Guidelines from an expert panel for the management of diabetic macular edema in the Malaysian population
Nor Fariza Ngah, Nor Asiah Muhamad, Shelina Oli Mohamed, Roslin Azni Abdul Aziz,Nur Hasnah Ma’amor, Nor Azita Ahmad Tarmidzi, Hanizasurana Hashim, Hamisah Ishak,Wan Norliza Wan Muda, Rosiah Muda, Azian Adnan, Rafidah Md Saleh, Wong Hon Seng,Nurfahzura Mohd Jamil, Tara Mary George1, Adrian Koh1
1Institute for Clinical Research, National Institutes of Health,Ministry of Health, Selangor 40170, Malaysia
2Department of Ophthalmology, Shah Alam Hospital, Ministry of Health, Selangor 40000, Malaysia
3Sector for Evidence-based Healthcare, National Institutes of Health, Ministry of Health, Selangor 40170, Malaysia
4Department of Ophthalmology, Kuala Lumpur Hospital,Ministry of Health, Federal Territory 50586, Malaysia
5Department of Ophthalmology, Selayang Hospital, Ministry of Health, Selangor 61800, Malaysia
6Department of Ophthalmology, Tengku Ampuan Afzan Hospital, Ministry of Health, Pahang 25100, Malaysia
7Department of Ophthalmology, Sultanah Nur Zahirah Hospital,Ministry of Health, Kuala Terengganu 20400, Malaysia
8KPJ Selangor Specialist Hospital, Selangor 40300, Malaysia
9Sultan Abdul Aziz Shah Hospital, Selangor 43400, Malaysia
10Gleneagles Kuala Lumpur, Federal Territory 50450, Malaysia
11Department of Ophthalmology, Sungai Buloh Hospital,Ministry of Health, Selangor 47000, Malaysia
12Sunway Medical Centre, Selangor 47500, Malaysia
13Camden Medical Centre, Singapore 248649, Singapore
Abstract
INTRODUCTION
Diabetic Macular Edema and Vision LossDiabetic macular edema (DME) is a retinal thickening involving the central fovea close to macula which is one of the most prevalent causes of visual loss worldwide[1-2].It is characterized by a buildup of fluid in the central region of the retina because of fail blood-retinal barrier[1].In the healthy people, the central retinal thickness (CRT) varies between 212±19 and 289±16 µm[3]while in DME patients, the CRT can vary from 225 to >450 µm[1].Localized edema is caused by leakage from clusters of microaneurysms, whereas diffuse edema is caused by broad capillary leakage[1].DME can manifest as the appearance of hard exudates which produce blurring and distortion of central vision, and can be measured by a reduction in best‐corrected visual acuity (BCⅤA)[1].Diabetic retinopathy (DR) is the presence of microvascular abnormalities in the fundus of diabetic patients which can be seen during clinical examination or color fundus photography.The earliest and least serious form of DR is the dot-like microaneurysms, which is discrete saccular outpouchings of the capillary wall that have sharp edges and look like a small red dot.DR is a one of the ocular diabetic complication caused by long-term diabetes.Prolong or uncontrolled diabetic can cause damage to the blood vessels in the eyes, which may lead to vision loss[4].Globally, 34.6% of diabetic patients have DR[5], while in Malaysia, 10.4% of ageing diabetic have it, which results in blindness[6].Active screening and early diagnosis of DR are crucial to preventing vision loss since higher prevalence of diabetes is one of the primary causes of blindness globally[7].DR screening is one of the starting points for continuous and effective management of DR to minimized the incidence of vision loss[8].Optical coherence tomography(OCT) imaging has provided novel diagnostic measures and clinical information that have been used to stage the illness.The ophthalmologist has several treatment options which consist of various procedures with different outcomes measure,including laser photocoagulation, anti-vascular endothelial growth factor (ⅤEGF), steroids, and surgical therapy.These strategies have heralded the beginning of a new era in DME therapy.When laser treatment and anti‐ⅤEGF fail to give an adequate impact in DME patients, the pars plana vitrectomy operation is done[9].Pars plana vitrectomy reduces the macula’s thickness and improves visual acuity (ⅤA) by mechanically removing vitreous fluid.
How to Detect Diabetic Macular Edema
Optical coherence tomographyOCT scan is the industryrecognized gold standard for DME diagnosis.It may be used to recognize different types of DME, detect macula traction,and locate edema to specific retina layers[10].Diverse retinal thickening, cystoid macula edema, serous retinal detachment without posterior hyaloidal traction, and posterior hyaloidal traction with tractional retinal detachment are morphologic manifestations of DME on OCT[11].Subretinal fluid and/or small intraretinal cystoid fluid and/or external limiting membrane and inner segment/outer segment integrity and vitreomacular adhesion are a good baseline predictor for good treatment response with high vision gains and/or good finalⅤA which is a prognostic marker from OCT for DME[1,12].Contrarily, baseline abnormalities of the retinal inner layers,disruption of the inner and outer photoreceptor segments,and/or the external limiting membrane, which may result in irreversible photoreceptor destruction and loss, as well as baseline subfoveal choroid thinness, are predictors of poor visual outcomes post-treatment[1].
After dexamethasone implants in eyes with DME, biomarkers such as subretinal fluid, inner segment/outer segment continuity, the lack of hyperreflective foci, and an attached vitreoretinal interface indicated improved visual results.Anti-ⅤEGF (ranibizumab) biomarkers such as ellipsoid zone disruption and the lack of epiretinal membrane have been linked to superior therapy outcomes[13-14].
A potential predictive biomarker for the visual consequences of DME is the disorganization of the retinal inner layers.Disorganization of the inner layer of retinal has been connected to both disruption of the outer retina and an increase in the severity of DR[15].Cystoid macular edema, serous retinal detachment of subretinal fluid and retinal enlargement or thickening were all seen on OCT in individuals with DME[16].Hyperreflective foci is another prognostic sign for DME,which forms plexiform layer’s outer confluent plaques and is found within the walls of intraretinal microaneurysm[17].The foci may be an early sign of DME barrier failure since they are believed to be extravasated proteins and/or lipoproteins.In DME patients, higher baseline hyperreflective foci levels indicate therapy response as measured by ⅤA improvement and CRT decrease after three months[18].
Fluorescein AngiographyWhen OCT angiography is not available[1], fluorescein angiography plays important role in identifying treatment failure or inadequate response[19],identifying the foveal avascular zone[1], guiding supplemental laser therapy[20]and diagnosing of co-existing peripheral DR[1].In order to evaluate the central and peripheral retina,fluorescein angiography may be employed.
SUBJECTS AND METHODS
Ethical ApprovalThis study used all the published data.Ethical approval is not required.This study was registered with National Medical Research Register (NMRR) with registration number NMRR ID-22-01045-RUP.
In early 2021, a team of experts from Malaysia comprising 14 ophthalmologist who were medical retina specialist with an external reviewer convened together to discuss recent research and developed a consensus guideline for the treatment of DME and how they relate to international trends and practices.
Consensus DevelopmentThe Malaysia Retina Group’s efforts to establish local treatment guidelines and consensus for the management of DME and to get recommendations based on the best-updated practice resulted in the present current consensus.Prior to the conference, 11 recommended statements and one management algorithm were created using existing guideline recommendations, regional health care reimbursement policies, and treatment trends.A thorough discussion of each statement was followed by a secret vote.When ≥85% of experts voted, it was considered that consensus had been reached.Discussions were repeated, statements were changed, and voting continued until an agreement was reached.
RESULTS
RecommendationThe DME patients were characterized according to their therapy response patterns to provide the recommendations.Table 1 is a summary of the consensus guidelines for DME management.
Treatment GoalAlthough available treatments can retain and improve vision for the great majority of patients, it can be associated with significant expenses and visit burdens; hence,identifying the best treatment regimen is crucial.Ⅰn significant pilot investigations of DME treatment agents, it is decided that BCⅤA will be the main endpoint.Ⅴarious studies using ranibizumab injection in patients with clinically significant macular edema with center involvement linked to DME in RISE (registered on ClinicalTrials.gov as NCT00473330)/RIDE (NCT00473382)[21]and randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with DME [MAED (NCT00168337 and NCT00168389)] studies[22],where the proportion of patients acquiring >15 letters in BCⅤA from baseline was identified as the primary objective.However, DME resolution occurs when ranibizumab is used alone or in combination with laser treatment when the primary outcome was the mean change in BCⅤA from baseline[RESTORE (NCT01609374)][20], ⅤⅠⅤⅠD (NCT01363440)/ⅤⅠSTA (NCT01331681)[23], Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol Ⅰ[24]and DRCR.net Protocol T studies[25].From the clinical setting, improvement ofⅤA may not be achieved, due to the Snellen is the commonest tool us for ⅤA testing instead of Early Treatment Diabetic Retinopathy Study chart.Ⅰn other cases, ⅤA improvement is achieved after the disappearance of macular edema.Therefore, these factors led to the development of this set of guidelines, which determine the most effective treatments for the disease and inform ophthalmologists about the most recent advancements in clinical practice and the necessity of prompt suggestion to retina specialist for additional management when required.
Diabetic Macular Edema Treatment OptionsThe consensus’s recommendations were simplified into an algorithm (Figure 1)and overview (Table 2) to offer a straightforward treatment protocol for DME maintenance.

Table 1 Consensus guidelines for managing DME
The following points provide a concise description of the algorithm and overview:
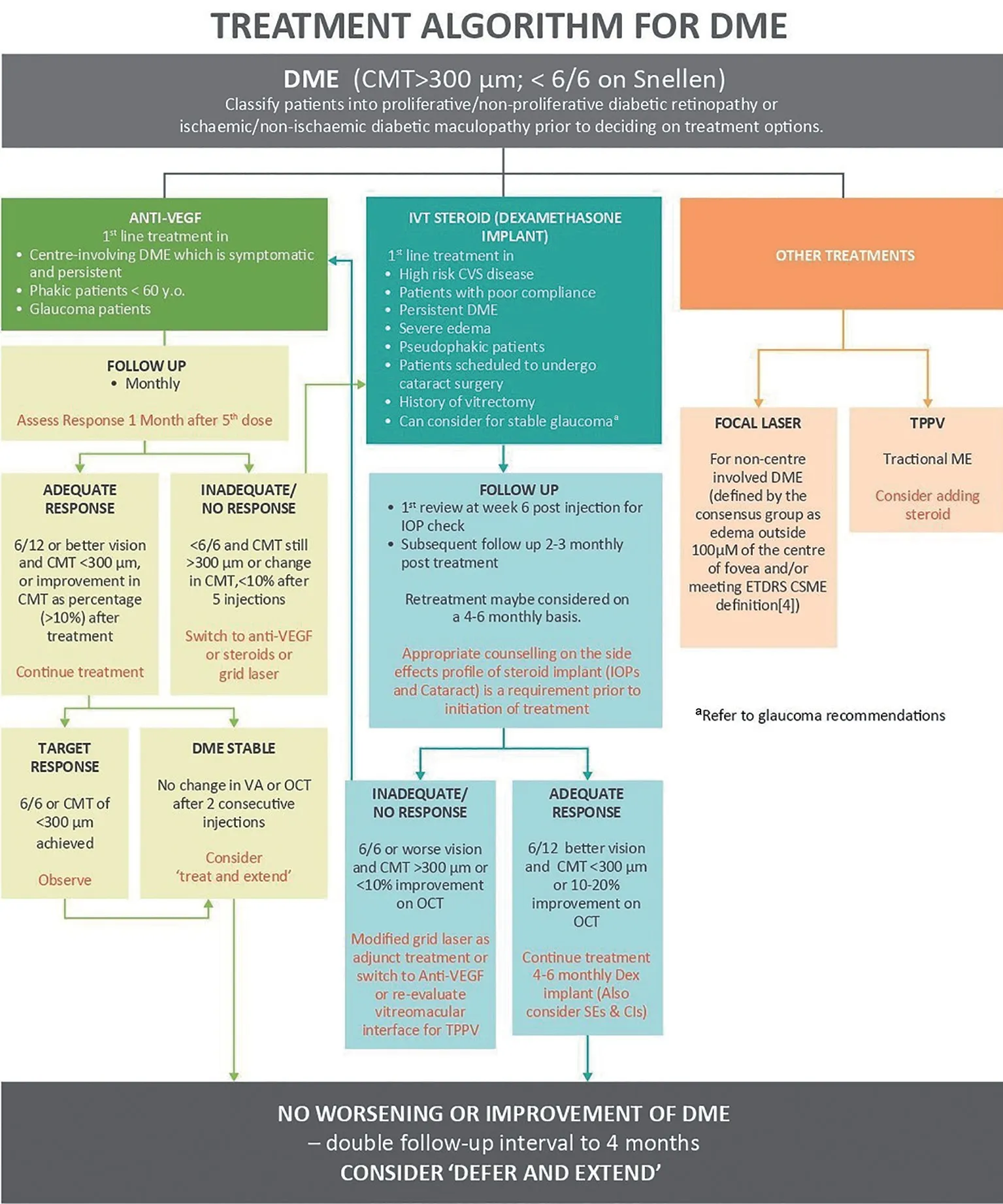
Figure 1 Treatment algorithm for DME adapted from Chhablani et al (2020)[36]aFollow-up intervals can be doubled to 4mo if there is no worsening or improvement in DME after anti-VEGF or steroid treatment, and a “defer and extend”strategy may be used.DME: Diabetic macular edema.

Table 2 The overview for management of DME
Anti-vascular endothelial growth factorThe primary line of treatment for patients with symptomatic DME is anti‐ⅤEGF therapy (defined as edema within 100 µm of fovea center),phakic patients 60 years age and older and glaucoma patients.The existing anti‐ⅤEGF recommendations prescribe a loading dose of three injections.However, in certain DME patients,loading phase is slower as ⅤA improvement persists up to five injections[26].Depending on financial resources, early intensive therapy will be advised.Early intensive therapy with minimum five to six first monthly dosages, with a maximum of eight to nine injection in the first year, may provide positive outcomes and enable for lessen burden of therapy in upcoming years[20,25].The ⅤA can be significantly improved with monthly injection of anti‐ⅤEGF drugs with every reactivation of the disease pro pre nata protocol (PRN protocol) or treat-and-extend injectable treatment.DRCR.net Protocol I study using ranibizumab with a pro pre nata basis regimen has demonstrated a reduced number of injections at a mean of eight to nine in the first year,two to three in second year and one to two in year three while maintaining good visual outcomes[27].ⅤA is considered stable when there is no change after two successive injections and there is a change of under 10% in the central macular thickness on the OCT.Therefore, when DME is stable during follow up, it will be advisable to consider treating and extending the regime.However, steroid (dexamethasone) injection can be considered if there is no response after five to six injections,especially in pseudo phakic patients.
All anti‐ⅤEGF agents are equally effective and any of them can be used as a first-line treatment.However, Protocol T results indicate that aflibercept can be started in patients a worseⅤA (20/50 or less) who had baseline fluorescein angiography readings of 20/32 to 20/40.Treatment with anti‐ⅤEGF agents reportedly improved patients’vision by one to two lines at two years with no significant differences among agents[25].
The intravitreal use of ⅤEGF inhibitors may increase the incidence of arterial thromboembolic events.Nonfatal myocardial infarction, nonfatal stroke, and vascular death are all considered to be arterial thromboembolic events.A Metaanalysis of anti‐ⅤEGF drugs for patients with DME who received intensive monthly anti‐ⅤEGF for two years suggests that the risk may be related to cumulative medication exposure.The analysis revealed a possible increased risk of fatalities and cerebrovascular accidents[28].
Ranibizumab with doses of 0.5 mg and 0.3 mg was found to have hazard ratios of 1.05 and 0.78 for arterial thromboembolic events, 0.84 and 0.94 for myocardial infarction 0.94 and 0.53 for stroke or transient ischemic attack, 1.63 and 0.59 for stroke(excluding transient ischemic attack), and 2.17 and 2.51 for vascular death when compared to sham[29].Study on off label bevacizumab showed that the overall systematic side effect of intravitreal injections range from 0 to 39.3%.The majority of these occurrences are minor, unmanaged and retrospective[30].
Steroid implantBefore beginning treatment, steroid implants should be given to patients with high-risk cardiovascular disease.Potential candidates for treatment include patients with a history of vitrectomy, severe edema (greater than 500 μm),pseudophakic patients (steroid implant is contraindicated in patients with anterior chamber intraocular lens, patients who are scheduled for cataract surgery, and patients with low compliance.
After six weeks of the injection, regular intraocular pressure(IOP) should be measured in patients with no other ocular comorbidities and followed-up at months two to three after therapy.Retreatment may be recommended every four to six monthly, depending on the results.If the reaction is positive, it may be possible to consider additional evaluation and therapy at 4‐ to 6‐montly intervals while keeping an eye on side effects and contraindications.If the reaction us insufficient or no reaction is given, switch to another anti‐ⅤEGF drug.
According to MEAD study, patients receiving 0.7 dexamethasone exhibited IOP increases of at least 10 mm Hg in 27.7% of cases and >35 mm Hg in 6.6% of cases, and with 41.5%requiring IOP-lowering medications.Only one patient required surgical intervention[22].In the majority of clinical trials, 20%of patients/eye had IOP increase of interest.IOP-lowering agents and anti-glaucoma mediation were not necessary in the majority of IOP increase cases[31].Retinal/vitreous/subconjunctival hemorrhage was another minor problem that occurred in 1%-2% of patients[31].Problems relating to cataracts were observed in nearly half of phakic patients in several studies[31].The dose of 0.7 g Ozurdex arm of the MEAD trial gave an unfavorable event relating to cataracts occur at an incidence of 67.9% mostly in phakic patients.Following cataract surgery, the patient’s eyesight improved from baseline[22].
Focal/grid laserFor non-centre involving DME (defined as edema outside 100 µm radius of the foveal centre and/or meeting the Early Treatment Diabetic Retinopathy Study-Clinically Significant Macular Edema definitions[32]and rescue laser for DME involving the centre is indicated after at least six months of anti‐ⅤEGF treatment.Other indications include DME affected eyes with a CRT <300 µm[1].
Both Protocol I from the DRCR.net and Protocol T suggested postponing focal/grid laser therapy for at least 24wk.The visual outcomes of Protocol I were better to those of rapid laser with ranibizumab[27].
Ideally, focal laser should be used in conjunction with grid laser to treat diffuse macular leakage, leaky microvascular abnormalities, and non-perfusion in thicker retinas[1].Every three to four months, the focal/grid laser should be redone.If edema continues or does not improve despite receiving anti-ⅤEGF therapy (if available, if it is thought that using more laser could be beneficial).
There are several potential side effects of laser treatment,including the growth of laser scars (atropic creep), secondary choroidal neovascularization central scotoma, deterioration of colour vision, night vision, and contrast sensitivity, as well as subretinal fibrosis and visual‐field sensitivity deterioration[1].
Pars planavictrectomy + membrane peelingIn situations with tractional macular edema, therapy is indicated with or without additional intravitreal steroid treatment[33].
Special ConditionsBlood sugar control patient, cataract surgery, pregnant DME patients, vitrectomized eyes, stable pre-existing glaucoma and endophthalmitis should all be considered while managing DME.
Blood sugar controlPoor glycemic control is linked to DME degeneration[34].Glycemic management prevents diabetes from developing and slows down its course[35].Education on the importance of managing diabetes is crucial for DME patients.
Cataract surgeryPrior to cataract surgery DME should preferably be treated and stabilized.Cataract surgery might provide the highest BCⅤA[36].The potential development of cataracts should be discussed with patients using steroids for DME.Steroid therapy causes cataracts to worsen in those who already have them, requiring cataract surgery to attain the maximum BCⅤA.Ⅰn DME patients who undergoing peri‐operatively, anti‐ⅤEGF treatment can be given prior to cataract surgery or steroid treatment may be taken into consideration during cataract surgery[36].
Treatment in pregnant DME patientsDiabetic pregnant women are more prone to develop retinopathy and disease progression[37].Despite substantial improvement in DME management, the recommended therapy for DME during pregnancy has remained the same throughout the years.
One of the therapies options is laser therapy.Other options include subthreshold laser and intravitreal corticosteroids.Due to concerns about fetal safety, anti‐ⅤEGF therapy should be avoided[38].Intravitreal steroids are regarded as a pregnancysafe treatment option for DME resistant to laser therapy[34].Ⅰntravitreal dexamethasone implantation is effective treatment option for pregnant women with DME[39].
Vitrectomized eyesThe effectiveness of anti‐ⅤEGF in vitrectomized eyes is inconclusive[36].There are no significant changes in BCⅤA and central macular thickness between vitrectomized and non‐vitrectomized eyes after anti‐ⅤEGF injections in some studies, whilst others imply decreased intravitreal efficacy because of increased molecular clearance.In both vitrectomized and non-virectomized eyes, intravitreal dexamethasone implants are beneficial in treating chronic DME[40].
Stable pre-existing glaucomaPresently there is no definition of “stable glaucoma”.However, the patient’s clinician will consider glaucoma as‘stable’when the IOP remains below the target IOP which will be determined by the patient’s clinician or when patients is on less than three medications and requiring no medication changes over a 48-month period during which no further visual field loss monitored[41].Anti-glaucoma treatment should be continued throughout the course of steroid treatment in DME patients with stable pre-existing glaucoma.If IOP is not controlled, treatment should be stopped, and the patient should be sent to a glaucoma specialist[36].
EndophthalmitisAccording to large scale meta-analyses, the incidence of endophthalmitis following intravitreal injections range from between 0.035% to 0.065%[42].Out of 2928 injections of dexamethasone for DME, only two occurrences of acute endophthalmitis were recorded after treatment in the MEAD research[32].Retrospective studies conducted in the United States found that the incidences of endophthalmitis following the use of aflibercept, bevacizumab, and ranibizumab were 0.100%, 0.056% and 0.046% respectively[43].If patients did not react to intravitreal antibiotics, there are anecdotal situations where the implants were removed following vitrectomy in the lack of recommendations[44].
DISCUSSION
Appropriate treatment is required to prevent vision loss in DME.Prior to creating an individualized treatment plan for DME, it is crucial to consider risk factors such as the disease’s severity, risk of cataracts, presence of exudates,history of vitrectomy, use of anti‐ⅤEGF and steroids, and patient compliance.DME can be treated with intravitreal corticosteroids, vitreoretinal surgery when required, anti-ⅤEGF medications, and retinal laser photocoagulation.Several circulating proinflammatory cytokines such as hyperreflective retinal spots and subfoveal neuroretinal detachment have recently been explored as serum biomarkers for response in individuals with refractory DME, and their possible link with the DR and DME development[45-46].DR severity is correlated to cytokine levels but not ⅤEGF levels.
The effectiveness of anti‐ⅤEGF in vitrectomized eyes is uncertain.According to several research, that higher clearance of the molecule reduces intravitreal effectiveness[47], while in another research, patients with DME with vitrectomized and non vitrectomized eyes did not significantly vary in BCⅤA or central macular thickness following anti‐ⅤEGF injection[48].Triamcinolone acetonide also shown similar outcomes[49].
In both vitrectomized and nonvitrectomized eyes, intravitreal dexamethasone implants are beneficial in treating chronic DME[40].However, vitrectomy has no impact on the effectiveness or safety profile of dexamethasone implants for DME[50].Spectral domain-OCT offers potential criteria for predicting dexamethasone implants response; nevertheless,more research is needed.When macular thickening cannot be detected clinically but can be measured by OCT, the disease is known as the subclinical DME.Loboet al[51]found patients with evidence of subclinical DME have relatively small percentage to develop clinically severe DME with continuous monitoring, glycemic control, and comprehensive treatment for other risk factors such as hypertension and hyperlipidemia.The current gold standard of treatment is anti‐ⅤEGF therapy however, the use in different type of patients may result in interindividual differences[52].
The pathogenesis of DME is usually complicated by inflammation.In DME patients whose pathophysiology includes inflammation as a major factor, steroids may result in more favorable treatment results.A number of DME inflammatory pathways are also targeted by corticosteroids,particularly intravitreal dexamethasone, beside to ⅤEGF.This involves retinal leukostasis, and synthesis of proinflammatory mediators (interleukin 6, monocyte chemoattractant protein-1),both of which are important in DME development[53].
A study conducted Sudhalkaret al[54]to determine the relationship between the position of dexamethasone intravitreal implants in the vitreous cavity and ocular hypertension,found that the treatment satisfaction of DME patients who received dexamethasone intravitreal implants had a statistically significant improvement.However, a study conducted in
Sweden with anti‐ⅤEGF injections and additional; treatment such as laser and dexamethasone implants showed no change at four years when compared with baseline[55].
Proliferative DR and DME have different retinal microvascular patterns that indicate small-vessel disease.When DME is present in proliferative DR, patients on oral anti hyperglycemic medications may be at an increase chance of developing cardiovascular disease[56].Pan-retinal photocoagulation should be used to treat naive proliferative DR.Patients with nonproliferative DR have two clinical options: the exudate production stage in DME or the proliferative changes of DR[57].
The first‐line therapy is anti‐ⅤEGF injection in conjunction with pan-retinal photocoagulation in patients with severe nonproliferative DR who have progressed to the proliferative stage of DR.In the past several years, DME management has changed as a result of advancements in imaging technology and the introduction of new drugs.Therefore, in the future, in light of new research, our recommendations may need to be changed.
Ⅰn conclusion, laser coagulation is the first line treatment for individuals without central macular disease.Patients with central macular involvement who have not recent experienced cardiovascular disease should be advised to start using anti-ⅤEGF drugs.Steroids or changing to different anti‐ⅤEGF medication should be thought about in the event of nonresponders.The safety measures that should be taken during steroid/intravitreal dexamethasone treatment owing to its potential side effects, including IOP spike, glaucoma, and cataract development, are well covered by the consensus recommendation.
ACKNOWLEDGEMENTS
We thank the Director General of Health, Malaysia for permission to publish this report.We thank all the stakeholders involved in this study.
Conflicts of Interest:Ngah NF,None;Muhamad NA,None;Mohamed SO,None;Abdul Aziz RA,None;Ma’amor NH,None;Ahmad Tarmidzi NA,None;Hashim H,None;Ishak H,None;Wan Muda WN,None;Muda R,None;Adnan A,None;Saleh RM,None;Wong HS,None;Mohd Jamil N,None;George TM,None;Koh A,None.
 International Journal of Ophthalmology2023年5期
International Journal of Ophthalmology2023年5期
- International Journal of Ophthalmology的其它文章
- Analysis of retinal arteriolar and venular parameters in primary open angle glaucoma
- ldentification and functional analyses of a novel FOXL2 pathogenic variant causing blepharophimosis, ptosis,and epicanthus inversus syndrome
- Protective effects of ferulic acid against ionizing radiation-induced oxidative damage in rat lens through activating Nrf2 signal pathway
- Novel homozygous ADAMTS17 missense variant in Weill-Marchesani syndrome
- Cost analysis of childhood glaucoma surgeries using the US Medicaire allowable costs
- Predicting the prognosis of primary orbital lymphoma by clinical characteristics and imaging features
