Fasting produces antidepressant-like effects via activating mammalian target of rapamycin complex 1 signaling pathway in ovariectomized mice
Zi-Qian Cheng , Jie Fan , Fang-Yi Zhao Jing-Yun Su Qi-Han Sun Ran-Ji Cui , Bing-Jin Li
Abstract Recent studies have shown that a 9-hour fast in mice reduces the amount of time spent immobile in the forced swimming test. However, whether 9-hour fasting has therapeutic effects in female mice with depressive symptoms has not been established. Therefore, in this study, we simulated perimenopausal depression via an ovariectomy in mice, and subjected them to a single 9-hour fasting 7 days later. We found that the ovariectomy increased the time spent immobile in the forced swimming test, inhibited expression of the mammalian target of rapamycin complex 1 signaling pathway in the hippocampus and prefrontal cortex, and decreased the density of dendritic spines in the hippocampus. The 9-hour acute fasting alleviated the above-mentioned phenomena. Furthermore, all of the antidepressant-like effects of 9-hour fasting were reversed by an inhibitor of the mammalian target of rapamycin complex 1. Electrophysiology data showed a remarkable increase in long-term potentiation in the hippocampal CA1 of the ovariectomized mice subjected to fasting compared with the findings in the ovariectomized mice not subjected to fasting. These findings show that the antidepressant-like effects of 9-hour fasting may be related to the activation of the mammalian target of the rapamycin complex 1 signaling pathway and synaptic plasticity in the mammalian hippocampus. Thus, fasting may be a potential treatment for depression.
Key Words: antidepressant; brain-derived neurotrophic factor; dendritic spine; fasting; hippocampus; LTP; mTOR complex 1; neural plasticity; ovariectomized mice; rapamycin 1Jilin Provincial Key Laboratory on Molecular and Chemical Genetic, The Second Hospital of Jilin University, Changchun, Jilin Province, China; 2Jilin Engineering Laboratory for Screening of Antidepressant, Changchun, Jilin Province, China
Introduction
Depression is a common and recurrent psychiatric disorder. It is listed by the World Health Organization as the single largest cause of disability worldwide (World Health Organization, 2017). However, therapeutic treatment via antidepressants has many shortcomings (Rheker et al., 2017; Kutzer et al., 2020; Li et al., 2022). Fasting has been found to effectively elevate mood (Molendijk et al., 2018; de Carvalho, 2022). For instance, fasting and calorie restriction were found to alleviate depressive symptoms in both basic and clinical studies (Lutter et al., 2008; Hussin et al., 2013). Moreover, a 9-hour fast in normal mice reduced the immobility time in behavioral tests, activated the cyclic adenosine monophosphate response element-binding protein-brainderived neurotrophic factor (BDNF) signaling pathway in the hippocampus and prefrontal cortex, and altered synaptic transmission in the hippocampus, as measured via subsequent transcriptome analysis (Li et al., 2014; Wang et al., 2019). Meanwhile, fasting had an estrogen-like effect in ovariectomized mice, indicating that it may share some of the functions of estrogen (Bigsby et al., 1997). Caloric restriction has been found to up-regulate the expression of estrogen receptors, but does not affect the expression of androgen receptors (Słuczanowska-Głąbowska et al., 2015).
Ovariectomy has been found to elicit depression-like behaviors while downregulating BDNF expression in the prefrontal cortex and hippocampus (Xu et al., 2015; Fan et al., 2017). Ovariectomy quickly withdraws estrogen and other ovarian hormones in female mammals, which effectively simulates the perimenopausal period in women (Gore et al., 2002; Greendale et al., 2011). Low estrogen levels have been found to induce depression-like behaviors in rodents (Walf and Frye, 2006). In addition, female rats are more sensitive to ketamine than male rats via a phenomenon related to the alternative activation of the mammalian target of rapamycin (mTOR) (Carrier and Kabbaj, 2013). Furthermore, acute 17α-ethinyl estradiol treatment induces significant antidepressant-like behaviors through mTOR activation in ovariectomized mice (Saeedi Saravi et al., 2017). Therefore, it is meaningful to explore the mechanism of mTOR-mediated treatment of estrogen deficiency-induced depression. The mTOR signaling pathway has been found to integrate signals from nutrients and growth factors, initiate downstream pathways through mTOR complex 1 (mTORC1) and mTORC2, and further regulate growthrelated cellular processes (Shimobayashi and Hall, 2014; Teng et al., 2019). Studies have indicated that the BDNF-mTORC1 signaling pathway is associated with the potential mechanisms underlying the rapid antidepressant-like effects of ketamine (Wohleb et al., 2017; Duman et al., 2019; Fogaça et al., 2019; Abdallah et al., 2020). The proposed mechanisms of fasting acting antidepressant drugs relate to an increase in BDNF expression and direct action on the mTORC1 signaling pathway, which includes extracellular signal-regulated protein (ERK), mTORC1, p70 ribosomal S6 kinase (p70S6), protein kinase B (AKT), and small ribosomal protein 6 (S6) (Li et al., 2010; Lepack et al., 2014; Duman et al., 2016; Rantamäki and Yalcin, 2016). Activation of the mTORC1 signaling pathway ultimately induces an increase in postsynaptic density protein 95 (PSD95) levels, promotes neurogenesis and synaptogenesis, and reverses brain volume atrophy, which can ameliorate depressive symptoms (Duman et al., 2016; Wohleb et al., 2017).
In the present study, we assessed whether a 9-hour fast produced antidepressant effects in female mice by activating the mTORC1 signaling pathway. We also measured synaptic plasticity-related proteins including BDNF and PSD95, dendritic spine density, and long-term potentiation (LTP) to clarify the antidepressant mechanisms of 9-hour fasting.
Methods
Animals
Adult female Institute of Cancer Research (ICR) mice (6–8 weeks, 30 ± 5 g) were purchased from Liaoning Changsheng Life Sciences Ltd. (Benxi, Liaoning, China; license No. SCXK (Liao) 2020-00015). The mice were maintained in standard laboratory conditions (23 ± 1°C, 40–50% relative humidity, 12-hour light-dark cycle, 06:00 to 18:00 lights), allowed to eat and drink ad libitum, and housed individually in plastic cages (25.5 cm × 15 cm × 14 cm). All experiments were conducted according to the Laboratory Animal Guidelines for the Ethical Review of Animal Welfare (GB/T 35892-2018) (General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China and Standardization Administration of the People’s Republic of China, 2018) and approved by the Institutional Animal Care and Use Committee of Jilin University (approval No. 2018086; February 26, 2018).The mice were randomly divided into five groups, including a sham group (n= 24, 8 mice for the behavioral tests, 11 mice for the western blot assay, and 5 mice for Golgi staining), ovariectomy (OV,n= 29, 8 mice for the behavioral tests, 11 mice for the western blot assay, 5 mice for Golgi staining, and 5 mice for electrophysiology), ovariectomy and fasting (OVF,n= 29, 8 mice for the behavioral tests, 11 mice for the western blot assay, 5 mice for Golgi staining, and 5 mice for electrophysiology), ovariectomy and fasting with rapamycin (OVF-R,n= 24, 8 mice for the behavioral tests, 11 mice for western blot assay, and 5 mice for Golgi staining), and ovariectomy with rapamycin (OVR,n= 24, 8 mice for the behavioral tests, 11 mice for the western blot assay, and 5 mice for Golgi staining) groups. See Figure 1A for a flow chart of the study design.
Ovariectomy
The surgical procedure for ovariectomy has been described in a previous report (Fan et al., 2017). Mice were anesthetized with 65 mg/kg pentobarbital sodium solution (10 mL/kg; Beijing Dingguo Changsheng Biotechnology Co. Ltd., Beijing, China). An incision approximately 1 cm in length was made on the ventrolateral side of the abdomen, above the ovary. Ovariectomy was performed bilaterally. Finally, vaginal smears were used to verify the ovarian extirpation. Sham-operated animals that did not undergo ovariectomy served as controls for the ovariectomized mice, and enabled us to verify the successful establishment of the depression model.
Fasting
A previous study indicated that a 9-hour fast (not a 3- or 18-hour fasting) had the most significant antidepressant-like effects on behavior in the forced swimming test (FST) (Li et al., 2014). Seven days after the ovariectomy, fasting was implemented between 00:00 and 09:00.
Drug administration
Rapamycin was purchased from Solarbio (Beijing, China) and dissolved in a mixed vehicle including 4% ethanol (Beijing Chemical Works, Beijing, China), 5% PEG 400 (Shanghai Shanpu Chemical Co. Ltd., Shanghai, China), and 5% Tween 80 (Beijing Dingguo Changsheng Biotechnology Co. Ltd., Beijing, China). The mixture of rapamycin (4.0 mg/kg) and vehicle (10 mL/kg) was injected intraperitoneally before the end of fasting.
Behavioral tests
All behavioral tests were conducted 7 days after the ovariectomy. Behavioral tests were performed 30 minutes after the administration of vehicle plus rapamycin or vehicle alone.
Open field test
Mice were placed individually in a black acrylic cylinder (diameter: 48.8 cm; height: 16 cm) with 19 equal squares demarcated by black lines. The horizontal and vertical locomotor activities of the mice were recorded for 6 minutes using a video camera. Horizontal locomotor activity was measured as the number of squares crossed with all four paws. The detailed experimental process is given in a previous report (Hall, 1934; Fan et al., 2017).
FST
The FST was performed after the open field test. The FST, originally described by Porsolt et al. (1977, 1978), is currently the most widely used method for assessing the antidepressant-like effects of drugs. In mice, one exposure to the FST is sufficient to generate a stable calculation of immobility that can be counteracted by acute pretreatment with an antidepressant (Petit-Demouliere et al., 2005; Yan et al., 2010). In this study, the mice were placed in a cylindrical acrylic container (diameter: 11 cm; height: 25 cm; depth: 20 cm). Immobility was defined as a lack of activity other than when the mouse exposed its head to the surface of the water (25 ± 1°C) via slight movements. Each FST trial lasted 6 minutes, and the immobility time was manually measured during the last 4 minutes (Fan et al., 2017). The immobility duration was recorded by an observer who was blinded to the treatment conditions.
Western blot assay
For western blotting, mice were deeply anesthetized with 65 mg/kg pentobarbital sodium solution 30 minutes after rapamycin administration. The mouse brains were removed with ophthalmic scissors, and then the hippocampus and prefrontal cortex tissues were collected. Whole protein extraction was conducted with homogenates of the hippocampus and prefrontal cortex tissue samples. The extracted samples were separated onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and subsequently transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Then, the membranes were incubated with 5% bovine albumin (Sigma, St. Louis, MO, USA) in Tris-buffered saline with Tween for 1 hour. The membranes were incubated with the following primary antibodies overnight at 4°C: estrogen receptor α (rabbit polyclonal, 1:1000; Affinity, Cincinnati, OH, USA, Cat# AF6058, RRID: AB_2834976), estrogen receptor β (rabbit polyclonal, 1:1000; Affinity, Cat# AF6469, RRID: AB_2835288), BDNF (rabbit polyclonal, 1:800; Santa Cruz Bio, Dallas, TX, USA, Cat# sc546, RRID: AB_630940), AKT (rabbit polyclonal, 1:1000; CST, Danvers, MA, USA, Cat# 9272, RRID: AB_329827), phosphor-AKT (pAKT; rabbit polyclonal, 1:1000; CST, Cat# 9271, RRID:AB_329825), ERK (rabbit polyclonal, 1:1000; CST, Cat# 9102, RRID: AB_330744), phosphor-ERK (pERK; rabbit polyclonal, 1:1000; CST, Cat# 9101, RRID: AB_331646), mTORC1 (rabbit monoclonal, 1:1000; CST, Cat# 2587, RRID:AB_2261091), p70S6 (rabbit polyclonal, 1:1000; CST, Cat# 9202, RRID:AB_331676), S6 (rabbit monoclonal, 1:1000; CST, Cat# 2217, RRID: AB_331355), 4E-binding protein 1 (4EBP1; rabbit polyclonal, 1:1000; CST Cat# 9452, RRID: AB_331692), PSD95 (mouse monoclonal, 1:1000; Abcam, Cambridge, UK, Cat# ab192757, RRID: AB_2750929), and β-actin (mouse monoclonal, 1:2000; Transgen Biotech, Beijing, China, Cat# HC201, RRID: AB_2860007). After extensive washing with Tris-buffered saline with Tween, the membranes were incubated with secondary antibodies (antirabbit: 1:2000; ZSBG-Bio, Beijing, China, Cat# ZB2301, RRID: AB_2747412; anti-mouse: 1:6000; ZSBG-Bio, Cat# ZB2305, RRID: AB_2747415) at room temperature (25 ± 1°C) for 40 minutes. The membranes were incubated with enhanced chemiluminescence (Millipore) supersensitive developer solution and observed using a Tanon-5200 chemiluminescence imaging system (Shanghai, China). Finally, optical density analysis (normalized by β-actin) was performed using ImageJ 1.50i software (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Golgi staining
Golgi staining was conducted as previously reported (Glaser and Van der Loos, 1981; Shors et al., 2001). Briefly, whole fresh brain tissues were collected 30 minutes after rapamycin administration for impregnation in Golgi-Cox solution (200 mL 5% potassium dichromate solution (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) + 200 mL 5% mercuric chloride solution (Tongren Chemical Plant, Tongren, Guizhou, China) + 200 mL 5% potassium chromate solution (Tianjin BASF Chemical Trade Co., Ltd., Tianjin, China) + 400 mL double distilled water (ddH2O)), and incubated in a dark box for 2 days. Then, the samples were moved to fresh Golgi-Cox solution for 14 days before dehydration in 30% sucrose solution for 3 days. Serial coronal sections (200 µm thick) were mounted for development. The mounted sections were washed three times with ddH2O, and then placed in a coplin jar filled with 25% ammonia solution for 60 minutes. The sections were then washed three times with ddH2O and incubated in fixer solution (Kodak; Rochester, NY, USA) for an additional 30 minutes to fix the stain. Next, the mounted sections were washed three times with ddH2O. The whole color development process was conducted under dark conditions. Finally, the sections were dehydrated via a gradient alcohol emersion process and made transparent after three repeated emersions in xylene. We counted eight neurons from each region of interest (CA1, CA3, and the dentate gyrus [DG]) in each mouse and quantitatively analyzed the dendritic branches emanating from the secondary dendrite in the neuronal apical tree. The number of spines was counted in a 10 µm segment and averaged from three segments in a neuron. The final value was the average of each group.
Electrophysiology
The mice were deeply anesthetized with 65 mg/kg pentobarbital sodium solution before the brain was removed. Coronal hippocampal slices (300-µm thickness) were cut for extracellular recordings using a Leica VT1200S vibratome (Leica, Heidelberg, Germany). The slices were transferred to nylon mesh submerged in artificial cerebrospinal fluid (119 mM sodium chloride (Beijing Chemical Works), 2.5 mM potassium chloride (Sigma), 2.5 mM calcium chloride (Sigma), 1.3 mM magnesium chloride (Sigma), 1 mM sodium dihydrogen phosphate (Sigma), 26.2 mM sodium carbonate (Sigma), and 11 mM glucose (Sigma)) that had been equilibrated with 95% O2and 5% CO2for at least 60 minutes at room temperature. After this recovery period, the slices were transferred to a recording chamber (Molecular Devices, Foster City, CA, USA) where they were perfused with artificial cerebrospinal fluid at a rate of 2 mL/min. Field excitatory postsynaptic potentials were evoked using a monopolar electrode (Vitalsense Scientific Instruments Co., Ltd., Wuhan, China) that was filled with artificial cerebrospinal fluid and placed in the stratum radiatum of the CA1 via stimulation of the Schaffer collateral afferents, as previously described (Talani et al., 2011). LTP was elicited by highfrequency stimulation (100 Hz tetanic stimulation, three trains, 1 second). Data were collected and analyzed using pClamp 10 software (Molecular Devices).
Statistical analysis
The sample size was largely determined by similar previous publications (Liu et al., 2018; Aguiniga et al., 2019; Wu et al., 2019; Cruz et al., 2021). Mice that died during the study (one in the ovariectomy and fasting group and two in the ovariectomy group) were excluded from the analysis. The evaluators were blinded to group membership. Data are expressed as the mean ± standard error of the mean (SEM) and were analyzed via a one-way analysis of variance using GraphPad Prism 6.01 software (GraphPad Software, San Diego, CA, USA, www.graphpad.com).Post hoccomparisons were performed using Tukey’s honestly significant difference test. AP-value less than 0.05 was considered statistically significant.
Results
A 9-hour fasting induces antidepressant-like behavior
According to our analysis of the behavioral test data, the 9-hour fasting did not affect the number of line crosses (F(4,35)= 0.2075,P= 0.9325; Figure 1B) or instances of rearing (F(4,35)= 0.2717,P= 0.8942, Figure 1C) in the open field test. However, the FST results showed significant differences in immobility time among the five groups (F(4,33)= 8.799,P< 0.0001, Figure 1D). Compared with the sham group, the OV mice exhibited a significantly increased immobility time (P= 0.0483). Fasting reduced this (P< 0.0001) to a level below that in the sham group. After rapamycin administration, the immobility time of OVF-R mice that had undergone fasting treatment was significantly increased to near sham levels (P= 0.0235). However, rapamycin alone reduced the immobility time in the OVR group (P= 0.0011, Figure 1D), which was unlike its effect in OV animals. These data indicate that general changes in activity induced by the 9-hour fasting were not responsible for the differences in immobility time observed in the FST.
A 9-hour fasting induces an increase in estrogen receptor β expression in the hippocampus and prefrontal cortex
The western blot analysis showed that, compared with the sham group, OV mice exhibited reduced estrogen receptor α expression in the hippocampus (F(4,19)= 2.734,P= 0.0595, one-way analysis of variance;P= 0.0398, post hoc analysis; Figure 2A) and prefrontal cortex (F(4,14)= 9.224,P= 0.0007, one-way analysis of variance;P= 0.0422,post hocanalysis; Figure 2B). Furthermore, the ovariectomy reduced the levels of estrogen receptor β in the hippocampus (F(4,12)= 7.229,P= 0.0033, one-way analysis of variance;P= 0.0237,post hocanalysis; Figure 2C) and prefrontal cortex (F(4,15)= 7.904,P= 0.0012, one-way analysis of variance;P= 0.0488,post hocanalysis; Figure 2D) of OV mice. In addition, compared with OV mice, the 9-hour fasting increased the level of estrogen receptor β in the hippocampus (P= 0.0030, Figure 2C) and prefrontal cortex (P= 0.0183, Figure 2D) of OVF mice. Rapamycin administration increased estrogen receptor α levels in the prefrontal cortex of OVR mice (P= 0.0053, Figure 2B), but did not modulate the effect of fasting on estrogen receptor β expression.
A 9-hour fasting induces an increase in mTORC1 signaling pathway activity and synaptic protein expression in the hippocampus
We assessed the effect of the 9-hour fasting on the protein levels in the mTORC1 signaling pathway, BDNF levels, and synaptic proteins in the hippocampus and prefrontal cortex via western blotting (Figures 3 and 4).
We performed an analysis of variance to evaluate changes in mTORC1 expression in the hippocampus, including the effects of fasting and rapamycin (F(4,19)= 8.029,P= 0.0006). Compared to sham group, the ovariectomy significantly decreased mTORC1 levels in the mouse hippocampus (P= 0.0268,vs.sham group) in the OV group. In contrast, fasting improved mTORC1 levels in the hippocampus (P= 0.0227), and this effect was reversed by rapamycin (P= 0.0318). Compared with OV group, rapamycin alone produced a significant increase in mTORC1 levels in OVR mice (P= 0.0029; Figure 3B).
We also measured the effects of the 9-hour fasting and rapamycin treatment on the expression of other parts of the mTORC1 signaling pathway, BDNF, and synaptic proteins in the hippocampus. An analysis of variance revealed significant effects of the 9-hour fast on the expression of mTORC1 upstream activators and downstream effectors (BDNF:F(4,19)= 6.309,P= 0.0021, Figure 3C; AKT:F(4,31)= 8.271,P= 0.0001, Figure 3D; ERK:F(4,31)= 8.256,P= 0.0001, Figure 3F; phosphorylated ERK:F(4,35)= 11.64,P< 0.0001, Figure 3G; p70S6:F(4,27)= 10.68,P< 0.0001, Figure 3H; S6:F(4,24)= 7.532,P= 0.0004, Figure 3I; and PSD95 (a representative synaptic protein):F(4,27)= 7.440,P= 0.0004, Figure 3J). As shown in Figure 3, compared with sham group, the ovariectomy significantly decreased the expression of mTORC1 upstream activators, including BDNF (P= 0.0204, Figure 3C), AKT (P= 0.0147, Figure 3D), ERK (P= 0.0298, Figure 3F), phosphorylated ERK (P= 0.0213, Figure 3G), and downstream effectors, such as p70S6 (P= 0.0244, Figure 3H) and PSD95 (P= 0.0072, Figure 3J) in the OV group. Compared with the findings in the OV group, fasting increased the levels of AKT (P= 0.0481, Figure 3D), ERK (P= 0.0043, Figure 3F), phosphorylated ERK (P= 0.0210, Figure 3G), p70S6 (P= 0.0484, Figure 3H), and PSD95 (P= 0.0369, Figure 3J) in OVF mice. Rapamycin reversed the effect of fasting and decreased the levels of BDNF (P= 0.0327, Figure 3C), AKT (P= 0.0050, Figure 3D), ERK (P= 0.0174, Figure 3F), p70S6 (P= 0.0153, Figure 3H), S6 (P= 0.0090, Figure 3I), PSD95 (P= 0.0065, Figure 3J), and phosphorylated ERK (P= 0.0002, Figure 3G).
A 9-hour fasting induces an increase in mTORC1 signaling pathway activity and synaptic protein expression in the prefrontal cortex
In addition to the hippocampus, we examined changes in the mTORC1 signaling pathway, BDNF levels, and synaptic protein levels in the prefrontal cortex. We observed substantial differences in the mTORC1 signaling pathway activity, BDNF levels, and synaptic proteins, as shown in Figure 3. An analysis of variance confirmed significant differences in mTORC1 levels in the prefrontal cortex among the five groups (F(4,27)= 11.42,P< 0.0001). Compared with the sham group, OV group exhibited decreased mTORC1 levels in the prefrontal cortex (P= 0.0113). Fasting normalized mTORC1 levels in the prefrontal cortex (P= 0.0478), and this effect was reversed by rapamycin (P= 0.0002, Figure 3L).
We also analyzed the effects of the 9-hour fasting and rapamycin treatment on the expression of the mTORC1 signaling pathway, BDNF, and synaptic proteins in the prefrontal cortex. An analysis of variance revealed that fasting had a significant effect on the expression of mTORC1 upstream activators and downstream effectors (BDNF:F(4,18)= 6.888,P= 0.0015, Figure 3M; AKT:F(4,34)= 5.390,P= 0.0018, Figure 3N; phosphorylated AKT:F(4,23)= 3.608,P= 0.0201, Figure 3O; ERK:F(4,27)= 7.371,P= 0.0004, Figure 3P; phosphorylated ERK:F(4,31)= 9.725,P< 0.0001, Figure 3Q; p70S6:F(4,25)= 4.128,P= 0.0106, Figure 3R; S6:F(4,26)= 3.869,P= 0.0135, Figure 3S; and PSD95:F(4,33)= 5.709,P= 0.0013, Figure 3T). Compared with the sham group, the OV group exhibited a significant decrease in the expression of BDNF (P= 0.0079, Figure 3M), AKT (P= 0.0070, Figure 3N), phosphorylated AKT (P= 0.0488, Figure 3O), ERK (P= 0.0315, Figure 3P), phosphorylated ERK (P= 0.0253, Figure 3Q), and downstream effectors p70S6 (P= 0.0415, Figure 3R) and PSD95 (P= 0.0069, Figure 3T). Compared with the OV group, fasting increased the levels of BDNF (P= 0.0491, Figure 3M), AKT (P= 0.0489, Figure 3N), ERK (P= 0.0033, Figure 3P), phosphorylated ERK (P= 0.010, Figure 3Q), p70S6 (P= 0.0104, Figure 3R), and PSD95 (P= 0.0160, Figure 3T) in the OVF group. Rapamycin reversed the effects of fasting and decreased the levels of S6 (P= 0.0459, Figure 3S) and PSD95 (P= 0.0403, Figure 3T). Rapamycin alone did not lead to any differences compared with the findings in the OV groups for any of the assessed proteins.
A 9-hour fasting induces an increase in the density of dendritic spines in the hippocampal CA1, CA3, and DG regions
To analyze the effect of the 9-hour fasting on spine density, we conducted Golgi staining. We quantitatively assessed the changes in spine density induced by 9-hour fasting or rapamycin treatments (Figure 4). We found substantial differences in dendritic spine density in the hippocampal CA1 (F(4,19)= 42.94,P< 0.0001, Figure 4B), CA3 (F(4,20)= 27.79,P< 0.0001, Figure 4D), and DG (F(4,20)= 22.73,P< 0.0001, Figure 4F) regions of the hippocampus across the treatment groups. Compared with the sham group, the OV mice exhibited a significant decrease in dendritic spine density in the hippocampal CA1 (P= 0.0006, Figure 4B), CA3 (P= 0.0005, Figure 4D), and DG (P= 0.0037, Figure 4F) regions of the hippocampus. The dendritic spine density in the hippocampal CA1 (P= 0.0383, Figure 4B), CA3 (P= 0.0016, Figure 4D) and DG (P= 0.0473, Figure 4F) regions of OVF mice was higher following the 9-hour fasting compared with that in the mice in the OV group. Compared with the OVF group, the inhibition of mTORC1 with rapamycin significantly suppressed the effects of fasting on dendritic spine density, as evidenced by a significant decrease in the dendritic spine density in the hippocampal CA1 (P< 0.0001, Figure 4B), CA3 (P< 0.0001, Figure 4D), and DG (P< 0.0001, Figure 4F). In addition, the density of hippocampal CA1 (P< 0.0001, Figure 4B) region in OVR mice was lower than that in the OV group.
A 9-hour fasting increases synaptic plasticity in the hippocampal CA1 area
To further understand the fasting-induced consequences of changes in spine density and synaptic protein expression in the hippocampus of OV mice, we conducted electrophysiological investigations of the effects of fasting on LTP. Sections were prepared from OVF mice, and then compared with those from OV mice using extracellular field excitatory potential recordings of postsynaptic potentials. We found that a 100-Hz tetanic stimulation induced robust LTP of Schaffer collateral-CA1 synapses in the OV mice. There was a substantial difference in the slope of the LTP between OV and OVF mice (Figure 4). Compared with the findings in the OV mice, fasting significantly increased the slope of LTP in the hippocampal CA1 (P= 0.0005, Figure 4H) in initial observations. This increase in LTP slope in the OVF group gradually returned to the levels seen in the OV mice over an hour. The levels in the OV group remained elevated over the baseline but did not change during the same time frame. The above results indicate that fasting led to increased synaptic plasticity in the hippocampal CA1 area.

Figure 1|Fasting reverses depression-like behavior induced by ovarian removal.

Figure 2|Effect of rapamycin on the fasting-induced increase in estrogen receptor β expression in the hippocampus and prefrontal cortex.

Figure 3|Fasting increases protein expression in the mTORC1 signaling pathway in the HP and PFC of ovariectomized mice.
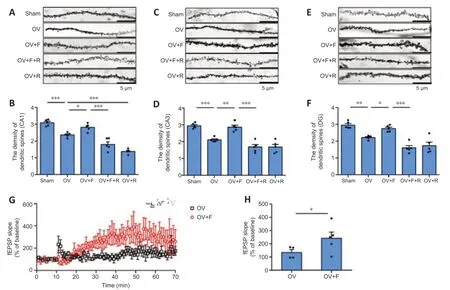
Figure 4|Fasting increases the density of dendritic spines in the hippocampal CA1, CA3, and DG regions, and enhances LTP in the hippocampal CA1 subregion.
Discussion
Consistent with previous studies, we found that after the mice were subjected to an ovariectomy, the immobility time in the FST was significantly increased (Liu et al., 2012; Fan et al., 2017). Furthermore, fasting produced an antidepressant-like effect without changing locomotor activity. These antidepressant-like effects of fasting were reversed by an mTORC1 inhibitor (rapamycin). In addition, as in a previous study (El-Khatib et al., 2020), the levels of estrogen receptors α and β in the mouse hippocampus and prefrontal cortex were significantly reduced following ovarian removal, and a 9-hour fasting increased the level of estrogen receptor β in the hippocampus and prefrontal cortex of ovariectomized mice. Estrogen receptor β agonists have been found to reduce passive floating and immobility in animals in the FST (Donner and Handa, 2009). Estrogen receptor β knockout mice showed reduced depression-like behaviors, and this reduction could be reversed by 17β-estradiol, while estrogen receptor α knockout mice showed no significant changes in depression-like behaviors (Rocha et al., 2005). Therefore, the estrogen deficiency-induced increase in immobility time in the FST might be linked to estrogen receptor β. When rapamycin reversed the antidepressant-like effect of 9-hour fasting, we found no difference in estrogen receptor β expression. The above results suggest that 9-hour fasting produces antidepressant-like effects, and that the mTORC1 signaling pathway might be involved in this process. In our study, rapamycin alone reduced the immobility time in the FST. This might be related to the drug dose and individual differences in drug absorption among mice. Studies have found that rapamycin has potent anti-inflammatory effects, which may be directly connected to the antidepressant-like effects (Abdallah et al., 2020; Aghaie et al., 2021). Although rapamycin enabled us to inhibit the mTORC1 signaling pathway in this study, additional work is needed to explore the effects of different doses in terms of reversing the antidepressant-like effect of acute fasting.
We examined the expression of proteins in the mTORC1 signaling pathway, BDNF levels, and levels of synaptic proteins via western blotting to explore the molecular mechanisms of the antidepressant-like effects of the 9-hour fasting. Consistent with the results of the behavioral tests, fasting activated the mTORC1 signaling pathway in the prefrontal cortex and hippocampus. In addition to elevating the levels of mTORC1 protein, fasting also enhanced the expression of BDNF, AKT, ERK, phosphorylated ERK, p70S6, and PSD95 protein in both the hippocampus and prefrontal cortex. Rapamycin significantly reversed the effect of fasting on the expression of mTORC1, BDNF, phosphorylated ERK, p70S6, S6, and PSD95 protein in the hippocampus and that of mTORC1 and PSD95 protein in the prefrontal cortex. These findings are consistent with previous studies showing that the mTORC1 signaling pathway is implicated in the mechanisms of the antidepressant-like effect of fasting (Gerhard and Duman, 2018). In addition, NV-5138, GLYX-13, hydrogen sulfide, zinc, and other fast-acting antidepressants have also been found to depend on the mTORC1 signaling pathway (Pałucha-Poniewiera et al., 2014; Szewczyk et al., 2015; Hou et al., 2017; Liu et al., 2017; Hasegawa et al., 2019). Compared with a 9-hour fasting, 21 days of paroxetine or escitalopram prevented a chronic stress-induced decrease in phospho-mTORC1 in the hippocampus (Seo et al., 2017). The above findings suggest that the mTORC1 signaling pathway is strongly implicated in the antidepressant-like effects of fasting. ANA-12 (a TrkB antagonist) reversed the effect of calorie restrictioninduced reduction of cognitive decline, as measured by the escape latency in the Morris water maze (Kishi et al., 2015). Further studies found that calorie restriction activated the BDNF/TrkB signaling pathway, further increasing neuroprotection and neurogenesis in the hippocampus and prefrontal cortex (Kishi et al., 2015; Huang et al., 2018). Compared with the findings in mice fed ad libitum, the ratio of full-length to truncated TrkB was approximately 25% higher in the hippocampus of calorie-restricted mice, while the levels of full-length and truncated TrkB did not vary in the prefrontal cortex (Lee et al., 2002). However, we only investigated BDNF levels in the present study. Thus, whether the BDNF-TrkB signaling pathway is involved in the antidepressantlike effects of 9-hour fasting requires further exploration.
Our western blot experiments revealed that fasting had the greatest modulation effect in the hippocampus. As a result, we chose to focus on hippocampal neurons for our analysis of synaptogenesis. We performed Golgi staining to examine the density of dendritic spines in the hippocampal CA1, CA3, and DG regions. Consistent with the western blot results, we found that 9-hour fasting was associated with increased dendritic spine density in all areas examined, and rapamycin treatment reversed this effect. The above results suggest that synaptogenesis induced by mTORC1 signaling pathway activation may be linked to the antidepressant-like effects of 9-hour fasting in ovariectomized mice. To further explore the effects of 9-hour fasting on synaptic plasticity, we conducted electrophysiological experiments. LTP is an important measure of synaptic plasticity, and reflects changes in synaptic plasticity in the hippocampal CA1 area (Holderbach et al., 2007; Watson et al., 2016). Interestingly, intermittent fasting enhanced LTP at hippocampal synapses (Baik et al., 2020). In contrast, excessive caloric intake reduced hippocampal dendritic spine density, LTP at Schaffer collateral-CA1 synapses, and BDNF levels (Stranahan et al., 2008). Consistent with the above results, our findings indicate that fasting significantly increased the slope of LTP in the CA1 of ovariectomized mice. These results suggest that fasting leads to an increase in synaptic plasticity in the CA1.
Fasting has been used to prevent and treat metabolic syndromes in humans, and has been found to improve cognition (Butler et al., 2015). Feeding mice two meals per day (resulting in approximately 11 hours of fasting between meals) prolongs their lifespan in a comparable way to the classic once-daily calorie restriction diet (Mitchell et al., 2019). Long-term calorie restriction has been found to induce an antidepressant-like response, with a decrease in immobility time in the FST (Lutter et al., 2008; Zhang et al., 2015; Wang et al., 2021). This effect is associated with a decrease in excitatory synaptic transmission in the prefrontal cortex (Wang et al., 2021). Moreover, acute or intermittent rapamycin therapy has been reported to have some metabolic similarities to caloric restriction (Kezic et al., 2018). However, in our study, the inhibition of mTORC1 signaling via a single dose of rapamycin reversed the antidepressant-like effects induced by acute fasting. Because the current research on caloric restriction is focused on dietary restriction (i.e., restricting intake by 20–60% of normal levels) and intermittent fasting (Ingram and Roth, 2015), follow-up studies are needed to investigate whether long-term fasting and the activation of mTOR signaling affect depression-like behaviors. Limitations: In this study, we demonstrated that the antidepressant-like effects of 9-hour fasting are related to mTORC1 signaling pathway activation and hippocampal neuroplasticity. However, we only examined mice with ovariectomy (which induces an estrogen deficiency)-induced depressive symptoms. The effects of 9-hour fasting on other mouse models of female depression still need to be explored. Furthermore, this study only investigated the effects of short-term fasting on depression induced by ovarian removal, and did not assess the effects of long-term fasting on depression. In addition, only the FST was used in this study to explore the changes in depressionlike behaviors. As depression-like behaviors are diverse, other behavioral tests (e.g., tail suspension test, sucrose preference test, elevated plus maze, etc.) are needed to investigate the antidepressant-like effects of acute fasting. Another limitation of this study is that our sample size was relatively small. A clinical trial is necessary to examine the antidepressantlike effects of fasting in humans. Fasting is a low cost intervention with few side effects that will likely have potential as a depression treatment. When examining the antidepressant-like effects of energy restriction in mice to the clinical population, the following points should be considered: (1) individual differences in weight/size; (2) active nocturnal activity in miceversusinactive daytime sedentary behavior in humans; (3) differences in the eating patterns of mice and humans; (4) different substrate processing and oxidation patterns between these species (Hawley et al., 2020). Further research is needed before the observed antidepressant-like effects induced by energy restriction can be applied to humans.
In summary, our results show that fasting can produce an antidepressantlike effect. This effect may be linked to activation of the mTORC1 signaling pathway, up-regulation of BDNF expression in the hippocampus and prefrontal cortex, and increases in the levels of synaptic proteins, spine density, and synaptic plasticity in the CA1. The observed effects were reversed by rapamycin, indicating that fasting may modify neural plasticity through activating mTORC1 signaling. This study adds to our understanding of the mechanisms underlying the antidepressant-like effects of fasting, and provides a foundation for the development of fasting as a novel, non-pharmacological treatment for depression.
Author contributions:All authors contributed to the study conception and design, manuscript revision reviewed and approved the final version of the manuscript. Material preparation, data collection and analysis: BJL, ZQC, JF; manuscript draft: ZQC, JF.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Anna O. Giarratana, Rutgers Robert Wood Johnson Medical School, USA.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

