不可切除肝细胞癌的经肝动脉化疗栓塞术联合靶向药物或程序性死亡受体1及其配体单抗治疗进展
彭秋菊 戴涛 谢贵波 陈金军 程笑 晏媛

摘要:
經肝动脉化疗栓塞术(TACE)被国内外指南推荐用于不可切除肝细胞癌(uHCC)患者的治疗,是uHCC患者最常用的治疗方法之一。TACE治疗HCC常用的化疗药物包括表柔比星、顺铂、氟尿嘧啶等,不过哪种化疗药物更优效还不清楚。本文总结了近5年关于使用不同化疗药物的TACE方案治疗uHCC患者的研究。TACE联合索拉非尼显著改善中晚期HCC患者的生存,已被中国临床肿瘤学会指南推荐用于这类患者,TACE联合其他酪氨酸激酶抑制剂(TKI)的疗效也成为研究热点。研究提示TACE联合仑伐替尼较TACE联合索拉非尼治疗晚期HCC患者的中位无进展生存期显著更高、中位总生存期有提高的趋势。而由于靶受体或下游信号的变异,分子靶向药物耐药仍然是一个挑战性问题。TKI结合免疫检查点抑制剂的治疗对uHCC患者可能是一个有希望的策略。一些研究初步提示TACE联合TKI及程序性死亡受体1及程序性死亡受体及其配体(PD-1/PD-L1)单抗的三联治疗在改善uHCC患者生存方面有较佳的疗效。本文综述了近5年TACE联合靶向药物、TACE联合PD-1/PD-L1单抗治疗uHCC患者的疗效与安全性研究。
关键词:
癌, 肝细胞; 化学栓塞, 治疗性; 分子靶向治疗; 免疫检查点抑制剂; 药物疗法, 联合
基金项目:
国家自然科学基金(82070650); 国家科技部重大专项(2018ZX10723203, 2018ZX10302206)
Research advances in transcatheter arterial chemoembolization combined with targeted agents or anti-PD-1/PD-L1 monoclonal antibody in treatment of patients with unresectable hepatocellular carcinoma
PENG Qiujua, DAI Taoa, XIE Guiboa, CHEN Jinjunb, CHENG Xiaob, YAN Yuana. (a. Department of Pharmacy, b. Department of Hepatology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China)
Corresponding author:
YAN Yuan, 616756657@qq.com (ORCID:0000-0002-1044-2457)
Abstract:
Transcatheter arterial chemoembolization (TACE) is recommended by domestic and international guidelines for the treatment of patients with unresectable hepatocellular carcinoma (uHCC), and it is one of the most common treatment methods for patients with uHCC. The chemotherapy drugs commonly used in TACE for HCC include epirubicin, cisplatin, and fluorouracil, while it is still unclear which chemotherapy drug has a better clinical effect. This article summarizes the studies of different TACE regimens using different chemotherapy drugs in the treatment of patients with uHCC in the recent five years. TACE combined with sorafenib can significantly improve the survival of patients with advanced HCC and has been recommended for the treatment of such patients by Chinese Society of Clinical Oncology guidelines, and the efficacy of TACE combined with other tyrosine kinase inhibitors (TKI) has become a research hotspot. Studies have shown that compared with TACE combined with sorafenib in the treatment of patients with advanced HCC, TACE combined with lenvatinib can achieve a significantly longer progression-free survival time and a tendency of increase in median overall survival time. However, due to the variation of target receptors or downstream signals, resistance to molecular-targeted agents is still a challenging problem. TKI combined with immune checkpoint inhibitors may be a promising strategy for the treatment of patients with uHCC. Some studies suggest that triple therapy using TACE combined with TKIs and anti-PD-1/PD-L1 monoclonal antibody has better efficacy in improving the survival of patients with uHCC. This article reviews the studies of the efficacy and safety of TACE combined with targeted agents and TACE combined with anti-PD-1/PD-L1 monoclonal antibody in the treatment of patients with uHCC in the recent five years.
Key words:Carcinoma, Hepatocellular; Chemoembolization, Therapeutic; Molecular Targeted Therapy; Immune Checkpoint Inhibitors; Drug Therapy, Combination
Research funding:National Natural Science Foundation of China (82070650);National Science and Technology Major Project (2018ZX10723203, 2018ZX10302206)
最新的全球癌症负担数据[1]显示,2020年的全球肝癌新发病例与新增死亡病例分别约90.57万例与83.02万例。这一年,肝癌在中国是发病率与死亡率均排列前十的肿瘤病种,新发肝癌患者约41万例,新增肝癌死亡患者约39万例[1],严重威胁人民的生命健康。肝细胞癌(HCC)占原发性肝癌的75%~85%[2]。经肝动脉化疗栓塞术(TACE)治疗有助于减轻化疗药物的全身毒副作用、提高局部肿瘤部位的药物浓度。目前TACE被国内外权威指南推荐用于不可切除肝细胞癌(uHCC)患者,是uHCC患者最常用的治疗方法之一[3-4]。TACE联合索拉非尼已被推荐用于中晚期HCC患者[3],最近一些文献报道了TACE联合抗程序性死亡受体1(programmed cell death protein 1,PD-1)及其配体(programmed cell death 1 ligand 1,PD-L1)单抗用于HCC患者的研究结果。TACE是否联合靶向药物、免疫检查点抑制剂是HCC患者TACE的重要预后因素。不过TACE中使用的化疗药物如何选择还不清晰,TACE联合靶向药物或PD-1/PD-L1单抗的临床研究数据不断更新,本文对近5年TACE单独、TACE联合靶向或PD-1/PD-L1单抗治疗uHCC患者的疗效与安全性研究作一综述,以期为临床治疗方案的选择提供参考。
1 含不同化疗药物的TACE方案治疗uHCC患者的疗效与安全性
TACE是指将碘化油化疗药物乳剂或载药微球、补充栓塞剂(如聚乙烯醇颗粒)等经肿瘤供血动脉支注入的治疗[3],它是一种局部治疗。依据栓塞剂不同,TACE分为传统TACE(cTACE)与载药微球TACE(DEB-TACE)。DEB-TACE即预先加载化疗药物的药物洗脱微球栓塞治疗的方法。
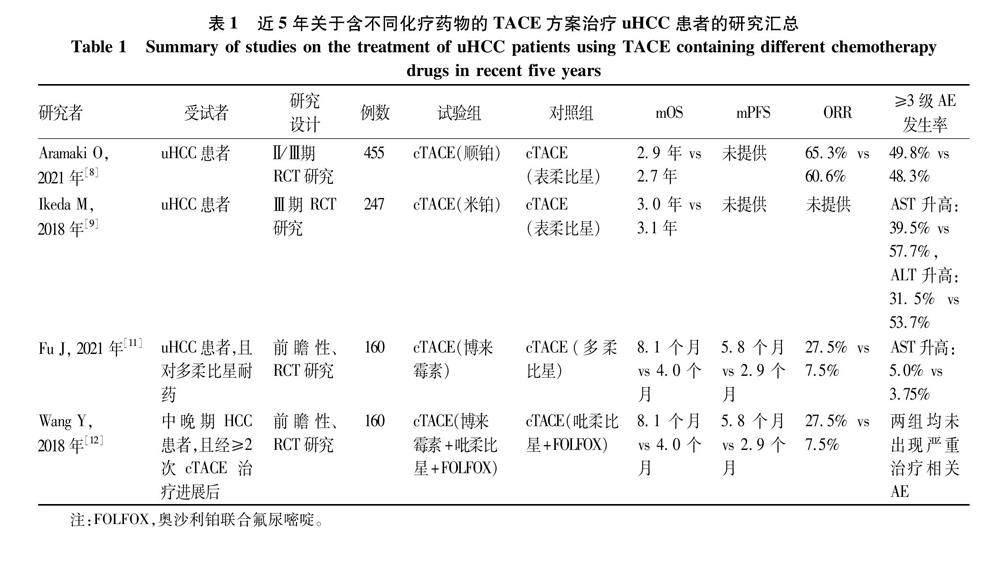
HCC患者TACE治疗中常见化疗药物包括蒽环类、顺铂、丝裂霉素、氟尿嘧啶等,其中蒽环类常用的包括多柔比星、表柔比星、伊达比星等[5]。表1总结了近5年关于不同化疗药物的TACE方案治疗uHCC患者的研究。2002年报道的两项重要RCT[6-7]分别证实了使用顺铂的cTACE与使用多柔比星的cTACE均比对症治疗显著改善uHCC患者的生存。ACE500研究[8]纳入日本uHCC患者,研究发现接受含顺铂的cTACE治疗组与含表柔比星的cTACE治疗组的中位总生存期(mOS)无显著差异,两组的客观缓解率(ORR)也无明显差异。另外这项研究显示顺铂组与表柔比星组的严重不良事件(AE)发生率相近。Ikeda等[9]发现含有米铂对比含有表柔比星的cTACE治疗uHCC患者的mOS无显著差异,不过米铂组的≥3级肝脏转氨酶升高发生率较表柔比星组低。该项研究未证实含米铂的TACE较含表柔比星的TACE治疗延长uHCC患者生存期,另外当前米铂仅在日本上市,含米铂的TACE适用于HCC患者也仅在日本获批,米铂在uHCC患者中的应用人群还比较局限。
依据改良实体瘤疗效评价mRECIST标准,评价为疾病进展,而患者肝功能、体力状况等一般情况符合TACE要求,建议进行后续的TACE治疗[10]。Fu等[11]研究纳入了多柔比星耐药的uHCC患者,结果显示cTACE(博来霉素)治疗组的ORR、mOS、中位无进展生存期(mPFS)均显著高于cTACE(多柔比星)治疗组,且两组患者的术后并发症无显著差异。一致的是,一项研究[12]纳入中晚期HCC且经cTACE治疗进展后的患者,分别给予含有或不含有博来霉素的cTACE治疗(博来霉素组:博来霉素+吡柔比星+奥沙利铂+氟尿嘧啶;对照组:吡柔比星+奥沙利铂+氟尿嘧啶),结果显示含博来霉素组较不含博来霉素组有显著更长的mPFS与mOS,以及更高的ORR,该研究中未出现严重治疗相关AE。研究提示博来霉素的临床疗效和安全性较好,可作为uHCC患者cTACE治疗的二线用药选择。
目前TACE治疗中化疗药物的用量没有标准推荐,临床一般根据患者的体表面积、体力状况、肿瘤负荷、既往用药史、联合用药情况等选择用量[10]。一项研究[13]比较了含多柔比星不同剂量(低剂量组50 mg vs 高剂量组100 mg)的TACE治疗中期HCC患者的疗效,共纳入28例受试者,研究发现两组患者的反应率与mOS均无显著差异,不过低剂量组比高剂量组患者的栓塞后发热与疼痛的持续时间均更短。
2 TACE联合靶向药物治疗uHCC患者的疗效与安全性
有研究[14]显示TACE联合靶向药物较TACE单独治疗uHCC患者的总生存期更高。研究[15]发现TACE治疗后HCC患者的血清血管内皮生长因子(VEGF)水平明显升高,这可能增加肿瘤复发或转移风险[16]。而索拉非尼、仑伐替尼、阿帕替尼等TKI,具有抑制VEGF受体VEGFR1、VEGFR2与VEGFR3的作用,TACE与TKI联合使用可能在HCC患者中发挥协同抗肿瘤作用。表2总结了近5年关于TACE联合靶向药物治疗uHCC患者的前瞻性研究。
2.1 TACE联合索拉非尼 TACE联合索拉非尼已被中国临床肿瘤学会(CSCO)指南推荐用于HCC患者(CNLC分期Ⅱb期)[3]。TACTICS研究[17],是一项多中心随机对照试验(RCT)研究,发现TACE联合索拉非尼比TACE单独治疗uHCC患者的mPFS延长11.7个月。亚组分析显示mPFS的显著获益主要体现在巴塞罗那(BCLC)分期B期患者中,而在BCLC分期A期、C期的患者中未发现显著差异。一项研究[18]对START研究数据进一步分析发现,TACE联合索拉非尼较TACE单独治疗早中期uHCC患者的反应率与mOS均显著更高。相似的是,Xu等[19]研究发现,与TACE单用或索拉非尼单药治疗相比,TACE联合索拉非尼的治疗显著提高中晚期肝癌患者的疾病控制率(DCR)。一项Meta分析纳入了14项关于TACE联合索拉非尼治療晚期HCC患者的疗效与安全性研究[20](受试者数n=1670),结果提示TACE联合索拉非尼较TACE单独治疗显著改善ORR、DCR及1年生存率,不过联合组发生的AE也更多,其中最常见的包括疲劳、手足皮肤反应和腹泻,这些AE都是可耐受的。综上分析,与TACE单独治疗比较,TACE联合索拉非尼治疗uHCC患者的生存获益更大,且不良反应可耐受。
2.2 TACE联合仑伐替尼 Ding等[21]比较了TACE联合仑伐替尼与TACE联合索拉非尼一线治疗伴有门静脉瘤栓的uHCC患者的疗效,结果显示TACE联合仑伐替尼组较TACE联合索拉非尼组的ORR与
mPFS均显著更高,两组的mOS无显著差异,不过前一组较后一组的mOS有升高趋势,两组患者出现的AE及其发生率是相似的。该研究是单中心、小样本量的RCT研究,还有待进一步研究证实。
2.3 TACE联合阿帕替尼 一项单中心RCT研究[22]发现,TACE联合阿帕替尼较TACE单独治疗中晚期HCC患者的mPFS延长6.5个月(P<0.05)。之后多个回顾性研究探究了这一主题,一项Meta分析纳入了23项相关研究[23],结果表明与TACE单独治疗比较,TACE联合阿帕替尼治疗中晚期HCC患者显著提高1年生存率、ORR与DCR。一项回顾性研究[24]结果提示,TACE联合阿帕替尼对比TACE联合索拉非尼治疗晚期HCC患者的反应率与mOS均无显著差异。相似的是,Qiu等[25]的回顾性研究结果显示这两种治疗方法治疗晚期HCC患者的mOS差异不显著。另外,该研究显示TACE联合阿帕替尼较TACE联合索拉非尼治疗晚期HCC患者的mPFS更短。上述两项研究中的前者发现两种干预方式组最常见的不良反应是相似的,包括手足皮肤反应、腹泻、高血压、蛋白尿与疲劳[24]。而后者[25]发现3/4级AE在TACE联合阿帕替尼组比TACE联合索拉非尼组更常见,TACE联合阿帕替尼组的高血压、口腔或肛门溃疡和蛋白尿发生率较高,而TACE联合索拉非尼组的腹泻和脱发发生率较高。TACE联合阿帕替尼是否不劣于TACE联合索拉非尼有待前瞻性的大样本研究进一步证实。
2.4 TACE联合其他TKI 安罗替尼、舒尼替尼与阿昔替尼作用于抗VEGF/VEGFR途径,其联合TACE治疗可能提高TACE治疗在uHCC患者中的疗效,减少肿瘤转移风险。一项回顾性研究[26]结果显示,TACE联合安罗替尼较TACE单独治疗显著提高uHCC患者的ORR、mPFS、半年生存率及1年生存率,且治疗期间受试者未出现治疗相关的4级AE或死亡。Turpin等[27]研究发现,在uHCC患者中,TACE联合舒尼替尼较TACE单独治疗带来更长的mOS与mPFS。不过Xu等[28]的研究发现,与TACE联合索拉非尼比较,TACE联合舒尼替尼治疗uHCC患者未显示出优效性,而TACE联合索拉非尼组的mPFS与mOS显著更长、ORR也更高,虽然手足综合征在TACE联合索拉非尼组更常见。一项单臂临床研究[29]发现,接受TACE联合阿昔替尼治疗的uHCC患者的ORR、两年存活率、mOS分别为68.2%、43.7%、18.8个月。初步提示TACE联合阿昔替尼在uHCC中有较好的抗肿瘤活性与安全性。
3 TACE联合PD-1/PD-L1单抗治疗uHCC患者的疗效与安全性
3.1 TACE联合PD-1/PD-L1单抗 抗肿瘤免疫治疗主要包括免疫检查点抑制剂与细胞免疫治疗,前者包括PD-1/PD-L1单抗与CTLA-4单抗。卡瑞利珠单抗是一种PD-1单抗,一项RCT[30]纳入98例肿瘤负荷大的HCC患者,研究发现与TACE单独治疗比较,TACE联合卡瑞利珠单抗的治疗显著提高ORR(55.1% vs 22.5%)与DCR(77.6% vs 32.7%)。该研究中,所有的受试者均出现短暂的肝损伤、恶心呕吐、发热与腹痛,对症治疗后症状均得到缓解,两组的AE发生率无显著差异。一致的是,一项回顾性研究[31]纳入82例中晚期HCC患者,研究结果显示TACE联合卡瑞利珠单抗较TACE单独治疗显著提高ORR(61.9% vs 35.7%)与DCR(92.9% vs 69.1%),同时显著改善mPFS(12.9个月 vs 8.4个月)与mOS(19.3个月 vs 13.6个月)。该研究中联合组69.1%的患者出现与卡瑞利珠单抗相关的1级反应性毛细血管增生症,未出现严重AE。上述真实世界研究的数据提示TACE联合卡瑞利珠单抗较TACE单独治疗改善uHCC患者生存,且安全性可控。
3.2 TACE联合靶向药物及PD-1/PD-L1单抗由于靶受体或下游信号的随机性变异,靶向治疗药物耐药仍然是uHCC治疗中的挑战性问题,TKI结合PD-1/PD-L1单抗的治疗对uHCC患者是一个有希望的策略[32]。在2022年美国临床肿瘤协会会议上,报告了一项关于TACE+仑伐替尼+卡瑞利珠单抗/信迪利单抗治疗HCC患者(BCLC分期B/C期)的前瞻性研究[33]的初步结果,显示在中位随访时间33.3周后,38例受试者转化为可手术切除的比率达50%,其中5例患者达完全病理缓解;48周的OS率与PFS率分别为96.4%与91.7%。该研究中57.9%受试者出现过3级治疗相关AE,未出现3级以上治疗相关AE。一项Meta分析纳入了该项研究与其他3个回顾性研究进行分析,结果提示TACE+仑伐替尼+抗PD-1单抗三联治疗较TACE单独治疗uHCC患者的手术转化率与ORR均更高,且三联治疗较双联治疗(TACE+放疗/TKI/肝动脉灌注化疗)在uHCC患者中的手术转化率(42% vs 19%)与ORR(71% vs 40%)均更高[34]。目前还有一些三联治疗的研究正在进行中,LEAP-012研究[35]将比较TACE+仑伐替尼+帕博利珠单抗的三联治疗与TACE+安慰剂治疗在中期uHCC患者中的疗效与安全性,另外TACE+贝伐珠单抗+阿替利珠单抗方案治疗中期HCC患者的单臂Ⅱ期临床研究正在开展[36]。
4 总结
研究表明,使用含顺铂或多柔比星或表柔比星的cTACE均能改善uHCC患者生存,且出现严重不良AE的概率相似,尚不能确定cTACE使用其中哪种化疗药物对uHCC患者效果更佳,临床可综合评估患者肾功能、心脏功能、骨髓造血功能等指标之后选择TACE治疗中合适的化疗药物。虽然CSCO指南推荐TACE联合靶向治疗用于中晚期HCC患者,不过仅明确推荐TACE联合索拉非尼[3]。最近的研究发现TACE联合仑伐替尼较TACE联合索拉非尼给晚期HCC患者带来显著更高的ORR与mPFS,不过前一种较后一种治疗在延长mOS方面未显示出明显优效性,仅有提高的趋势。TACE联合靶向药物(仑伐替尼与阿帕替尼)治疗uHCC患者的前瞻性研究樣本量均较小,未来开展扩大样本量的RCT将为临床提供更强的参考依据。初步研究提示TACE联合靶向药物及抗PD-1单抗的三联治疗对于uHCC患者似乎是一种更有前景的治疗策略,且安全性可控,不过疗效有待未来三联治疗的RCT结果进一步证实,继续深入阐明抗PD-1单抗影响靶向药物治疗uHCC患者疗效的作用机理也是需要的。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:彭秋菊、晏媛完成课题设计;彭秋菊完成文献筛选,文献数据整理与文章初稿写作;晏媛、戴涛负责指导文章写作;谢贵波、陈金军、程笑负责修改文章;陈金军提供基金支持。
参考文献:
[1]SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660.
[2]BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492.
[3]Chinese Society of Clinical Oncology. Guidelines for diagnosis and treatment of primary liver cancer (2022 edition)[M]. Beijing: Peoples Medical Publishing House, 2022.
中國临床肿瘤学会. 原发性肝癌诊疗指南(2022年版)[M]. 北京: 人民卫生出版社, 2022.
[4]NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)[EB/OL]. Hepatobiliary Cancers. Version 1.2022. https://www.nccn.org/.
[5]RAOUL JL, FORNER A, BOLONDI L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence[J]. Cancer Treat Rev, 2019, 72: 28-36. DOI: 10.1016/j.ctrv.2018.11.002.
[6]LO CM, NGAN H, TSO WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma[J]. Hepatology, 2002, 35(5): 1164-1171. DOI: 10.1053/jhep.2002.33156.
[7]LLOVET JM, REAL MI, MONTAA X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial[J]. Lancet, 2002, 359(9319): 1734-1739. DOI: 10.1016/S0140-6736(02)08649-X.
[8]ARAMAKI O, TAKAYAMA T, MORIGUCHI M, et al. Arterial chemoembolisation with cisplatin versus epirubicin for hepatocellular carcinoma (ACE 500 study): A multicentre, randomised controlled phase 2/3 trial[J]. Eur J Cancer, 2021, 157: 373-382. DOI: 10.1016/j.ejca.2021.08.027.
[9]IKEDA M, KUDO M, AIKATA H, et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial[J]. J Gastroenterol, 2018, 53(2): 281-290. DOI: 10.1007/s00535-017-1374-6.
[10]Chinese College of Interventionalists, Chinese Medical Doctor Association. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma [J]. Chin J Intern Med, 2021, 60(7): 599-614. DOI: 10.3760/cma.j.cn112137-20210425-00991.
中國医师协会介入医师分会临床诊疗指南专委会. 中国肝细胞癌经动脉化疗栓塞(TACE)治疗临床实践指南(2021年版)[J]. 中华内科杂志, 2021, 60(7): 599-614. DOI: 10.3760/cma.j.cn112137-20210425-00991.
[11]FU J, WANG Y, ZHANG J, et al. The safety and efficacy of transarterial chemoembolisation with bleomycin for hepatocellular carcinoma unresponsive to doxorubicin: a prospective single-centre study[J]. Clin Radiol, 2021, 76(11): 864.e7-864.e12. DOI: 10.1016/j.crad.2021.07.013.
[12]WANG Y, FU JX, ZHANG JL, et al. Transarterial chemoembolization with bleomycin treatment for moderate-advenced hepatocellular carcinoma[J]. Nat Med J China, 2018, 98(39): 3166-3170. DOI: 10.3760/cma.j.issn.0376-2491.2018.39.008.
王燕, 付金鑫, 张金龙, 等. 博来霉素经肝动脉化疗栓塞治疗中晚期肝癌的临床观察[J]. 中华医学杂志, 2018, 98(39): 3166-3170. DOI: 10.3760/cma.j.issn.0376-2491.2018.39.008.
[13]BESSAR AA, FARAG A, ABDEL MONEM SM, et al. Transarterial chemoembolisation in patients with hepatocellular carcinoma: low-dose doxorubicin reduces post-embolisation syndrome without affecting survival-prospective interventional study[J]. Eur Radiol Exp, 2021, 5(1): 10. DOI: 10.1186/s41747-021-00204-6.
[14]ZHANG Z, WU Y, ZHENG T, et al. Efficacy of transarterial chemoembolization combined with molecular targeted agents for unresectable hepatocellular carcinoma: A network meta-analysis[J]. Cancers (Basel), 2022, 14(15) . DOI: 10.3390/cancers14153710.
[15]SCHICHO A, HELLERBRAND C, KRGER K, et al. Impact of different embolic agents for transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels[J]. J Clin Transl Hepatol, 2016, 4(4): 288-292. DOI: 10.14218/JCTH.2016.00058.
[16]SERGIO A, CRISTOFORI C, CARDIN R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness[J]. Am J Gastroenterol, 2008, 103(4): 914-921. DOI: 10.1111/j.1572-0241.2007.01712.x.
[17]KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69(8): 1492-1501. DOI: 10.1136/gutjnl-2019-318934.
[18]LEE TY, LIN CC, CHEN CY, et al. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: A post hoc analysis of the START trial[J]. Medicine (Baltimore), 2017, 96(37): e7655. DOI: 10.1097/MD.0000000000007655.
[19]XU X, MENG Q. Drug effect analysis of sorafenib combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma[J]. Pak J Pharm Sci, 2018, 31(4(Special)): 1751-1755.
[20]CAI R, SONG R, PANG P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis[J]. BMC Cancer, 2017, 17(1): 714. DOI: 10.1186/s12885-017-3707-5.
[21]DING X, SUN W, LI W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study[J]. Cancer, 2021, 127(20): 3782-3793. DOI: 10.1002/cncr.33677.
[22]LU W, JIN XL, YANG C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial[J]. Cancer Biol Ther, 2017, 18(6): 433-438. DOI: 10.1080/15384047.2017.1323589.
[23]ZHAO S, ZHANG T, DOU W, et al. A comparison of transcatheter arterial chemoembolization used with and without apatinib for intermediate- to advanced-stage hepatocellular carcinoma: a systematic review and meta-analysis[J]. Ann Transl Med, 2020, 8(8): 542. DOI: 10.21037/atm.2020.02.125.
[24]CAO Y, SUN T, GUO X, et al. Sorafenib versus apatinib both combined transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: A comparative retrospective study[J]. Front Oncol, 2021, 11: 673378. DOI: 10.3389/fonc.2021.673378.
[25]QIU Z, SHEN L, JIANG Y, et al. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study[J]. Ann Transl Med, 2021, 9(4): 283. DOI: 10.21037/atm-20-5360.
[26]GUO W, CHEN S, WU Z, et al. Efficacy and safety of transarterial chemoembolization combined with anlotinib for unresectable hepatocellular carcinoma: A retrospective study[J]. Technol Cancer Res Treat, 2020, 19: 1533033820965587. DOI: 10.1177/1533033820965587.
[27]TURPIN A, de BAERE T, HEURGU A, et al. Liver transarterial chemoembolization and sunitinib for unresectable hepatocellular carcinoma: Results of the PRODIGE 16 study[J]. Clin Res Hepatol Gastroenterol, 2021, 45(2): 101464. DOI: 10.1016/j.clinre.2020.05.012.
[28]XU Q, HUANG Y, SHI H, et al. Sunitinib versus sorafenib plus transarterial chemoembolization for inoperable hepatocellular carcinoma patients[J]. J BUON, 2018, 23(1): 193-199.
[29]CHAN SL, YEO W, MO F, et al. A phase 2 study of the efficacy and biomarker on the combination of transarterial chemoembolization and axitinib in the treatment of inoperable hepatocellular carcinoma[J]. Cancer, 2017, 123(20): 3977-3985. DOI: 10.1002/cncr.30825.
[30]ZHANG S, ZHAO Y, HE L, et al. Effect of camrelizumab plus transarterial chemoembolization on massive hepatocellular carcinoma[J]. Clin Res Hepatol Gastroenterol, 2022, 46(4): 101851. DOI: 10.1016/j.clinre.2021.101851.
[31]YU SL, LIU DH, WANG C, et al. TACE combined with camrelizumab for treatment of advanced hepatocellular carcinoma [J]. Chin J Interv Imaging Ther, 2022, 19(7): 391-395. DOI: 10.13929/j.issn.1672-8475.2022.07.003.
于士龙, 刘东辉, 王储, 等. TACE联合卡瑞利珠单抗治疗中晚期肝细胞癌[J]. 中国介入影像与治疗学, 2022,19(7): 391-395. DOI: 10.13929/j.issn.1672-8475.2022.07.003.
[32]MOU L, TIAN X, ZHOU B, et al. Improving outcomes of tyrosine kinase inhibitors in hepatocellular carcinoma: New data and ongoing trials[J]. Front Oncol, 2021, 11: 752725. DOI: 10.3389/fonc.2021.752725.
[33]ZHANG X, ZHU X, LIU C, et al. The safety and efficacy of transarterial chemoembolization (TACE) + lenvatinib + programmed cell death protein 1 (PD-1) antibody of advanced unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2022, 40(4_suppl): 453-453. DOI: 10.1200/JCO.2022.40.4_suppl.453
[34]LI W, PEI Y, WANG Z, et al. Efficacy of transarterial chemoembolization monotherapy or combination conversion therapy in unresectable hepatocellular carcinoma: A systematic review and meta-analysis[J]. Front Oncol, 2022, 12: 930868. DOI: 10.3389/fonc.2022.930868.
[35]LLOVET JM, VOGEL A, MADOFF DC, et al. Randomized phase 3 LEAP-012 study: Transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment[J]. Cardiovasc Intervent Radiol, 2022, 45(4): 405-412. DOI: 10.1007/s00270-021-03031-9.
[36]WANG K, YU HM, XIANG YJ, et al. Transcatheter arterial chemoembolization plus atezolizumab and bevacizumab for unresectable hepatocellular carcinoma: a single-arm, phase II trial[J]. Future Oncol, 2022, 18(30): 3367-3375. DOI: 10.2217/fon-2022-0188.
收稿日期:
2022-10-31;錄用日期:2022-12-18
本文编辑:王亚南
引证本文:
PENG QJ, DAI T, XIE GB, et al.
Research advances in transcatheter arterial chemoembolization combined with targeted agents or anti-PD-1/PD-L1 monoclonal antibody in treatment of patients with unresectable hepatocellular carcinoma
[J]. J Clin Hepatol, 2023, 39(7): 1740-1746.
彭秋菊, 戴涛, 谢贵波, 等.
不可切除肝细胞癌的经肝动脉化疗栓塞术联合靶向药物或程序性死亡受体1及其配体单抗治疗进展
[J]. 临床肝胆病杂志, 2023, 39(7): 1740-1746.

