Survival of rat sciatic nerve segments preserved in storage solutions ex vivo assessed by novel electrophysiological and morphological criteria
Liwen Zhou, Monzer Alatrach, Ted Zhao, Paul Oliphint, George D. Bittner
Abstract Most organ or tissue allografts with viable cells are stored in solutions ex vivo for hours to several days. Most allografts then require rapid host revascularization upon transplantation to maintain donor-cell functions (e.g., cardiac muscle contractions, hepatic secretions). In contrast, peripheral nerve allografts stored ex vivo do not require revascularization to act as scaffolds to guide outgrowth by host axons at 1–2 mm/d, likely aided by viable donor Schwann cells. Using current storage solutions and protocols, axons in all these donor organ/tissue/nerve transplants are expected to rapidly become non-viable due to Wallerian degeneration within days. Therefore, ex vivo storage solutions have not been assessed for preserving normal axonal functions, i.e., conducting action potentials or maintaining myelin sheaths. We hypothesized that most or all organ storage solutions would maintain axonal viability. We examined several common organ/tissue storage solutions (University of Wisconsin Cold Storage Solution, Normosol-R, Normal Saline, and Lactated Ringers) for axonal viability in rat sciatic nerves ex vivo as assessed by maintaining: (1) conduction of artificially-induced compound action potentials; and (2) axonal and myelin morphology in a novel assay method. The ten different storage solution conditions for peripheral nerves with viable axons (PNVAs) differed in their solution composition, osmolarity (250–318 mOsm), temperature (4°C vs. 25°C), and presence of calcium. Compound action potentials and axonal morphology in PNVAs were best maintained for up to 9 days ex vivo in calcium-free hypotonic diluted (250 mOsm) Normosol-R (dNR) at 4°C. Surprisingly, compound action potentials were maintained for only 1–2 days in UW and NS at 4°C, a much shorter duration than PNVAs maintained in 4°C dNR (9 days) or even in 25°C dNR (5 days). Viable axons in peripheral nerve allografts are critical for successful polyethylene glycol (PEG)-fusion of viable proximal and distal ends of host axons with viable donor axons to repair segmental-loss peripheral nerve injuries. PEG-fusion repair using PNVAs prevents Wallerian degeneration of many axons within and distal to the graft and results in excellent recovery of sensory/motor functions and voluntary behaviors within weeks. Such PEG-fused PNVAs, unlike all other types of conventional donor transplants, are immune-tolerated without tissue matching or immune suppression. Preserving axonal viability in stored PNVAs would enable the establishment of PNVA tissue banks to address the current shortage of transplantable nerve grafts and the use of stored PEG-fused PNVAs to repair segmentalloss peripheral nerve injuries. Furthermore, PNVA storage solutions may enable the optimization of ex vivo storage solutions to maintain axons in other types of organ/tissue transplants.
Key Words: axonal morphometrics; axonal viability; calcium; osmolarity; peripheral nerve grafts; peripheral nerve injury; temperature; tissue storage solutions; tonicity; Wallerian degeneration
Introduction
Characteristics of most allograft vs nerve allograft transplants stored exvivo
Current protocols for transplantation of donor, non-neuronal organs (e.g., livers, hearts, kidneys) storedex vivoare designed to restore vascular connections that re-establish oxygenated blood flow (Petrenko et al., 2019). The clinical intention for some tissue grafts of non-neuronal origin (e.g., skin, fascia) also storedex vivois often to produce progressive revascularization via capillary ingrowth within days to weeks after transplantation to maintain donor cell viability (Ramsey et al., 2022). The clinical intention for other nonneuronal, acellular tissue allografts (e.g., bone, cartilage, and tendon) storedex vivois to maintain long-term structural integrity after transplantation with no requirement for immediate revascularization (Sohn and Oh, 2019). Immediate restoration and continued maintenance of function is the usual measure of success for all these transplanted organs or tissues that most commonly have been storedex vivofor 1–2 days in University of Wisconsin cold storage solution (UW solution) that maintains donor cell viability (Spiegel et al., 1999; Chen et al., 2019).
In contrast to the organs and tissues storedex vivodescribed above, the current surgical intent for segments of peripheral nerve storedex vivoas a vascularized or non-vascularized tissue transplant is to provide a scaffold that may help axonal regeneration by outgrowth from proximal cut ends of host axons connected to their soma (Allan, 2000; Houschyar et al., 2016; Bittner et al., 2018). Distal segments of peripheral nerve axons within these tissues are expected to be non-functional and undergo Wallerian degeneration (WD) within 3–7 days. None of the above examples of procedures for organs or tissues storedex vivoare expected upon transplantation to rapidly re-establish the normal functions of the peripheral nerve axons. Hence, storage solutions like UW have only been tested for their ability to preserve the structure, and not axonal function, of peripheral nerve grafts and how stored peripheral nerve allografts affect host immunological responses to the grafts (Hare et al., 1993; Ikeguchi et al., 2007). We have addressed the problem of how to assess and preserve axonal structure and function in anucleate segments of peripheral nerves inex vivostorage solutions.
Importance of nerve allograft transplants
The ability to preserve anucleate segments of axons in peripheral nerves is important because peripheral nerve injuries (PNIs) often produce nerve gap (segmental-loss) defects. Segmental-loss PNIs are the most common form of neural dysfunction (Bittner et al., 2022). Patients and experimental laboratory animals with nerve gap defects sequentially experience (1) immediate complete loss of sensory and motor functions mediated by the denervated structures followed by (2) rapid WD of severed distal axonal segments within a few days after injury, and (3) slow (~1 mm/d) natural regeneration by outgrowths that produce poor (if any) functional recovery after months to years, due in part to non-specific reinnervation and/or to muscle atrophy or deterioration of nerve target structures before reinnervation (Gaudet et al., 2011; Menorca et al., 2013; Scheib and Hoke, 2013; Kornfeld et al., 2019; Bittner et al., 2022). Current best clinical or experimental practices to repair segmental-loss PNIs employ (1) autografts obtained from other previously intact nerves, usually sensory in nature, (2) donor non-allogenic (decellularized/denatured) allografts, or (3) synthetic conduits (Mackinnon et al., 2001; Muheremu and Ao, 2015; Pan et al., 2020). All these natural or artificial conduits are micro-sutured to host epineurium and connective tissue at both ends of the gap to create a bridge across the gap to guide surviving proximal axons to distal nerve segments.
All these contemporary procedures to repair nerve gaps are problematic because they do not prevent WD of distal axonal segments and, therefore, solely rely on natural regeneration to produce slow and poor recovery of motor function and/or sensation. The lack of supporting cells in acellular nerve grafts and artificial conduits is believed to reduce their efficacy (Pan et al., 2020). Despite previous attempts using freshly harvested peripheral nerve transplants that have viable axons and other supporting cells, the repairs have not been successful; transplanted tissues were often rejected even with systemic immune suppression and major and/or minor histocompatibility complex matching (Mackinnon et al., 2001; Campbell, 2008).
Alternatively, a recently-developed repair strategy using polyethylene glycol (PEG) fusion of peripheral nerves with viable axons (PNVAs) to repair segmental-loss PNIs has produced excellent recovery of function and voluntary behaviors within weeks (Riley et al., 2015; Bittner et al., 2016a). Furthermore, PEG-fused PNVAs showed minimal immune response despite neither histocompatibility complex matching nor immune suppression (Mikesh et al., 2018a).
Rationale and importance of our study to assess axonal structure and function in nerve allografts stored ex vivo
We hypothesized that most or all organ storage solutions would maintain axonal viability. Our current study was designed to test ten different sterile storage solution conditions, all containing antimicrobial agents, for the survivability of sciatic nerve axons storedex vivoas a PNVA model system. Axonal survival in the PNVAs was measured by compound action potentials (CAPs) recordedex vivoand by a novel imaging method using histological analyses of axoplasm and myelin in thick sections. In general, lower osmolarity, lower temperature, and lack of calcium improved the preservation of PNVAs assessed by CAP conduction. UW and Normal Saline solutions least-preserved viability of stored PNVAs. Hypotonic (250 mOsm) diluted Normosol-R at 4°C best-preserved viability of stored PNVAs assessed by CAP conduction and our novel morphological assessments, including axonal and myelin integrity, reduction of WD, and tissue disintegration. Most importantly, an ability to maintain axonal viability in stored PNVAs would make possible the establishment of PNVA tissue banks and the use of PEG-fused PNVAs to repair segmental loss PNIs as briefly discussed in the previous subheading.
Methods
Animals
All experimental procedures were approved by standards set forth by the Institutional Animal Care and Use Committee at the University of Texas at Austin (IACUC protocol ID: AUP-2019-00225, approval date: June 9, 2019) and were conducted in accordance with the National Institutes of Health policies, including the Guide for the Care and Use of Laboratory Animals (8thed, National Research Council, 2011). Sprague-Dawley (SD) rats or Thy1-GFP transgenic SD rats (gifted by Washington University in St. Louis, USA) of the same sex at ages 3–12 months were housed 2–3 rats/cage and maintained on a 12-hour light/dark cycle with food and water givenad libitum. Female and male rats, weighing 225–300 g and 300–350 g, respectively were used in this study.
Nerve extraction
Sciatic nerve segments (PNVAs,n= 179) were harvested during all rat’s active cycles. Rats were anesthetized by 4% isoflurane (RXISO-250; Animal Health International, Roanoke, TX, USA)/oxygen mixture at 1.5 L/min. Following induction, rats were maintained on 2% isoflurane mixture. Prior to the nerve harvest, all surgical tools were autoclaved. The incision site was first cleanly shaved, followed by sterilization using several wipes of 70% ethanol followed by Betadine. A 4–5 cm incision was made through the biceps femoris to expose the sciatic nerve. Sciatic nerve segments 2.5–3 cm in length were excised and stored in 10 mL of designated storage solutions.
Electrophysiological testing
CAPs that were conducted from one to the other end of each PNVA were recordedex vivoimmediately following harvesting and daily afterward for up to 7 days using a PowerLab 4/35 (AD instruments, Sydney, Australia). PNVAs were placed in a customized 3D printed chamber (ABS polymer and Craftbot XL machine) with titanium wires hooked up to a set of stimulating electrodes at one end and a set of recording electrodes at the other end (Figure 1A). PNVAs were stimulated with incremental increases in voltage from 0-8 V using 0.1 ms square wave depolarizations given at 1 Hz with a 0.1 ms delay from a sweep-triggering pulse. The minimum threshold and maximum amplitude of CAPs for each PNVA were recorded for each day of graft storage (Figure 1B–F). To avoid nerve damage, stimulus amplitudes were limited to 2 V maximum on the first day and 8 V maximum at longer storage times. We tested for 2 additional days following the day when no CAP was detected in a PNVA to ensure that the axons in the PNVA were no longer conducting. PNVAs that did not conduct CAPs immediately after harvesting due to either instrumental error or harvesting problems were excluded from the study (n= 7).
Storage solutions
Fresh storage solutions containing 1x final concentration of penicillinstreptomycin (P4333; Sigma-Aldrich, St. Louis, MO, USA) were prepared and replaced daily. PNVAs were storedex vivoin one of seven different sterile storage solutions: (1) Normosol-R (NR) (0990-7967-09; ICU Medical, San Clemente, CA, USA), (2) diluted Normosol-R (dNR) consisting of NR diluted with 176 mL sterile ddH2O per liter of NR, (3) diluted Normosol-R with calcium (dNRc) consisting of dNR with 17 mg CaCl2per liter of dNR, (4) lactated Ringers (LR) (2B2323; Baxter Healthcare, Deerfield, IL, USA), (5) normal saline (NS) (0990-7983-09; ICU Medical) or made with 900 mg NaCl per liter of ddH2O and filter-sterilized, (6) diluted normal saline (dNS) consisting of NS diluted with 200 mL sterile ddH2O per liter of NS, and (7) University of Wisconsin Cold Storage Solution (UW) (NC0952695; Thermo Fisher Scientific, Hampton, NH, USA). Specifically, 100 mL of each solution contained:
1) NR: 526 mg NaCl, 222 mg C2H3NaO2, 502 mg NaC6H11O7, 37 mg KCl, 30 mg MgCl2
2) dNR: 447 mg NaCl, 189 mg C2H3NaO2, 427 mg NaC6H11O7, 31 mg KCl, 26 mg MgCl2
3) dNRc: same composition as dNR with the addition of 17 mg CaCl2
4) LR: 600 mg NaCl, 310 mg C3H5NaO3, 30 mg KCl, 20 mg CaCl2H4O2
5) NS: 900 mg NaCl
6) dNS: 750 mg NaCl
7) UW: 5000 mg pentafraction, 3583 mg C12H22O12, 340 mg KH2PO4, 123 mg H14MgO11S, 1783 mg C18H32O16·5H2O, 134 mg C10H13N5O4, 13.6 mg C5H4N4O, 92 mg C10H17N3O6S, 561 mg KOH, 5 mg NaOH (5N)
All experimental solution protocols are listed below in Table 1. The osmolarity of each was measured many times (> 10) on a well-calibrated freezing point osmometer (Model 3300; Advanced Instruments, Norwood, MA, USA). The measured osmolarity of many solutions was often different from the calculated osmolarity given on their commercial labels (Table 1). For example, the calculated osmolarity on the label assuming complete dissociation/hydration of NR was 294 mOsm, but the measured osmolarity was 268 mOsm. That is, the total osmotic concentration of many standard solutions is too high to assume complete hydration/no interaction between individual particles in an aqueous solution.
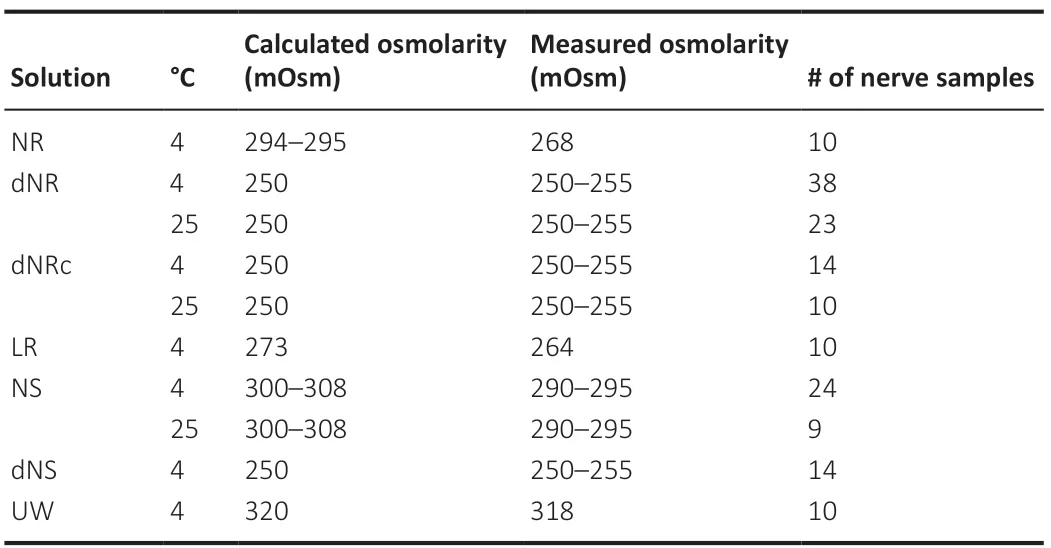
Table 1 |Storage solutions
Novel morphological analyses
PNVAs of similar length taken from similar regions of the thigh were fixed and embedded similar to previous descriptions (Mikesh et al., 2018; Smith et al., 2020). PNVAs (n= 17) were fixed overnight at room temperature in 2% paraformaldehyde/3% glutaraldehyde fixatives (15713, 16220; Electron Microscopy Sciences [EMS], Hatfield, PA, USA) in 0.1 M sodium cacodylate buffer (11653; EMS). Tissues were washed with buffer prior to trimming and post-fixing in 1% osmium tetroxide/1% potassium ferrocyanide (19150; EMS) in 0.1 M sodium cacodylate buffer for 4–5 hours. PNVAs were then washed in water, stained in 1% aqueous uranyl acetate (22400-1; EMS) for 2 hours, and then washed and held in water. PNVAs were dehydrated through graded ethanol, exchanged to absolute acetone, placed in increasing concentrations of Hard Plus Resin 812 (14115; EMS), and then embedded in fresh resin and polymerized at 60°C for at least 48 hours. Glass knife-thick sections (0.5 mm) were stained in toluidine blue (22050; EMS), and images were captured by Axiovert 200 M microscope (Zeiss, Oberkochen, Germany) using an Axiocam HR3 camera (Zeiss). For each PNVA sample, three regions of interest were given blinded to two investigators, and manually annotated for a total of at least 150 axons. Five different categories of axons were then reported as percentages of the total number of axons.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad, San Diego, CA, USA). PNVA survival data were analyzed using the Log-rank test and the Gehan-Breslow-Wilcoxon test. Morphological data were analyzed using two-way analysis of variance followed by Tukey’s multiplepost hoctest. A 95% confidence interval was used. Electrophysiological data were presented as survival curves. PNVA survival times measured by CAPs and the percentage of axons counted based on different morphological categories were presented as mean ± SEM.
Results
Study design
The current study was designed to determine how long axons in PNVAs remained viable as assessed by their functional ability to conduct action potentials recorded extracellularly (CAPs), as well as their morphological status as assessed by axoplasmic and myelin structures. A pilot study (n= 38) indicated no significant differences in the ability of PNVAs to conduct CAPs whether PNVAs were harvested before or after euthanasia with an intracardiac injection of saturated potassium chloride.
We tested four common solutions; several were tested at different temperatures (4°Cvs. 25°C), osmolarities, or calcium concentrations totaling 10 different protocols (Table 1). NR and LR were solutions we were currently using for acute (0–6 hours) storage of sciatic PNVAs used for PEG-fusion repair of ablation (segmental-loss) PNIs in previous publications (Riley et al., 2015; Mikesh et al., 2018; Ghergherehchi et al., 2019; Smith et al., 2020a, b). NS is a common solution utilized in clinics, and UW has been used in previous peripheral nerve graft studies as the primary storage solution (Levi et al., 1994; Evans et al., 1998).
Electrophysiological results
Figure 1 shows that as the storage time increased, PNVAs required higher stimulus voltages to elicit a CAP and the amplitude of the conducted CAP decreased. Figure 1B–F shows an example of recordings from a single preparation of a PNVA in 4°C dNR. CAPs on storage days 0 and 1 (Figure 1B) could be induced at low stimulus voltages (0.2–1 V). On day 1, the CAP decreased in amplitude but did not shift in its onset time. On days 2–3 (Figure 1C), CAP amplitude, duration, and onset time decreased and required higher voltage (2–6 V) to elicit. CAPS on storage days 4–5 were very similar to those of days 2–3, with an even shorter onset time with respect to the SA (Figure 1D). Starting on day 6 (Figure 1E), the amplitude of CAP further decreased and required a yet-higher stimulus voltage (6–8 V) to elicit. On storage day 8 (Figure 1F), the CAP was very small and difficult to distinguish from the SA. On storage day 9, no CAP was detectable.
Figure 2 shows survival curves of PNVAs stored in various conditions and solutions assayed by CAP conduction. Figure 2A–C shows no significant difference for PNVA survival in 4°C dNR between (1) female (n= 32) and male (n= 6) SD rats (Figure 2A), (2) wild-type SD rats (n= 10) and Thy1-GFP SD rats (n= 12) (Figure 2B), or (3) two different operating surgeons (MA (n= 10) and TZ (n= 22) (Figure 2C).
Since no significant differences of PNVA stored in 4°C dNR were observed for two different sexes, strains, or surgeons, a combined CAP conduction data from Figure 2A–C (n= 38) was plotted in Figure 2D–I. This 4°C dNR composite curve in Figure 2D–I appears to have an S-shaped survival curve over 10 days of storage. PNVAs in 4°C dNR on average conducted CAPs for 5.7 ± 0.27 days (Table 2). All stored PNVAs conducted CAPs on day 3. On day 4, almost all (94%) of stored PNVAs conducted CAPs. 74% of stored PNVAs conducted CAPs on day 5. Still almost half of all PNVAs (49%) conducted CAPs on day 6. Furthermore, CAPs survived in one PNVA up to 9 days. Because this 4°C dNR composite curve was the best of our 10 storage solution conditions for maintaining PNVAs (Figure 3), we used this curve to compare the effects of nerve storage on CAP conduction with all other solutions tested in this study in Figure 2D–I as listed below:
PNVA survival was significantly better in 4°C dNR than in 25°C dNR (P< 0.0001;n= 23; Figure 2D and Table 2). PNVAs in 25°C dNR on average conducted for 2.4 ± 0.29 days. While all PNVAs in 4°C dNR conducted CAPs on day 3, only 57% PNVAs in 25°C dNR conducted. On day 5, 74% of PNVAs in 4°C dNR conducted CAPs, while only 4.4% of PNVAs in 25°C dNR conducted CAPs. All PNVAs in 25°C dNR stopped conducting CAPs on day 6.
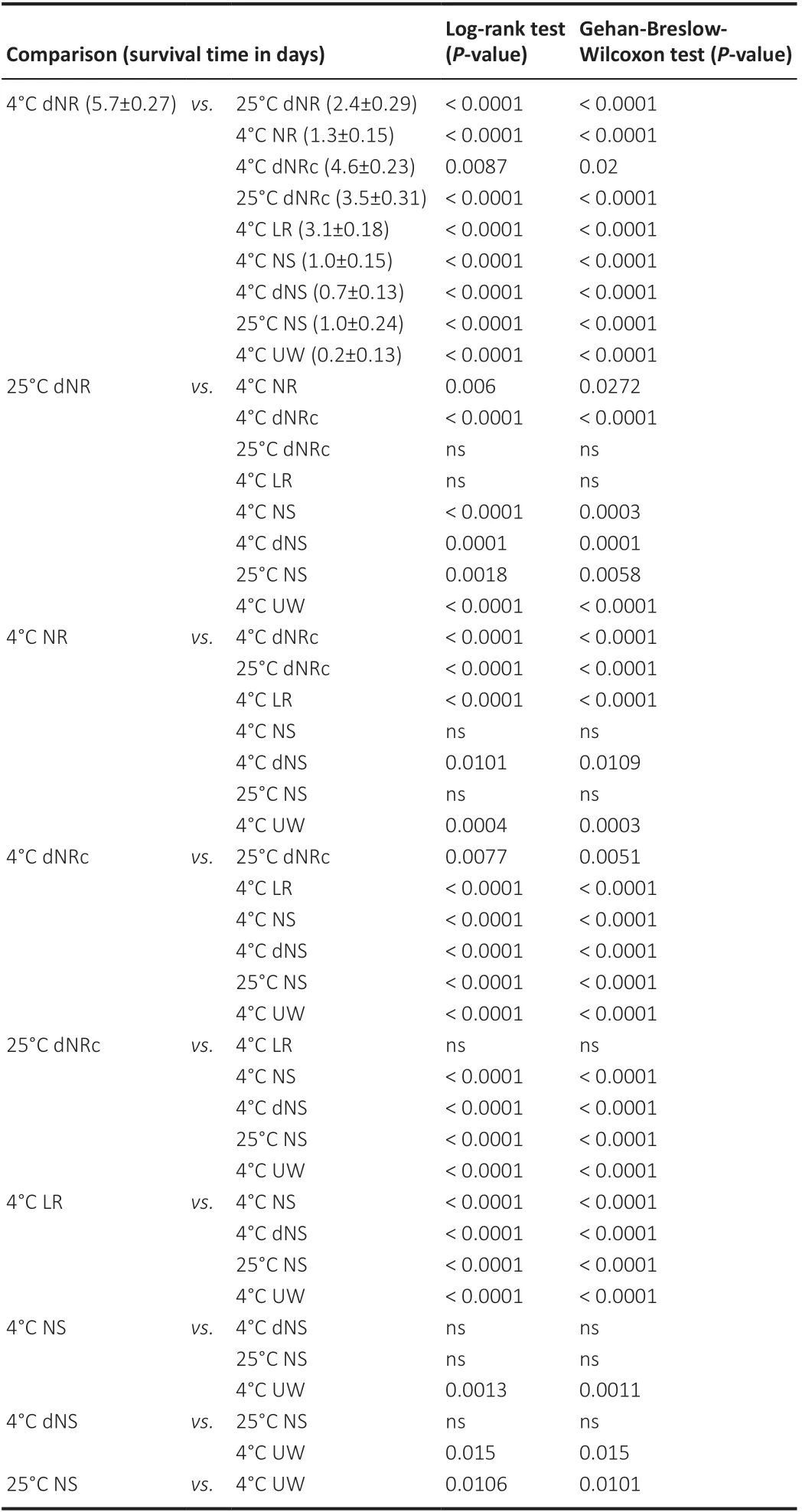
Table 2 |Summary statistics and statistical comparisons of PNVA survival time
PNVA survival was significantly better in 4°C hypotonic dNR than in 4°C isotonic NR (P< 0.0001;n= 10; Figure 2E). PNVAs in 4°C NR on averageconducted for 1.3 ± 0.15 days. While all PNVAs in 4°C dNR conducted CAPs up to day 3, only 30% of PNVAs in 4°C NR conducted CAPs on day 2. All PNVAs in 4°C NR stopped conducting on day 3.
PNVA survival was significantly (P= 0.009) impaired in 4°C dNRc (n= 14) and further impaired (P< 0.0001) in 25°C dNRc (n= 10) than in 4°C dNR (Figure 2F). PNVAs in 4°C dNRc on average conducted for 4.6 ± 0.23 days, and those in 25°C dNRc on average conducted for 3.5 ± 0.31 days. While all PNVAs in 4°C dNRc conducted CAPs on day 4, more than half of PNVAs stopped conducting on the following day, and by day 6 only 21% of PNVAs continued to conduct CAPs compared with 49% of PNVAs conducted in 4°C dNR. All PNVAs in 4°C dNRc stopped conducting on day 7. PNVA survival of CAP conduction is even less when stored in 25°C dNRc, where only 20% of PNVAs conducted on day 5. All PNVAs in 25°C dNRc stopped conducting on day 6.
PNVA survival of CAP conduction was significantly better in 4°C dNR than in 4°C LR (P< 0.0001;n= 10; Figure 2G). PNVAs in 4°C LR on average conducted for 3.1 ± 0.18 days. On day 3, 90% of PNVAs in 4°C LR conducted CAPs. However, a sharp drop in CAP conduction was observed on day 4, at which time only 20% of PNVAs were considered viable. All PNVAs in 4°C LR stopped conducting on day 5. PNVA survival of CAP conduction was not optimal in any NS-based storage conditions and was not significantly different among 4°C NS (n= 24), 4°C dNS (n= 14), and 25°C NS (n= 9; Figure 2H). CAPs in PNVAs stored in 4°C NS, 4°C dNS, and 25°C NS survived significantly (P< 0.0001) shorter than those stored in 4°C dNR. PNVAs in 4°C NS, 4°C dNS, and 25°C NS on average conducted for 1.0 ± 0.15, 0.7 ± 0.13, and 1.0 ± 0.24 days, respectively. On day 2, while all PNVAs in 4°C dNR conducted CAPs, all PNVAs in 4°C dNS stopped conducting, and only 17% of PNVAs in 4°C NS and 22% of PNVAs in 25°C NS conducted CAPs. On day 3, only 4.2% of PNVAs in 4°C NS conducted, while all PNVAs in 25°C NS stopped conducting. All PNVAs in 4°C NS stopped conducting on day 4. PNVA survival was significantly (P< 0.0001) better in 4°C dNR than in 4°C UW (n= 10; Figure 2I). PNVAs in 4°C UW on average conducted for only 0.2 ± 0.13 days. All PNVAs conducted CAPs immediately following harvest prior to storage in 4°C UW, but only 20% of PNVAs conducted on day 1. All PNVAs in 4°C UW stopped conducting on day 2.
Figure 3plots on one graph our electrophysiological survival data for all 10 storage conditions at all storage time points. Figure 3 shows the following:
At the end of day 1, all PNVAs conducted CAPs in five storage conditions: 4°C dNR, 4°C NR, 4°C LR, 4°C dNRc, and 25°C dNRc. For other storage conditions, 96% of PNVAs conducted CAPs in 25°C dNR. 70–80% of PNVAs conducted in 4°C NS, 4°C dNS, and 25°C dNS. Only 20% of PNVAs conducted CAPs in 4°C UW. At the end of day 4, almost all PNVAs conducted CAPs in 4°C dNR and 4°C dNRc (94% and 100%, respectively). Only 20–40% of PNVAs conducted in 4°C LR, 25°C dNR, and 25°C dNRc, and none of the PNVAs stored in the other 5 storage conditions conducted CAPs. At the end of day 7, only PNVAs stored in 4°C dNR conducted CAPs. At the end of day 10, all PNVAs stopped conducting CAPs in all storage conditions. Overall, 4°C dNR was the most optimal storage solution among all conditions tested.
Overall, PNVAs conducted CAPs for longer storage times when stored in solutions with lower osmolarity with the exception of dNS at 4°C. For example, PNVAs stored in 4°C LR conducted CAPs for significantly (P< 0.0001) longer times than 4°C UW (up to day 4 and 1, respectively). Lower temperature improved PNVA storage times in some solution conditions. As discussed above (Figure 2D and F), PNVAs in either 4°C dNR (P< 0.0001) or 4°C dNRc (P= 0.0077) conducted for a significantly longer time than their respective counterparts at 25°C. In contrast, PNVAs stored in 4°C NS and 25°C NS showed no significant difference in survival times as assayed by CAP conduction. Calcium had variable effects on PNVA storage times at different temperatures. PNVA stored in 4°C dNR remained viable significantly longer than those in 4°C dNRc (P= 0.009), but the survival curves of PNVAs stored in 25°C dNR and 25°C dNRc were not significantly different.
Results of novel morphological assessment
To characterize some of the unique features that represent less severe WD in PNVAs following multiple days of storage, we have developed a five-category classification system to describe axonal morphologies. Those five categories are (1) well-organized myelin and axoplasm, (2) abnormal myelin, (3) abnormal axoplasm, (4) abnormal myelin and axoplasm, and (5) disintegrating myelin. Figure 4 shows examples of this five-category classification system illustrated in individual panels as follows.
Figure 4Ashows axons that have well-organized myelin and axoplasm that have been termed “intact” in previous publications (Fox et al., 2005; Kerns et al., 2021). The white arrow in the top panel points to a large-diameter myelinated axon having many, non-convoluted myelin layers in which the myelin layers are compact (non-dissociated). The middle panel shows an axon similar to that of the top panel with some minor myelin dissociations and a slight retraction of the axoplasm. These small types of perturbations can even be seen in fresh nerves fixed immediately upon harvest. Therefore, this axon is counted as a well-organized axon in our classification system. The arrows in the bottom panel point to small-diameter axons having fewer myelin layers that are more convoluted, but well-organized myelin and axoplasm.
Figure 4Bshows axons with abnormal myelin sheaths. The red arrow in the top panel points to dissociated myelin wrapped around an axon with wellorganized axoplasm. The red arrows in the middle panel point to large spaces (more severe dissociations) within the myelin layers. The red arrow in the bottom panel points to an axon with an extensive myelin intrusion (bleb).
Figure 4Cshows axons with retracted axoplasm surrounded by a wellorganized myelin sheath. Some axons have two or more different points of retractions, or a single, much more pronounced retraction.
Figure 4Dshows axons that contain abnormalities present in both the myelin and axoplasm, to varying degrees of severity. The top panel shows an axon with severe myelin dissociation and axoplasmic retraction. The middle panel shows an axon with myelin intrusion and axoplasm retraction. The bottom panel shows an axon with myelin dissociation, myelin intrusion, and axoplasm retraction.
Figure 4Eshows axons with disintegrating myelin that is very poorly stained with toluidine blue. Since the visualization of myelin sheaths is achieved by osmium tetroxide binding to the double bonds in lipids, the faint staining of these axons suggests a severe degradation of the myelin sheath. Some axons completely lose all heavily stained myelin, while some retain remnants of their disassociating myelin sheath.
Comparing morphological results in different solutions
To assess the morphological correlates of PNVAs in 4°C dNR, we sampled PNVAs on storage days 0, 1, 3, and 5 (Figure 5A–E). We compared morphological data for 4°C dNR (the solution with the best CAP survival curve in Figure 3) with those for PNVAs stored 4°C NS (one of the storage solutions with the worst CAP survival curve) on day 1, since that is the last day when CAPs in most PNVAs remained viable in NS. Table 3 shows percentages of axons for each morphological category within each group; Table 4 shows statistical comparisons for each morphological category between groups.
Most axons in PNVAs in 4°C dNR fixed immediately following tissue harvest on storage day 0 (Figure 5F) had well-organized myelin and axoplasm (83 ± 2.7%), a few had abnormal myelin (13 ± 3.3%) or abnormal axoplasm (3.2 ± 1.8%), and almost none had abnormal myelin and axoplasm (0.6 ± 0.6%). None had disintegrating myelin (Table 3).
As the storage time in 4°C dNR increased, PNVAs exhibited increased signs of morphological degradation (Figure 5F). Two-way analysis of variance interaction was statistically significant (P< 0.0001), indicating storage duration affects the axonal composition of PNVAs. The percentage of wellorganized axons decreased significantly in PNVAs stored in 4°C dNR (P< 0.0001; Table 4), starting from 83 ± 2.7% (day 0) to 42 ± 6.2% (day 1) to 18 ± 3.1% (day 3) to 6.0 ± 3.1% (day 5) as reported in Table 3. This decrease correlated with a decrease in CAP amplitude from days 0–5 (Figure 1). In addition, as summarized in Table 3, the percentages of axons with abnormal myelin significantly increased between days 0 and 5 (13 ± 3.3% to 42 ± 8.0%;P=0.0005). Similarly, the percentages of axons with both abnormal myelin and axoplasm significantly increased between days 0 and 5 (0.6 ± 0.6% to 33 ± 9.9%;P< 0.0001). The percent of axons with abnormal axoplasm was not statistically different between days 0 and 5 (3.2 ± 1.8 % to 4.4 ± 1.9 %). Finally, the percentages of axons with disintegrating myelin were negligible between day 0 to 3 (all < 0.4 ± 0.4%) until day 5 at which time an increase (15 ± 4.0%) was noted that was not significant (P= 0.15).
As summarized in Table 3, PNVAs stored in 4°C NS on day 1 contained only 5.9 ± 2.6% of axons with well-organized myelin and axoplasm (Figure 5F). A few had abnormal myelin (3.1 ± 0.5%) or disintegrating myelin (0.9 ± 0.3%). The majority of the axons had abnormal axoplasm (62 ± 1.0%) or both abnormal myelin and axoplasm (28 ± 1.5%).Statistical differences were observed between PNVAs stored in 4°C dNR and 4°C NS on day 1 (Table 4). While 42 ± 6.2% of axons remained well-organized in 4°C dNR, only 5.9 ± 2.6% of axons in 4°C NS were well-organized (P< 0.0001). 4°C dNR contained significantly more axons with abnormal myelin (31 ± 6.1%vs. 3.1 ± 0.5%;P= 0.0007), while 4°C NS contained significantly more axons with abnormal axoplasm (62 ± 1.0%vs. 16 ± 5.1%;P< 0.0001). These differences were maintained when comparing PNVAs stored in 4°C NS to those in 4°C dNR until days 3 and 5 (P= 0.0003 or less; Table 4). Although not statistically significant (P= 0.06), a trend of more axons with both abnormal myelin and axoplasm was also observed in PNVAs stored in 4°C NS compared to those in 4°C dNR on day 1. Interestingly, PNVAs stored in 4°C NS on day 1 exhibit similar small percentages of well-organized axons as do those in 4°C dNR on day 5 (5.9 ± 2.6%vs. 6.0 ± 3.1%).

Figure 1|Recording chamber and CAPs.
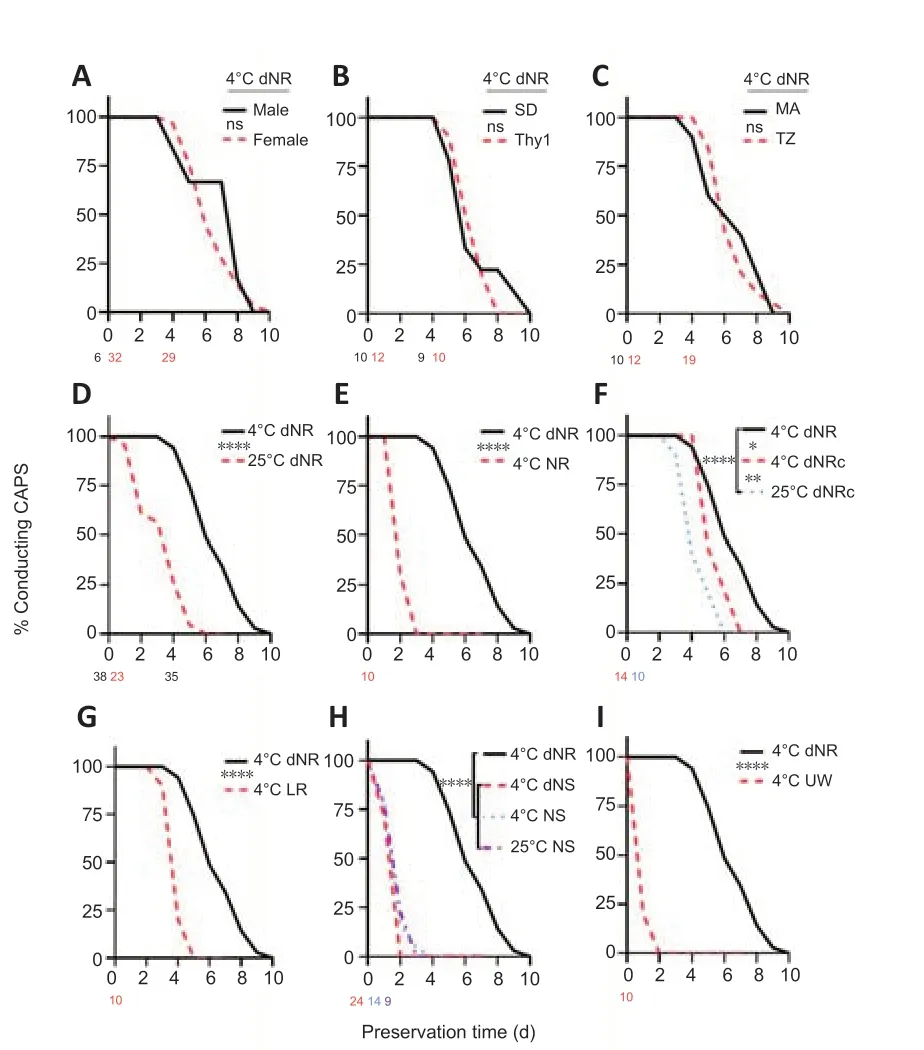
Figure 2|Nerve graft survival in various storage conditions.

Figure 3|Summary of % peripheral nerves with viable axons’ that conducted compound action potentials at 0–10-day storage time.
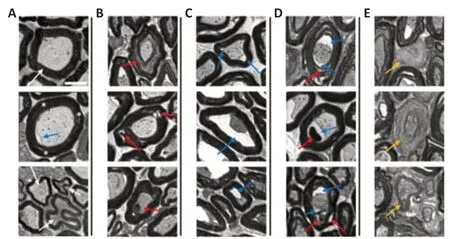
Figure 4|Representative histological sections of peripheral nerves with viable axons showing five axonal/myelin categories.

Figure 5|Morphological analyses of axons in stored peripheral nerves with viable axons.
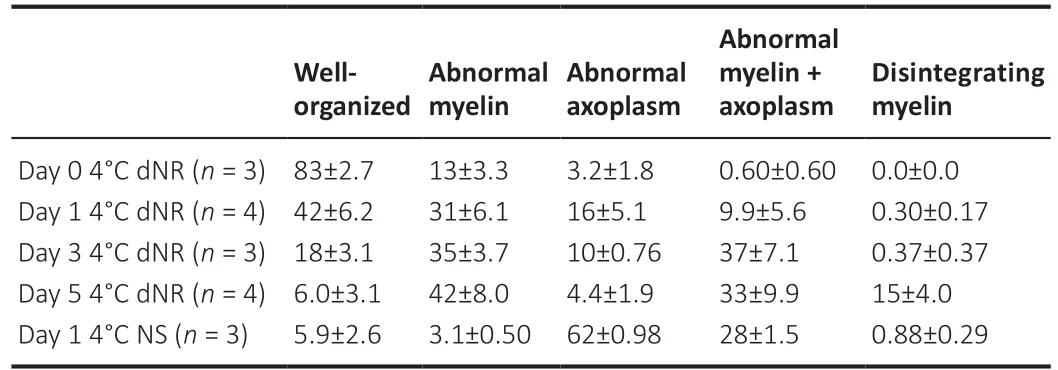
Table 3 |Summary statistics of peripheral nerves with viable axon morphology
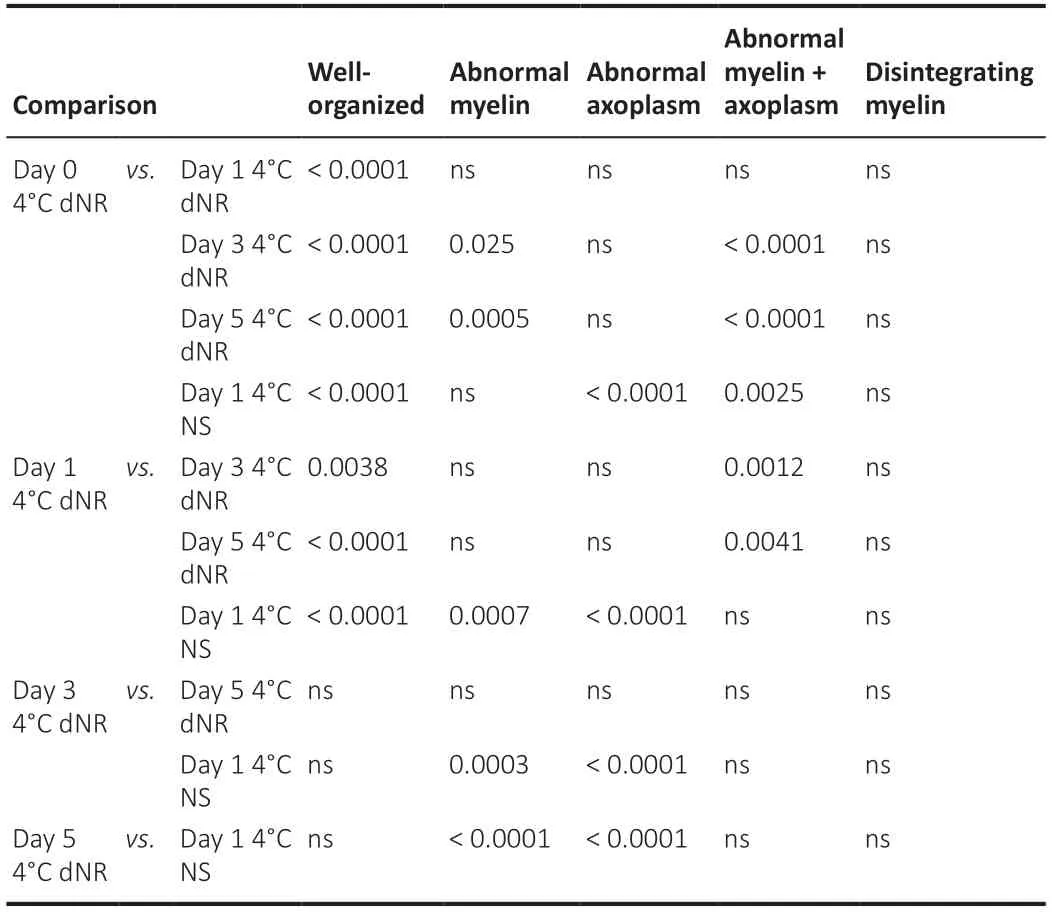
Table 4 | Statistical pair-wise comparisons summarizing PNVA morphology
Discussion
Summary of electrophysiological and morphological results
Our study used a novel electrophysiological (CAP recordings) criterion to assess the viability of PNVAs storedex vivo. First, CAP recordings of PNVAs were not significantly affected by the identity of the operating surgeon, the euthanasia procedure, and the strain or sex of the rat, even though sciatic PNVAs have somewhat larger diameters in males than females. Second, PNVA storage times were significantly improved by four conditions: (1) solution composition, (2) reduced tonicity, (3) lower temperature, and (4) low calcium concentration. Condition (1) and possibly (2) had stronger effects than (3–4). That is, as one example of the effect of solution composition, PNVAs stored in 4°C LR conducted CAPs for a significantly longer time (up to 4 days) than those stored in 4°C UW (up to 1 day). As an example of the effect of reduced tonicity, PNVAs stored in 4°C dNR conducted CAPs for a significantly longer time (up to 9 days) than those stored in 4°C NR (up to 2 days). As an example of the effects of lower temperature, PNVAs stored in 4°C dNR conducted CAPs for a significantly longer time (up to 9 days) than those stored in 25°C dNR (up to 5 days). As an example of the effects of low calcium concentration, PNVAs stored in 4°C dNR conducted CAPs for a significantly longer time (up to 9 days) than those stored in 4°C dNRc (up to 6 days).
Our novel, detailed morphometric assessment method reports the distinct morphological categories for PNVAs in 4°C dNR, including well-organized axons, axons with abnormal myelin dissociation or intrusion, axons with retracted axoplasm, axons with both abnormal myelin and axoplasm, and axons with disintegrating myelin. As storage time increased, the percentage of well-organized axons decreased significantly, but a small population of these axons still existed on day 5. The percentage of axons with abnormal myelin increased between day 0 and 1 but remained unchanged beyond day 1. Similarly, the percentage of axons with abnormal myelin and axoplasm increased between days 0 and 3 but remained unchanged beyond day 3. The percentage of axons with abnormal axoplasm did not vary significantly at any time point tested and was always low. Axons with disintegrated myelin sheath, indicative of tissue degradation, appeared on day 5. In contrast, PNVAs in 4°C NS exhibited a small population of well-organized axons on day 1, while the majority of the axonal population exhibited either abnormal axoplasm or abnormal myelin and axoplasm.
Most importantly, we have demonstrated, for the first time, preservation solutions and protocols that maintain axon viability in PNVAs for several days after harvest. We have just begun to examine how different storage solutions might affect axonal viability in PNVAs. We do not know how different cellular/biochemical mechanisms or pathways might be affected by differences in the four conditions (solution composition, measured osmotic strength, temperature, and presence of calcium) examined in this study. Other solutions (e.g., HTK, DMEM, Collin’s solution) reported as best for the storage of other types of tissue should also be investigated because they control additional variables such as the pH, sugars, amino acids, and electrolytes (Collins and Wicomb, 1992; Lassner et al., 1995; Spiegel et al., 1999; Jing et al., 2018) not examined in our study.
Storage solutions optimal for non-neuronal organs and tissues versus PNVAs
Storage solutions optimized for the storage of non-neuronal organs and tissues are not necessarily optimal for PNVA storage. For example, NS solution is often optimally used to store non-neuronal tissue grafts, including bone and vein grafts (Steiner and Ramp, 1988; Davies and Hagen, 1994). UW solution, created to prevent ischemic stress, is commonly used as an optimal solution to store non-neuronal organs, such as kidneys, livers, and pancreatic tissues (Belzer and Southard, 1988; Voigt and DeLario, 2013). Most unexpectedly in our study, 4°C NS or UW was decidedly suboptimal for PNVA storage as assessed by CAPs conduction, i.e., CAPs conducted for only 1 or 2 days. UW had previously been tested for nerve storage (Evans et al., 1998; Fox et al., 2005; Matsumoto et al., 2005; Ikeguchi et al., 2007), and intact myelin structures were observed after 7 weeks of cold storage. However, axonal viability was never tested. Among all the unmodified solutions tested in this study at 4°C (UW, NS, NR, and LR), LR maintained PNVA viability in PNVA for the longest storage times.
Storage solutions tonicity as a variable
Lower osmolarity of the storage solution often led to longer PNVA storage times with the exception of dNS at 4°C. For example, PNVAs stored in 4°C dNR (250–255 mOsm) continued to conduct CAPs for up to 9 days but only for 2 days in 4°C NR (268 mOsm). Our morphological data also suggested higher tonicity may lead to the retraction of axoplasm and axolemma from the myelin sheath. In 4°C dNR (250–255 mOsm), 37% of axons exhibited retracted axoplasm after 5 days of storage, whereas 90% of axons exhibited retracted axoplasm just after 1 day of storage in 4°C NS (290–295 mOsm). This may explain why UW performed the worst in PNVA storage at 4°C. UW contains lactobionic acid and raffinose, which were specifically added to create hypertonicity and cause cell desiccation prior to cryopreservation (Belzer and Southard, 1988; Chen et al., 2019). This principle of design may be detrimental to axonal viability in PNVA storage.
Storage temperature as a variable
Lower temperature often led to better PNVA storage times. For example, when assayed for CAP conduction, 4°C dNR and 4°C dNRc outperformed 25°C dNR and 25°C dNRc, respectively. Our result is consistent with previous reports of tissue storage (including nerve allografts) at different cold temperatures to reduce cell metabolism and activities and tissue degradation (Belzer and Southard, 1988; Levi et al., 1994; Evans et al., 1999; Fox et al., 2005; Ostrozka-Cieslik et al., 2018). However, the temperature was not necessarily the primary determinant variable for axonal viability since 4°C NS and 25°C NS had similar PNVA storage times based on CAP conduction. Additionally, 4°C UW, NS, dNS, and NR all had poorer PNVA storage times for viable CAP conduction than dNR at 25°C.
Calcium concentration as a variable
PNVA storage time assayed by CAP conduction was longer in calcium-free dNR at 4°C than calcium-containing dNRc at 4°C. This result was consistent with (Schlaepfer, 1974) in which calcium-containing solutions induced granular disintegration of axoplasmic microtubules and neurofilaments in stored rat nerve segments. However, calcium, like temperature, was not the dominant factor for axonal viability when comparing different solutions of different compositions, osmolarities, and temperatures when assessed by CAP conduction. As one example, 4°C UW had poorer PNVA storage times than 4°C calcium-containing LR. As another example, 25°C dNR had poorer PNVA storage times than 4°C dNRc.
Previous axonal morphometric studies on WD
We know no previous publications describing axonal morphometrics of nerves maintainedex vivo, perhaps because nerve grafts are often regarded simply as mechanical conduits to guide axonal regeneration. Previous morphometrics studies have characterized WDin vivo. For example, Kerns et al. (2021) described three categories of myelinated axons during WD: spared, partial/moderate degenerated, and advanced degenerated. However, that classification system did not separate populations of axons with obvious morphological differences in myelin and axoplasm and did not capture all the characteristics displayed in stored nerves demonstrated in this study.
PNVAs versus conventional conduits used in repairs of segmental-loss PNIs
Axons in nerve grafts have been expected to undergo WD, and the collapsed basal lamina tubes have been expected to guide axonal regeneration by outgrowth from the host proximal nerve with the help of supporting cells (Pan et al., 2020). Hence, previous nerve storage studies focused on maintaining the viability of the supporting cells rather than axons and the integrity of the graft structure. For example, Lassner et al. (1995) compared cold preservation of rat nerve grafts in various solutions to assess Schwann cell and fibroblast growthex vivo, axon counts, and myelination following transplantation. These repair strategies using various conduit materials always led to slow and poor behavioral recovery.
As an alternate and novel approach, we propose to use PNVAs that are PEGfused to repair segmental-loss PNIs. In PEG-fused PNVAs, the open cut ends of axons at both ends of the donor PNVA are joined to/ fused with open cut ends of host axons that are still viable at the proximal and distal ends of the gap where the PNVA has been inserted (Riley et al., 2015). When compared to conduits, autografts, or other current growth guides, PEG-fused PNVAs have the following advantages: (1) PNVAs can be selected to be predominantly motor, sensory, or mixed sensory/motor, depending on the nature of the lesioned nerve. (2) PNVAs can be size- or shape-matched to the lesioned nerve. (3) PNVAs do not produce additional host morbidity as do autografts. (4) PNVAs exhibit complex biological features of intact peripheral nerves. (5) PEGfused PNVAs need not be tissue matched or immune-suppressed to avoid immune rejection and restore many voluntary behaviors within weeks (Bittner et al., 2016b, 2018; Mikesh et al., 2018b; Smith et al., 2020; Roballo et al., 2022).
Conclusions
Maintaining axonal viability in PNVAs is important because viable donor axons in PNVAs can be PEG-fused with viable proximal and distal ends of host axons to repair segmental-loss PNIs that produce excellent recovery of sensory/motor functions and voluntary behaviors within weeks. Such PEGfused PNVAs, unlike other donor transplants, are immune-tolerated without tissue matching or immune suppression. An inability to store PNVAs for more than a day would be a limiting factor to expanding the potential of PEG-fusion technology to repair segmental-loss PNIs. Therefore, longer PNVA storage times could enable the establishment of PNVA tissue banks and the use of stored, PEG-fused PNVAs to repair segmental-loss PNIs. Furthermore, optimal storage solutions and conditions for PNVAs might be relevant for the storage of other donor tissue grafts that contain nerves, such as limb transplants or even heart transplants.
Acknowledgments:We thank Dr. Susan MacKinnon (Washington University) for providing animals used in this study. We thank Dr. David Jackson (Qerity) for careful readings of drafts and final versions of the manuscript and advice on protocol design.
Author contributions:Electrophysiology and TEM thick sections: LZ. Electrophysiology: MA and TZ. TEM thick sections: PO. Supervised the project: GDB. All authors designed experiments, analyzed and interpreted data, prepared the manuscript, and approved the final manuscript.
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr. Bittner has assigned all of his economic interests in a licensed PEG-fusion patent estate to a third party that affects in no way any data analyses or text in this manuscript.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Corrigendum
- Extracellular vesicles as a potential therapeutic for agerelated macular degeneration
- Fasting produces antidepressant-like effects via activating mammalian target of rapamycin complex 1 signaling pathway in ovariectomized mice
- Suppressing high mobility group box-1 release alleviates morphine tolerance via the adenosine 5'-monophosphate-activated protein kinase/heme oxygenase-1 pathway
- Transcriptional regulatory network during axonal regeneration of dorsal root ganglion neurons: laser-capture microdissection and deep sequencing
- 5-Hydroxytryptamine: a potential therapeutic target in amyotrophic lateral sclerosis

