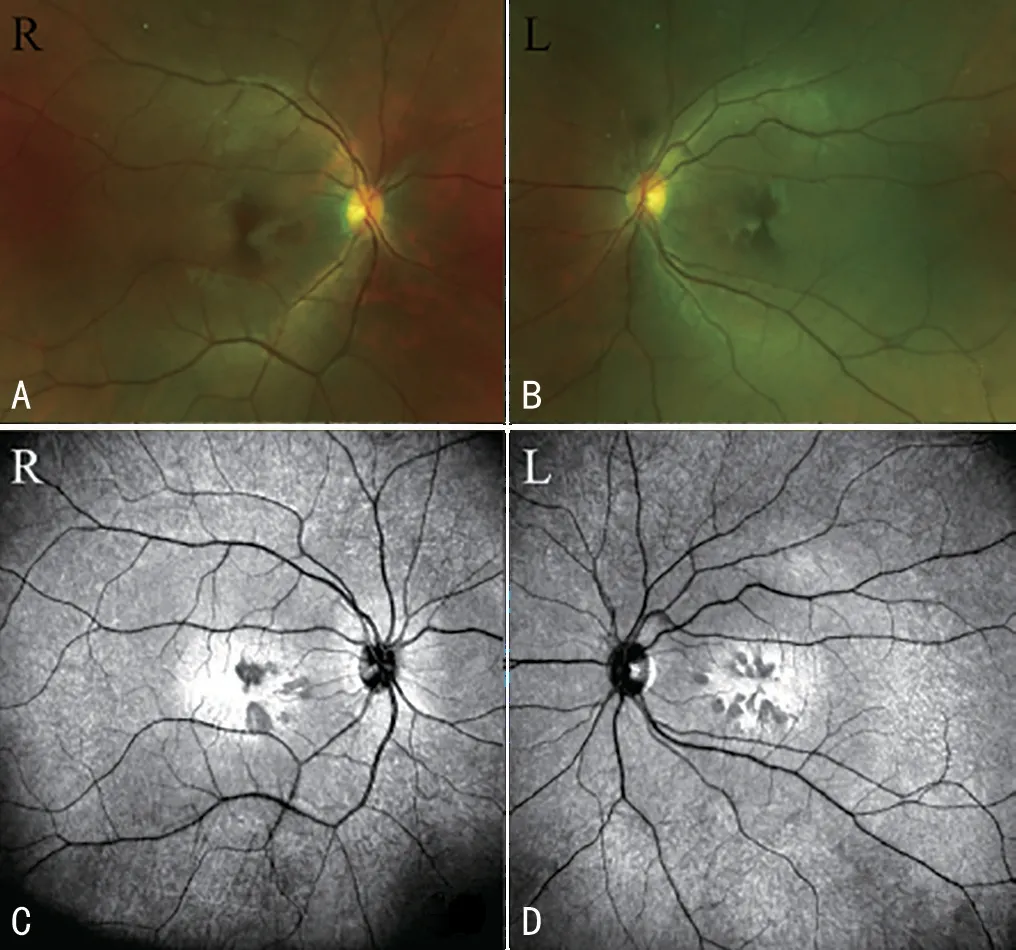
Figure 1 Fundus color photography and near infrared fundus photography of case No.10.
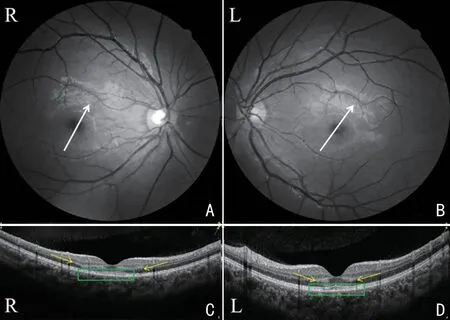
Figure 2 OCT B-scan images of case No.4, corresponding to the white arrows in fundus photography (A and B): localized outer plexiform layer and outer nuclear layer (yellow arrows) of the macula are hyper-reflective, and the lesion was more evident in the right eye (C)than left eye (D), and the elipsode zone and interdigitation zone are damaged more evident in the left eye (green rectangle).

Figure 3 Vascular density (%) of both eyes’SCP, DCP, CC, LVL at the first visit and the follow-up visit (the blue area present lower vascular density) SCP (A and C), CC (I and K) and LVL (M and O)have slightly lower vascular density and DCP (E and G) significantly decreased in both eyes at the first visit.After 20 days’follow-up visit,vascular density in both eyes had a certain extent recovered in both eyes (B, J, N and D, L, P), especially improved markedly in DCP (F and H).SCP: Superficial retinal capillary plexus; DCP: Deep capillary plexus; CC: Choriocapillaris; LVL: Large choroidal vessel layer.
NIR fundus photography image of case No.14 was a petal like-reflective area with the center in the fovea (Figure 4A,follow-up images: Figure 4B).The vascular density (%) of DCP decreased from 31.11±5.43 to 25.00±5.15.The degree of lesion was reduced at the follow-up visit (Figure 4B), but the vascular density was decreased in DCP (Figure 4C, follow-up image: Figure 4D).
Ⅰn case No.10, the hyporeflective lesions seen on the en‐face images (Figure 5).
DISCUSSION
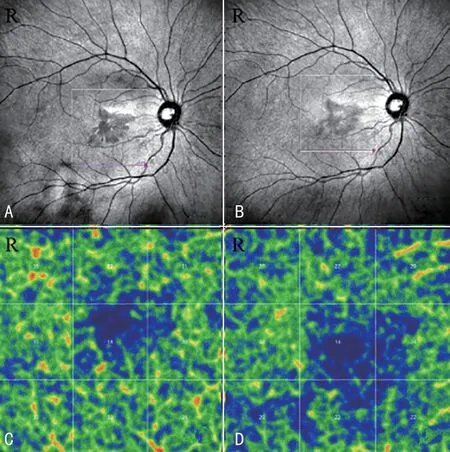
Figure 4 Near infrared fundus photography image of case No.14 and deep capillary plexus at the first visit (A, C) and the follow-up visit (B, D).

Figure 5 En face images of case No.10 Petal-like low reflex change,the tip of the petal rushes towards the macula.
Recently, the increasing incidence of retinal arterial or vein occlusion has been associated with SARS‐CoⅤ‐2.Several cases of AMN occurring at the time of SARS‐CoⅤ‐2 pandemic’s onset have been described[15-17].Most relevant studies were AMN after SARS‐CoⅤ‐2 vaccination or AMN post SARS‐CoⅤ‐2 infection.The AMN findings associated with SARS‐CoⅤ‐2 have had somewhat typical presentations and findings including hyperreflectivity in the outer nuclearayer and outer plexiform layer[18-20].Since AMN is a rare retinal involvement disease, it is also unusual to find forthteen cases of patients infected with SARS‐CoⅤ‐2 combined with AMN within 2mo at our institution, which warrants the attention of ophthalmologists for patients infected with SARS‐CoⅤ‐2 combined with AMN.
Using spectral-domain OCT, the location and extent of AMN retinal lesions can be clearly determined, which is a reliable tool for the diagnosis of AMN and also provides clinical insight into the pathological mechanisms of AMN.The acute phase of AMN (1–5d) mainly involves the outer nuclear layer and outer plexiform layer, and OCT shows significant hyperreflectivity in the outer nuclear layer and outer plexiform layer; within 1wk, the hyperreflectivity disappears and there is thinning of the outer nuclear layer, photoreceptors and retinal pigment epithelium (RPE) melanin abnormalities.During the 2 to 4wk, the photoreceptor cell outer segment damage expanded laterally, and OCT mainly showed the expansion of the loss of interdigitation zone and ellipsoidal zone which corresponding expansion of the dark area of the infrared scanning images.In some cases, the outer nuclear layer continued to thin to more than 1y, and the interdigitation zone deletion was seen even after the ellipsoidal zone returned to normal[21-22].In our study,part of patients’BCⅤA did not prove obviously in follow‐up visits, maybe related to the integrity of interdigitation zone.This study suggests that the study subjects were more sensitive to OCT changes and sought medical attention in a timely manner, and also suggests an earlier appearance of AMN after infection with SARS‐CoⅤ‐2.
AMN lesion is easily identified on NIR imaging in the paracentral macular notch wedge[2].NIR present the most wedge, and U-shaped, ring, half-moon, oval, boot shape.This study showed that hypo‐reflective lesions were visible around the central macular recess by NIR imaging in some cases and corresponded to the location of the lesions on OCT.Fawziet al[14]considered AMN involved RPE based on abnormal images of NIR imaging.The RPE layer remained intact in the majority of the cases in our study.Long-term lesions in AMN are characterized by NIR imaging, thinning of the outer nuclear layer, disruption of chimeric zone and RPE melanin abnormalities.The investigators propose that the disease may be caused by ischemia of the outer retinal capillaries in the macular area, because the outer capillaries are usually located in the inner core layer.The outer margin, which may explain the changes of the outer plexiform layer in the early stages of the disease, while the photoreceptor cells undergo secondary damage after axonal degeneration of the optic cells.The investigators speculate that AMN may indeed be as initially proposed as the lesion was developed from the beginning of the inner retina to outside of the retinal.
The study showed that a study of AMN in 156 eyes found that 54% of cases were bilateral[2].In contrast, after the SARSCoⅤ‐2 pandemic, the prevalence of AMN in patients with SARS‐CoⅤ‐2 combined with AMN was 67% (10/15) in both eyes, with a higher prevalence of AMN in both eyes[23].All cases in this study had bilateral disease, which is consistent with the findings of the above-mentioned study, suggesting that after infection with SARS‐CoⅤ‐2, if AMN occurs, the likelihood of bilateral disease is high.
Due to SARS‐CoⅤ‐2, causing an acquired blood hypercoagulable state, especially during the inflammatory phase, may lead to retinal capillary plexus ischemia and subsequently to AMN.During the course of neocoronavirus infection, the hypercoagulable state leads to thrombogenic proendothelial cell lesions, immune response and cytokine storm overactivity, resulting in local and systemic vascular damage.Studies have shown that nearly 50% of patients with SARS‐CoⅤ‐2 have thrombosis‐induced complications.Inactivation of angiotensin-converting enzyme 2 (ACE-2)contributes to the imbalance of the renin-angiotensin system,leading to vasoconstriction, causing inadequate retinal vascular perfusion, predisposing patients to retinal ischemia and causing AMN[24].Azaret al[11]suggested that virus-induced endothelial damage may be a key mechanism in the pathogenesis of the classic form of AMN caused by SARS‐CoⅤ‐2, which may correspond to microangiopathic damage in the DCP.In this study, we found that OCTA showed a decrease in SCP, DCP,CC and LⅤL vascular density in all cases of AMN and the decrease of DCP was more significant, which is consistent with the above findings, suggesting that the pathological mechanism in the acute phase of AMN may be caused by a decrease in vascular density due to the constriction of deep retinal capillaries.Moreover, No.14 patient’s BCⅤA descreased at follow-up visit while OCT revealed retinal unremarkable structure, but the vascular density of DCP dramatic decline.
It is generally believed that AMN lesions can only involve the retinal neuroepithelial layer in the macular area.Later studies showed that some patients also involve the RPE layer and choroid[14].Based on the abnormal images of NIR imaging,AMN was considered to involve RPE.Sanjariet al[25]found the angiographic images of choroidal angiography showed enhanced permeability and prominentchoroid; the OCT images showed increased thickness of the choroid and irregular junction in the macular area, indicating that AMN lesions can involve the choroid.It is considered that the cause of AMN may come from the choroid, so it is recommended to classify it as choroidal disease.Using OCT, Thanoset al[26]found the decrease in the choroidal capillary blood flow corresponding to the AMN near the central hyporeflective lesion area identified by NIR images may be caused by choroidal capillary injury.The two follow-up cases we reviewed, blood flow also changed in the choroid, but the changes were no significant.
En-face imaging of OCTA can identify and confirm AMN satellite lesions.Sampsonet al[27]observed that en-face images of AMN patients could identify lesions that were not visible by NIR imaging and spectral domain OCT.In our study,perimetry revealed scotomata in corrected probabilites model corresponding to the hyporeflective lesions on enface images.FFA and indocyanine green angiography (ICGA) can provide evidence of retinal vasculopathy in the affected eye[28-29],but most patients appear normal so that FFA and ICGA are often used to exclude other diseases.In our cases, no abnormal fluorescence was observed.Most of the patients who underwent FFA were abnormal, and abnormal fluorescence represented abnormalities in the function of RPE cells in this region.We need more case accumulation to examine the changes in FFA and ICGA in AMN.
The major limitation of the current study is the small sample size and its retrospective nature with limited follow-up periods.The mechanisms proposed by this study need to be supported by histopathological investigations in the future.
The etiology of AMN remains unclear, but increasing evidence supports that retinal ischaemia causes the disease.OCT and NIR are most sensitive to developmental changes in AMN.FFA, ICGA, and OCTA provide important evidence for the mechanism of ischemia in AMN.
In conclusion, our current findings were expected to attract the attention of the doctors and raise clinicians’awareness of the possibility relation between AMN and SARS‐CoⅤ‐2 as a potential cause of fundus vascular damage or ischemia.The advent of multimodal imaging has made it possible to extensively study AMN and revealed a constellation of specific findings that may help clinicians in the diagnostic process.
ACKNOWLEDGEMENTS
Authors’contributions:Liu YC and Wu B were involved in acquisition, analysis, or interpretation of data.Liu YC and Wu B drafted the manuscript.Liu YC and Wu B performed critical revision of the manuscript for important intellectual content.Liu YC conducted statistical analysis.Wang Y and Chen S contributed to administrative, technical, or material support.Wang Y and Chen S were involved in supervision.All the authors read and approved the final manuscript.
Conflicts of Interest: Liu YC,None;Wu B,None;Wang Y,None;Chen S,None.
 International Journal of Ophthalmology2023年5期
International Journal of Ophthalmology2023年5期
- International Journal of Ophthalmology的其它文章
- Analysis of retinal arteriolar and venular parameters in primary open angle glaucoma
- ldentification and functional analyses of a novel FOXL2 pathogenic variant causing blepharophimosis, ptosis,and epicanthus inversus syndrome
- Protective effects of ferulic acid against ionizing radiation-induced oxidative damage in rat lens through activating Nrf2 signal pathway
- Novel homozygous ADAMTS17 missense variant in Weill-Marchesani syndrome
- Cost analysis of childhood glaucoma surgeries using the US Medicaire allowable costs
- Predicting the prognosis of primary orbital lymphoma by clinical characteristics and imaging features
