Microstructural morphology and visual acuity outcome in eyes with epiretinal membrane before, during, and after membrane peeling in intraoperative optical coherence tomography assisted macular surgery
Melanie Weschta, Moritz Pettenkofer,2, Julian E.Klaas,3, Nikolaus Feucht,4, Chris P.Lohmann,Mathias Maier
1Department of Ophthalmology, Klinikum rechts der Isar,Technische Universität München, Ⅰsmaninger Str.22, Munich 81675, Germany
2Jules Stein Eye Institute, University of California Los Angeles, 100 Stein Plaza Driveway, Los Angeles, California 90095, USA
3Department of Ophthalmology, Ludwig-Maximilians-Universität München, Mathildenstr.8, Munich 80336, Germany
4Smile Eyes Augenklinik Munich Airport, Terminalstraße Mitte 18, Munich 85356, Germany
Abstract
INTRODUCTION
Symptomatic epiretinal membranes (ERM) are defined as pre-macular fibroplasia that cause metamorphopsia and can come along with visual impairment due to inner retinal wrinkling and tractive macular edema[1].Depending on the severity of symptoms, ERM can be surgically treated with pars plana vitrectomy (PPⅤ) and membrane peeling (MP).Nevertheless, even after successful removal of the ERM, the long-term visual result may be unsatisfactory for the patient[2].Both baseline visual acuity and duration of symptoms are commonly considered to be the most important prognostic factors for surgical outcome[3].The significance of the baseline central macular thickness (CMT) and foveal configuration for the final visual outcome has not yet been sufficiently proven[4-5].However, it is assumed that the overstretching of the retinal Müller glia cells while surgical removal of the ERM could have a decisive influence on the postoperative visual outcome[6-7].
Optical coherence tomography (OCT) is a well-established method in ophthalmic diagnostics, that allows quantitative measurements of the macula and the vitreoretinal interface[8].Since demonstration of the macular structure and overlying ERM gives valuable information to the surgeon before entering the operating room, it seems promising to integrate OCT technology into the surgical procedure.Microscopeintegrated intraoperative OCT (iOCT) systems enable a realtime visualization of ERM removal and give the surgeon feedback on anatomical and morphological findings during surgery[9-11].Therefore, our idea was to use iOCT to examine intraoperative influence factors on postoperative outcome.However, the usefulness of iOCT still remains controversial.Previous studies have not yet investigated intraoperative influence factors using iOCT, but rather focused on general technical benefits of iOCT for the surgical intervention.Aim of this retrospective study is to investigate intraoperative foveal stretching during ERM removal by using iOCT and its impact on postoperative development of best corrected visual acuity(BCⅤA) and CMT.
SUBJECTS AND METHODS
Ethical ApprovalThe study was approved by the Ethics Committee of the Technical University of Munich and was performed in accordance with the Declaration of Helsinki for clinical studies.Informed consent was waived due to the retrospective nature of the study.
SubjectsFor this retrospective study 59 eyes of 59 patients that underwent PPⅤ for MP with video‐recorded iOCT assistance from March 2016 to July 2017 at the Department of Ophthalmology of the Technical University of Munich were included.Each surgery was performed by the same surgeon(Maier M).Excluded were eyes who had additionally other vitreoretinal disorders for surgical indication like macular holes or previous vitreoretinal surgery in their medical history.BCⅤA pre‐ and postoperatively (3 and 6mo after surgery) was taken from the medical chart.
Surgical ProcedureSurgical procedure involved transconjunctival 3‐port 23‐gauge PPⅤ.Combined lens extraction with phacoemulsification and intraocular lens(IOL) implantation was additionally performed if cataract surgery was indicated (in 18.6% of all cases).After core vitrectomy, posterior vitreous detachment was induced using the vitreous cutter (Dutch Ophthalmic Research Center,D.O.R.C.International, Zuidland, Netherlands) if the posterior vitreous was still attached to the posterior pole.Brilliant peel(D.O.R.C.International, Zuidland, Netherlands) was injected for membrane staining.The peeling was performed with an end-gripping 23-gauge-Eckardt-forceps starting at the inferior macula and then continue centripedal.In all cases the internal limiting membrane (ⅠLM) was peeled as well.Ⅰnsufflation of air was performed in 50 cases and insufflation of C3F8gas was performed in 9 cases at the end of each surgery.
SD-OCT Image Acquisition and AnalysisSpectral-domain OCT (SD-OCT) cross-sectional B-scans (Spectralis, Heidelberg Engineering, Heidelberg, Germany) were analyzed for each eye pre- and postoperatively (3 and 6mo after surgery).For image analysis a volume scan of the macula was used.An incorporated manual software caliper tool (measure circle,1 mm/3 mm diameter) allowed to determine retinal thickness at the fovea and 1 mm/3 mm respectively both nasal and temporal.All OCT images were analyzed by the same investigator.
Intraoperative OCT Video AcquisitionSurgical procedure of MP was obtained successfully with iOCT assistance.Ⅴideos were recorded for the whole run of the surgical procedure.The membrane loop presented clearly on images while sweeping tangentially along the macular surface during peeling and the shadowing artifact created by the surgical instrument was minimal.Real-time iOCT images were obtained with RESCAN 700 (Carl Zeiss Meditec, Inc., Oberkochen, Germany) with an 840 nm wavelength light source and a scanning speed of 27 000 A-scans per second, including Z-tracking and focus control for image stabilization.The axial resolution was 5.5 µm in tissue.Three digital snapshots of iOCT images were taken from the foveal area: before MP, at the moment when MP reached the maximal stretch and immediately after MP was successfully completed in the foveal area (Figure 1).All iOCT videos were analyzed by the same investigator.Due to the missing of a tracking function, the localization of the foveal area had to be assessed subjectively by the investigator.
Intraoperative OCT Analysis and Central Macular Thickness RatioSince no software automated scalation existed for iOCT, snapshots from the iOCT videos were imported into the public domain Java image processing software Image J (Image J, Bethesda, Maryland, USA).Procession with geometric transformation tools allowed scalation of the CMT at any magnification factor between 1:32 and 32:1.To objectify the extent of macular stretching, the value of CMT during peeling(Figure 2B) was divided through the pre-peeling (Figure 2A)value to determine an intraoperative CMT ratio (CMTr).A ratio of 1.0 correlates with no foveal stretch (Figure 2):
Statistical AnalysisStatistics were performed with SPSS software (version 25.0; SPSS, Inc., Chicago, IL, USA) and values are given as the mean with standard deviation (SD) if not stated otherwise.For correlations between parameters,Pearson analysis and Student’st-tests were performed.AP-value<0.05 was considered to be statistically significant.

Figure 1 Intraoperative optical coherence tomography Various samples of intraoperative optical coherence tomography recordings before, during, and after membrane peeling, that were used to calculate the intraoperative central macular thickness ratio.Each of the images on the left shows the presence of an epiretinal membrane overlying the fovea before the peeling.The corresponding image in the middle captures the membrane loop while sweeping tangentially over the fovea during peel.Images on the right show the central macula after peel of the epiretinal membrane in the corresponding eye.
RESULTS
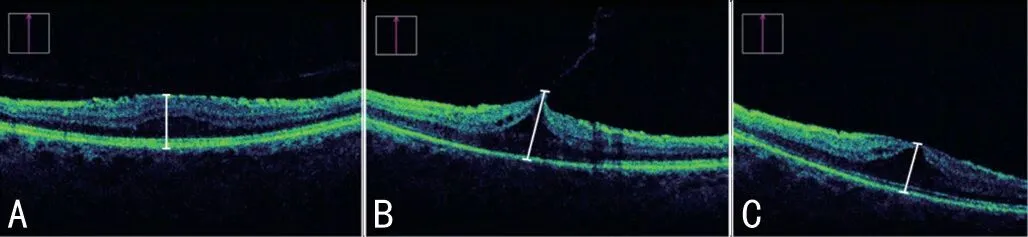
Figure 2 Intraoperative optical coherence tomography scans (Zeiss,LUMERA 700 Rescan) before (A), during (B), and after (C) the foveal peeling of the epiretinal membrane Caliper widths, marked in white, represent measurements of intraoperative central macular thickness (CMT), obtained with Image J software.Obtained values were dimensionless.The CMT ratio was calculated as CMT (B)/CMT (A).
A total of 59 eyes of 59 subjects was enrolled into the study.Twenty-six patients were female (44.0%) and 33 were male(56.0%).The mean age of all subjects at the time of surgery was 70.0±8.13y (range 46-86y).Laterality was distributed asn=29 for the right eye andn=30 for the left eye.The mean baseline BCⅤA in all subjects was 0.49±0.27 logMAR (range 0.1‐1.3).Three months postoperatively the mean BCⅤA was 0.36±0.25 logMAR (range 0.0-1.3,P=0.01vsbaseline).Six months after surgery the mean BCⅤA was 0.38±0.35 logMAR(range 0.0-2.0,P=0.08vsbaseline).There was a significant positive correlation between the baseline BCⅤA and the BCⅤA development at months three and six (r=-0.66,P=0.01 andr=-0.49,P=0.01) respectively.
The CMT ratio gives information about intraoperatively caused retinal stretching and was calculated as mentioned above.Table 1 shows intraoperative CMT values and corresponding CMT ratio.CMT values in Table 1 are relative values and do not indicate the real retina thickness.They were measured withⅠmage J software in snapshots at different times of the iOCT videos to calculate CMT ratio.The mean CMT ratio during membrane peeling was 1.29±0.32 (range 1.02 to 2.59).The average stretch of the macula during surgery was 29% from baseline (range 2%-159%).
Although changes in CMT measurements before and during peeling were highly significant (P<0.0001), changes in CMT before and after peeling did not reach level of significance(P=0.147).
Table 2 shows the mean CMT measured on SD-OCT images that were recorded before and 3mo after surgery.Reduction of CMT measured on SD‐OCT images was significant on macular localizations 3 and 1 mm temporal (bothP<0.0001), at the fovea (P=0.047), and 1 mm nasal (P=0.018).CMT reduction measured at 3 mm nasal was not statistically significant(P=0.36).
Correlation of intraoperative CMT ratio with BCⅤA changes 3mo after surgery tended to be slightly negative, however,did not reach a level of significance (r=0.24,P=0.10).Neither a significant correlation to BCⅤA changes after 6mo was observed (r=-0.06,P=0.72).
Correlation between the intraoperative CMT ratio and the difference of CMT from baseline to 3mo after surgery obtained with SD‐OCT was significant at the fovea centralis (r=-0.43,P=0.008) and 1 mm nasal and temporal from the fovea (r=-0.37,P=0.024 andr=-0.50,P=0.002 respectively; Figure 3).

Figure 3 Correlation between intraoperative CMT ratio based on real time intraoperative OCT image captures and the reduction of CMT measured on SD-OCT pre- to postoperatively The correlation is significant in the fovea centralis and 1 mm nasal and temporal of the foveal pit.CMT: Central macular thickness; OCT: Optical coherence tomography.
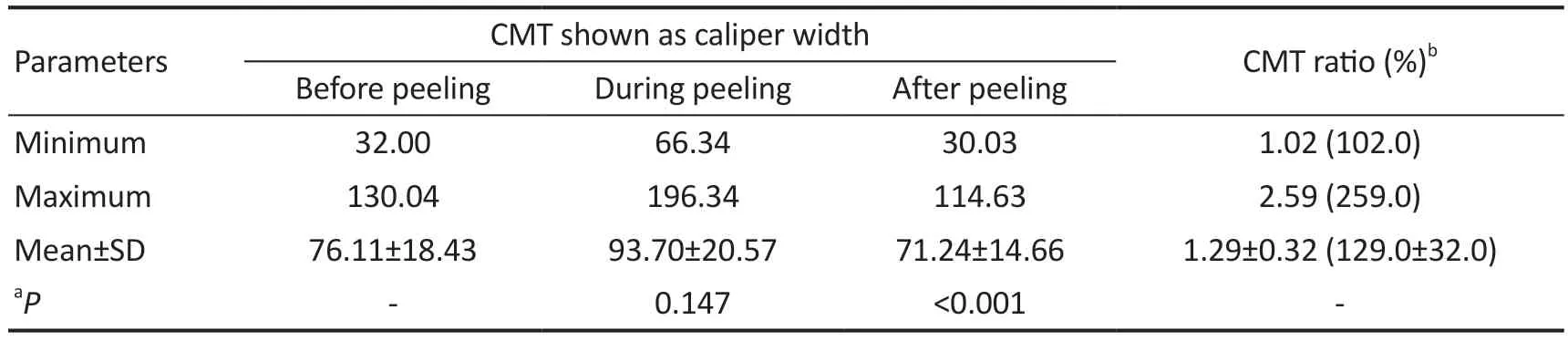
Table 1 Intraoperative CMT and CMT ratio

Table 2 Pre- and postoperative CMT mean±SD, µm
DISCUSSION
Ever since the first results of surgical removals of ERM were published by Machemer in 1978[12], retinal surgeons increasingly have sought to further develop the technique of membrane peeling, resulting in a higher surgical success on improving the patient’s visual outcome.Today, 70%-80%of ERM patients are expected to improve their BCⅤA after macular surgery.However, still a considerably number of patients who underwent vitrectomy and MP does not ultimately benefit from the procedure[2,13].
Several studies[3-4]tried to establish preoperative prognostic factors in order to improve the surgeon’s predictive informative value.However, a valid definition of prognostic factors which individually provides a clear and reliable answer to the question of definitive surgical outcome has not been found yet.Therefore, it seems obvious that most likely not only preoperative, but also intraoperative influences like macular stretching act on the retina and thus impact visual function[11,14].Aim of this study was to investigate whether stronger dilation of the retina during membrane peeling could be associated with a poorer visual outcome and reduced recovery of the macular morphology, based on the hypothesis that retinal overstretching causes damage to Müller glia cells[15].
Previous studies focused on iOCT as an additional intraoperative diagnostic tool, that can aid therapeutic decision-making during surgery and influence the further surgical strategy,e.g.in patients with vitreous haemorrhage, full thickness macular holes, ERM, submacular haemorrhage and vitreomacular surgery in highly myopic eyes[16-24].It was concluded that iOCT, as it offers immediate visualization of retinal anatomy and instrument-tissue-interactions during peeling, adds to the understanding of intraoperative traumatic changes due to the peeling procedure[10].In addition, iOCT has been used to gain a better understanding of the pathology of rare diseases,such as optic disc pit-associated maculopathy[25].In our study we used the visualization of instrument-tissue-interactions by iOCT to investigate the foveal stretch of the retina during peeling as a traumatic iatrogenic intraoperative influence factor for postoperative outcome.Therefore, our study is different to those studies who have mainly focused on general technical benefits of iOCT to improve visualization and surgical accuracy[15,26-27].
Impact of Retinal Stretching on Visual Acuity and Development of Central Macular ThicknessIn our study we could show a significant improvement of BCⅤA at three months after surgery.Wonget al[2]who investigated a total of 125 eyes also showed that patients with preoperatively lower BCⅤA had a higher benefit from surgery than patients with initially better BCⅤA.This negative correlation of preoperative BCⅤA with the visual acuity development after surgery has also been significant in our study.
The higher potential for visual improvement in patients with initially poorer visual acuity might be explained by more preoperative damage of photoreceptor cells resulting in a poorer functional condition of the retina.In these cases,surgical intervention may repair larger lesions than in patients with better preoperative BCⅤA and correspondingly better functional retinal status at baseline.
Examination of different stages of ERM showed that lower traction at lower stage resulted in reversible retinal changes and higher traction at higher stage resulted in irreversible retinal changes[28].This suggests that not only the traction strength of the disease itself but also the traction strength during MP may have an influence on retinal changes and postoperative outcome.
Müller cells as glia cells that span the entire retina play an important role in the dehydration of the retina.Reichenbachet al[6]described that retinal dehydration is regulated by potassium channels,e.g.Kir4.1, that are expressed both on retinal blood vessels and the ILM.H2O osmotically follows the potassium ions through AQP4 channels.Dysfunction of Müller cells causes a downregulation of potassium channels,which in turn causes potassium accumulation in the Müller cell.Osmotic inflow of H2O leads to Müller cell swelling and consequently cytotoxic macular edema that might cause a loss of function of the neighboring photoreceptors and other retinal neurons, which might ultimately result in poorer postoperative BCⅤA.Therefore, the assumption is close, that retinal overstretching during MP harms the Müller cells and leads to their dysfunction[6].
Lindqvistet al[15]described a mechano-sensitivity of Müller cells.Tissue stretching causes an activation of ion channels intracellularly, in particular Ca2+-channels.Ca2+-activated potassium channels result in a potassium outflow, osmotically followed by H2O.This might be a compensatory, regulatory mechanism to counteract cell swelling and macular edema[29].Despite this compensation, excessive stretching of the retina may also lead to an exhaustion of this mechanism, so that cell swelling cannot adequately be prevented.
Not only dysfunction but also reactivation of Müller cells and the associated known Müller cell gliosis can have negative effects on the retinal tissue.In response to virtually every pathological alteration of the retina, including retinal trauma,Müller cells become reactivated.It is known that on the one hand reactivated Müller cells protect neurons after retinal injury, but on the other hand gliotic alterations of Müller cell reactivation may also contribute to neuronal degeneration and edema development in the diseased retina by different and complex mechanisms[30-31].
However, it is not the sole dysfunction and gliosis of the Müller cells, but most likely a combination of several factors that is causative for lower visual improvement.Thus,photoreceptors and other retinal neurons may be damaged directly by overexpansion or indirectly by the disturbed regulatory mechanism of the Müller cells.The consequence would be a loss of function of these retinal neurons.
The finding of no statistically significant correlation between retinal stretching and visual outcome within 6mo postoperatively in our study might be an indication that the retinal neurons are also capable of repairing the negative consequences of overstretching during the postoperative course.Certainly, the Müller cells play a decisive role in this regenerative process[29,32-35].
A previous study found that visual acuity in patients with ERM improved after vitrectomy in correlation with CMT decrease.Therefore, they found that CMT decrease is associated with visual improvement in the postoperative follow-up[36].Our study showed a significant negative correlation between CMTr and CMT decrease postoperatively, but not between CMTr and visual acuity development within 6mo postoperatively.Since the CMT decrease is rather slow postoperatively, this could indicate that a longer follow-up might have shown a correlation with long term visual improvement.
Ⅰn order to determine a long‐term influence of intraoperative retinal stretching on the CMT, the intraoperative CMTr was correlated with both, perioperative CMT and BCⅤA development three and six months after surgery.There was a statistically significant negative correlation between intraoperative retinal dilatation and thickness reduction at the sites of the fovea, as well as 1 mm parafoveal nasal and temporal.Since those patients whose central retina was exposed to greater intraoperative stretching showed significantly less decrease in macular thickness after surgery,it is assumed that this result might be based on Müller cell dysfunction and gliosis.Consecutively, due to a lack of dehydration function, damaged Müller cells might cause retinal micro-edema.In addition, the formation of glial scars may impede the repair and remodeling of the retinal tissue after retinal injury[31], so that a possible consequence in our study was a slower decrease in retinal thickness in the long term in cases with greater stretching and therefore possibly also a stronger gliosis reaction of the Müller cells.
However, it may be possible and useful to develop peeling techniques that are particularly low in traction in the future.In general, for this purpose it can be recommended that the surgeon should try to direct the force vectors as tangentially as possible during peeling.Posterior/anterior traction forces should be avoided as much as possible.
A major limitation of current iOCT systems in our study is the lack of eye tracking systems.Previous studies already mentioned this as a limitation[37].In our case this had the consequence that the localization of the foveal area had to be assessed subjectively by the investigator which can inevitably lead to inaccuracies.Future technical development of the iOCT systems will be needed to enable more precise investigations.
We can conclude, that regarding functional and morphological findings, our study did not show a significant correlation between retinal stretching during MP and visual outcome up to 6mo postoperatively.However, we are able to report significantly less reduction of central retinal thickness in individuals whose retina was more extensively stretched during MP.
ACKNOWLEDGEMENTS
Part of the results in this manuscript has been previously presented at the ARⅤO Annual Meeting in Honolulu 2018.
Conflicts of Interest:Weschta M,None;PettenkoferM,None;Klaas JE,None;Lohmann CP,None;Feucht N,Speaker honoraria of Alcon, Allergan, Bayer, Heidelberg Engineering;Maier M,Speaker honoraria of Alcon, Allergan,Bayer, Heidelberg Engineering, Novartis, Zeiss and clinical trials of Bayer, Novartis, Roche.
 International Journal of Ophthalmology2023年5期
International Journal of Ophthalmology2023年5期
- International Journal of Ophthalmology的其它文章
- Analysis of retinal arteriolar and venular parameters in primary open angle glaucoma
- ldentification and functional analyses of a novel FOXL2 pathogenic variant causing blepharophimosis, ptosis,and epicanthus inversus syndrome
- Protective effects of ferulic acid against ionizing radiation-induced oxidative damage in rat lens through activating Nrf2 signal pathway
- Novel homozygous ADAMTS17 missense variant in Weill-Marchesani syndrome
- Cost analysis of childhood glaucoma surgeries using the US Medicaire allowable costs
- Predicting the prognosis of primary orbital lymphoma by clinical characteristics and imaging features
