Brain organoids are new tool for drug screening of neurological diseases
Jin-Qi Zhou , Ling-Hui Zeng , Chen-Tao Li Da-Hong He Hao-Duo Zhao Yan-Nan Xu Zi-Tian Jin Chong Gao
Abstract At the level of in vitro drug screening, the development of a phenotypic analysis system with highcontent screening at the core provides a strong platform to support high-throughput drug screening. There are few systematic reports on brain organoids, as a new three-dimensional in vitro model, in terms of model stability, key phenotypic fingerprint, and drug screening schemes, and particularly regarding the development of screening strategies for massive numbers of traditional Chinese medicine monomers. This paper reviews the development of brain organoids and the advantages of brain organoids over induced neurons or cells in simulated diseases. The paper also highlights the prospects from model stability, induction criteria of brain organoids, and the screening schemes of brain organoids based on the characteristics of brain organoids and the application and development of a high-content screening system.
Key Words: brain organoids; disease modeling; high-content system; multiple omic analysis; network pharmacology; neurodegeneration; phenotypic fingerprint; psychiatric diseases; stem cells; traditional Chinese medicine drug screening
Introduction
With the accelerating progress of social aging and increasing personal life stresses, neurodegeneration and psychiatric diseases have become a major health issue globally. The complex disease mechanisms contributing to the progression of neurological diseases mean effective treatments to cure such disorders are lacking. In terms of drug exploration, traditional Chinese medicine (TCM) is attracting increasing attention for the huge resource of neuroprotective compounds from the herbs of therapeutic formulas, which were demonstrated to attenuate the symptoms of diseases including cognitive impairment or motor dysfunctions (Han et al., 2017; Yang et al., 2017; Pei et al., 2020). However, a reliable disease model that reflects the phenotypes and underlying mechanism(s) of the diseases is still lacking and has become a bottleneck for identifying the effective compounds from TCM. Neurodegenerative disorders like Alzheimer’s disease (AD) and Parkinson’s disease (PD) are mostly sporadic with complex etiologies (Dorszewska et al., 2016; Nagatsu et al., 2019). As such, animal models carrying the familial mutations would miss the key targets for the treatments although they mirror some of the disease phenotypes. As for psychiatric diseases like depression, autism spectrum disorder, and schizophrenia, the biological hallmarks of the patients are too complex to fit the rodent models. In addition, the non-human primate models are too costly to offer many samples for high-throughput screening, although they do exhibit behavioral patterns that are more similar to those of humans. Considering the vast array of natural products contained in TCM formulas, reliablein vitrodisease models to match the requirement of high-throughput screening as well as the patient-specific phenotypes would be the first choice of drug exploration in the future.
Compared with the experimental disease animal models that struggle to meet the requirements of high-throughput screening,in vitrotwo-dimensional (2D) cultured cells, including neuron-like cell lines or rodent primary neurons, are suitable for high-throughput screening. However, these 2D cells are limited in offering a multiple cellular interactions system under three-dimensional (3D) conditions, which reflects the real growth environments of cells in physiological conditions. The technology of induced pluripotent stem cells (iPSCs) and functional neuronal induction offers patient-derived neurons for the study of disease mechanisms as well as drug screening (Zhang et al., 2013; Kajihara et al., 2021; Lagomarsino et al., 2021). By comparing the RNA sequencing and proteomic profiling of iPSC-derived neurons and brain tissue from 53 patients with AD, Lagomarsino et al. (Lagomarsino et al., 2021) confirmed that protein networks are associated between induced neurons and the patient’s brain. This finding indicated that the induced neurons could be applied to mimic neurodegeneration (Lagomarsino et al., 2021). In addition to the functions of induced neurons in representing patients’ pathological features, organoids provide an ideal disease model with a 3Din vitro-like tissue (Rossi et al., 2018). Furthermore, technological combination between iPSC reprogramming and the generation of brain organoids has resulted in a new disease model for neurological or psychiatric diseases (Lancaster et al., 2013; Clevers, 2016; Di Lullo and Kriegstein, 2017; Qian et al., 2019; Cherskov and Sestan, 2021). Through self-organization or biomaterials-based approaches, scientists have developed a series of induction methods for organoids to construct a brain-like architecture that contains multiple tissue types including different functional neurons, glial cells, etc. (Chhibber et al., 2020; Cherskov and Sestan, 2021). However, several key questions are yet to be clarified before engaging brain organoids in TCM drug screening. In this review, we summarize the development of brain organoid generation and its potential application in neurological or psychiatric disease modeling. We also discuss the screening strategy of TCM with brain organoids and highlight several issues that need to be further considered before engaging organoids as the main platform for TCM compound screening.
Retrieval Strategy
Articles in this review were retrieved by electronic search of the MEDLINE database for literature describing human organoids including generation, optimization, and disease modeling. Literature mainly during recent 10 years key words were screened with the keywords of brain organoids, induced pluripotent stem cells, neurodegenerative diseases (or disease including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis), psychiatric disease (or particular disease including depression, autism, and Rett disorder), traditional Chinese medicine, drug screening and high content system. Additionally, the MEDLINE database was also searched for articles related to 2D cell screening, high-content system strategy, and network pharmacology.
Progress of Brain Organoids
Generation of brain organoids
For brain organoids, stem cell-derived neural progenitor cells could be formed by induced embryonic bodies (EBs) under adherent conditions (Zhang et al., 2001; Caffrey et al., 2021; Cordella et al., 2022; Scott and Huang, 2022). Cortical organoids could be generated from embryonic stem cells (ESCs) by serum-free culture of embryoid body-like aggregates (Eiraku et al., 2008). By embedding EBs into Matrigel droplets, Lancaster et al. (2013) developed human-derived unguided brain organoids with heterogeneous neural identities. Such methods paved the way to mimic brain development and brain disorder models. With the help of a bioreactor, Qian et al. (2016) developed guided brain-region-specific organoids and proved their application for modeling fetal brain deficits under Zika virus infection. Subsequently, many researchers updated the culture method by slicing cerebral organoids to avoid the insufficient nutrient and oxygen supply at the core area for long-term culture (Giandomenico et al., 2019; Qian et al., 2020). Besides functional neurons, single-cell sequencing also indicates the glial cell diversity in brain organoids (Dang et al., 2021). Moreover, a new differentiation method of human iPSCs was used to generate 3D brain organoids that contained oligodendrocytes as well as neurons and astrocytes, called human oligodendrocyte spheroids (Marton et al., 2019). To date, various types of specific organoids to mimic different regional features have been developed, including midbrain (Jo et al., 2016), hypothalamus (Huang et al., 2021), bloodbrain barrier (Pellegrini et al., 2020), cerebellum (Ballabio et al., 2020), and spinal cord (Winanto et al., 2019; Faustino Martins et al., 2020). The abovementioned organoids provide tissue-specific screening tools for studying the region-specific deficits in related neurological or psychiatric diseases. Meanwhile, there has been great progress in improving the induction rate and the stability of brain organoids. For instance, a recent report indicated that the short-term SMAD protein (a homolog of the Drosophila protein Mothers against decapentaplegic, and the Caenorhabditis elegans protein Sma) and Wnt inhibition could provide an optimal condition to build a rich cortical cell repertoire for mirroring fundamental molecular and cytoarchitectural features of cortical development (Rosebrock et al., 2022). This progress in the induction and long-term culture of brain organoids has improved the application of brain organoids as the testing platform in drug discovery.
Chimeric brain organoids for expanding disease model phenotypes
A growing number of approaches have been developed for improving the structure and tissue integrity of organoids. However, it is still not clear whether brain organoids can generate cell identities beyond the ectodermal progenitors. For example, several tissue types like vascular structure or microglia were not included in brain organoids but are known to play key roles in the development of the neurological or psychiatric diseases (Zlokovic, 2011; Hickman et al., 2018; Sweeney et al., 2018). Recently, a complex vascular-like network in brain organoids was developed by ectopically expressing human ETS variant 2 in ESCs (Cakir et al., 2019). Moreover, mixing cultures of human umbilical vein endothelial cells or iPSC-derived endothelial cells during the differentiation of organoids also generated vascularized brain organoids (Mansour et al., 2018; Shi et al., 2020). Microglia, which are developed from mesodermal progenitors, are necessary for the development of neurological or psychiatric diseases (Salter and Stevens, 2017; Hickman et al., 2018). Ormel et al. (Ormel et al., 2018) demonstrated that brain organoids generated from the Lancaster protocol could produce innate microglia and function with an inflammatory response. Furthermore, extrinsic xenotransplantation of human microglia into cerebral organoids indicated that microglia decreased interferon signaling, which thereby accelerated the synchronized oscillatory network in brain organoids (Popova et al., 2021). To minimize organoid necrosis and hypoxia, Ao et al. (2021) constructed a tubular device channel to control the perfusion of nutrients and oxygen and incorporate microglia into organoids. Such models were proven to have broad uses in modeling multiple inflammatory conditions including brain injury, neural diseases, autoimmune disorders, and infectious diseases (Ao et al., 2021). The same methods have also been utilized in brain tumor studies. Introducing oncogenic mutations in cerebral organoids could reproduce the glioblastoma-like and central nervous system primitive neuroectodermal tumor-like neoplasms (Bian et al., 2018). By re-seeding patient-derived glioblastoma cells with brain organoids, Krieger et al. (2020) developed a chimeric organoid model that was applicable for studying glioblastoma invasion. Herein, the direct introduction of specific genes or cellular types could expand the application of brain organoids to explore therapeutic targets and effective drugs against neurological or psychiatric diseases.
Multiple organoids-on-a-chip and assembloids
The interactions between different systems or brain regions are also widely recognized as key contributors to the progress of neurodegenerative or psychiatric diseases. Therefore, establishing interaction(s) among brain organoids with other organs would promote the exploration of therapeutic targets. One strategy is the application of assembloids, which were developed by physically binding different organoids together. For instance, assembloids generated by fusing dorsal and ventral forebrain organoids could overcome the unidentical and random distribution of brain regions and achieve the arrangement of the dorsal-ventral axisin vitro, which benefits the analysis of complex neurodevelopmental defects involving neuronal circuits (Bagley et al., 2017). A 3D cortex-motor assembloid was generated by assembling cerebral cortex, hindbrain/spinal cord, and skeletal muscle spheroids. Such assembloids provide the projection from upper motor neurons to lower motor neurons and dramatically enhanced spontaneous muscular contraction, which indicated the formation of the motor system (Andersen et al., 2020). Moreover, assembloids of the cortex-ganglionic cortex showed the formation of the brain network and mimicked the epileptiform-like activity in Rett syndrome (Samarasinghe et al., 2021).
Another concern regarding neurological diseases is that the potential therapeutic targets might be in other organs that are not connected to brain. For example, the liver has been strongly suggested as the key contributor to different neurological diseases like AD, PD, and depression (Mohamed et al., 2014; Ciulla et al., 2019; Bassendine et al., 2020). Organoids chips that link the different organ-specific organoids by microfluid provides potential resolution for this concern (Zhou et al., 2021). Multiple organoids chips provide a platform to study the interactions of distant organs. With the support of biomaterial technologies such as microfluid, 3D printing, and hydrogel shaping, brain organoids could grow on biochips with different kinds of tissuespecific organoids. This model mimics the communication of brain tissue and other organs and can be used to study the development of pathologies in neurological or psychiatric diseases on a systematic level (Karzbrun et al., 2018; Mittal et al., 2019; Bang et al., 2021).
Modeling Neurodegenerative or Psychiatric Diseases with Brain Organoids
Despite the advantage of brain organoids in modeling developmental disorders, the brain organoids require a relatively long culture time to allow complete maturation of different tissue types to mimic the neurodegeneration. A recent study showed that AD-like phenotypes including amyloid beta aggregation, tau phosphorylation, and neural loss could be observed in brain organoids exposed to patients’ serum on day 50 after induction (Chen et al., 2021). Another study using air-liquid sliced brain organoids showed that organoids carrying the c9orf72 hexanucleotide repeat mutation presented a series of associated phenotypic changes including accumulating P62, dipeptide repeats aggregation, and DNA damage after day 100 post-slicing (Szebényi et al., 2021); these phenotypic changes are as seen in frontotemporal dementia. It was noticed that this air-liquid culture improved neuronal survival and axonal growth during induction of organoids, which is beneficial in establishing various morphologies such as long-range projection, growth-cone turning, and decussation (Giandomenico et al., 2019). For PD modeling, Jo et al. (2016) reported a 3D organoid midbrain model containing functional midbrain dopaminergic neurons, which could produce human-specific neuromelanin. Such midbrain organoids displayed mature dopaminergic functions after 65 days post-induction (Jo et al., 2016). Following introduction of the LRRK2G2019S mutation, Kim et al. (2019) induced the PD-specific midbrain organoids and observed the relative pathological changes including loss of the dopaminergic (DAergic) neurons, increased apoptosis, and aggregation of α-synuclein after day 60. By comparing midbrain organoids from healthy controls (H-lines), patientspecific iPSCs (LRRK2G2019S), and mutation-corrected isogenic controls, Smits et al. (2019) discovered the loss of PD-associated dopaminergic (DAergic) neurons was present in patient-specific organoids from day 35. Additionally, at this time point, the PD-midbrain showed an increased generation of DA progenitors, but such phenotype disappeared on day 70 (Smits et al., 2019). The brain organoids represented embryonic and neonatal developmental progress and could therefore be used as a model of brain developmental disorders. Lu et al. (2022) summarized the classic developmental diseases that could be represented with cerebral organoids including autosomal recessive primary microcephaly, autism spectrum disorders, Timothy syndrome, Tuberous sclerosis complex, and Down syndrome.
Recently, increasing attention has been focused on the functions of cerebral organoids as a model for psychiatric diseases. Compared with neurodegeneration with dramatical neural loss and protein aggregations (Hetz and Saxena, 2017), there are no obvious neuronal death occur in brain tissue, hence it lacks obvious phenotypes in organoids. Herein, the first step to apply brain organoids in modeling psychiatric diseases is exploring the stable biomarkers of psychiatric diseases in organoids to match the phenotypes in clinical or classic animal models. For example, decreasing adult hippocampal neurogenesis has been widely regarded as the biological hallmark and the antidepressant target for depression (Hill et al., 2015; Micheli et al., 2018; Kim and Park, 2021). Neurogenic deregulation in brain organoids, particularly the dysfunctions of radial glia-like neural stem cells, could be one depressive feature for organoids (Micheli et al., 2018; Sun et al., 2022). Indeed, a study on human brain organoids indicated the function of serotonin receptor 6 in regulating neural progenitor self-renewal and differentiation, which represents the depressive-like behavior and adult neurogenic deficits in serotonin receptor 6 knockout mice (Wang et al., 2021a). Therefore, neurogenesis could be a biological hallmark of depression in cerebral organoids. Increasing evidence indicates the critical roles of neural circuit networks in the development of psychiatric diseases (Gunaydin and Kreitzer, 2016; Theyel, 2018; McTeague et al., 2020). Based on such principles, fused organoids or assembloids might provide a disease model for psychiatric disorders. More importantly, it is also expected that cerebral organoids could provide the tool for understanding the biology underpinning psychiatric diseases.
Application of Organoids in Traditional Chinese Medicine Screening
Standardization of organoids during drug screening
For the drug screening system, the advantage of cultured cells is the relatively simple system and the small number of variations. For rodent models, stable animal strains such as Sprague-Dawley rats or C57B/L mice could ensure controllable variation within groups. However, compared with these two models, current cultured organoids exhibit difficulties establishing relatively stable phenotypes that can discriminate the disease-specific phenotypic features from the random abnormalities. Hence, before utilizing brain organoids for neurological or psychiatric drug screening, several critical issues need to overcome: 1) minimize the random variation during the generation and maintenance of organoids; 2) set the phenotypic criteria to separate the healthy and pathological conditions; and 3) determine the exclusive standards of the abnormal organoids that are due to random factors.
Emerging evidence indicates that organoids themselves could reflect the heterogeneity of the condition of the disease in the clinic (Tang et al., 2022). However, such an advantage could, in extreme cases, increase the instability of the organoids in drug screening. The heterogeneity of brain organoids is contributed by multiple factors, including different suppliers of culture medium components, unstable shear force in orbital shakers or bioreactors, as well as the different genetic backgrounds of iPSCs from donors. Taking brain organoids as an example, the variation could be from the formation of EB. As the culture progresses, the EB or the neural spheroids display different sizes because of the distinct proliferative and differentiative speeds of the progenitor cells (Hinman and Burke, 2018). Moreover, stabilizing a drug screening system involving organoids requires minimum interference during the culture as well as stable phenotypes between the control and disease models. Various approaches have been developed to avoid the randomization of the long-term culture of brain organoids. Qian et al. (2016) developed a new apparatus—SpinΩ—to control the shear force in brain-region organoids. Sliced sectional organoids or air-liquid interface brain organoids can also enhance the stability of brain organoids for long-term culture by avoiding insufficient oxygen and nutrient supply in the core area (Giandomenico et al., 2019; Qian et al., 2020). Mapping the phenotypic fingerprints of brain organoids could further enhance the power of the system in drug evaluation. Lukonin et al. (2020) reported the phenotypic landscape of intestinal organoid regeneration by analyzing the multivariate feature profiles with the help of over 2000 compounds targeting 800 unique molecules associated with key biological events. The phenotypic fingerprint was formed by combining the different morphological features of organoids including 4′,6-diamidino-2-phenylindole, aldolase B, lysozyme, mass, etc. (Lukonin et al., 2020). The same idea could be applied in establishing the phenotypic fingerprint of brain organoids matching with different biological events including neurogenesis, neurodegeneration, calcium homeostasis, etc. More importantly, establishing a control group for each batch of screening is another critical factor to decrease the instability of the organoid phenotypes. In the screening system, control groups should include healthy iPSCs or ESCs as well as isogenic control iPSCs with genetic correction. In disease modeling with organoids, healthy iPSCs or ESCs were used to control the generation process of organoids (Figure 1). By comparing healthy and isogenic controls, Hinman and Burke (2018) found that amyotrophic lateral sclerosis (ALS)-frontotemporal dementia brain organoids established aggregation of polyGA, congruent with that observed in patients’ brains. Furthermore, by comparing primary hepatocytes with longterm cultured organoids from the same donor, Huch et al. (2015) discovered that expanded cells are highly stable at the chromosome and structural levels, while single base changes occur at very low rates. This discovery indicates the relatively stable condition during maintenance of the organoids. Moreover, using isogenic iPSCs could help to exclude the interference from different genetic backgrounds of iPSCs.
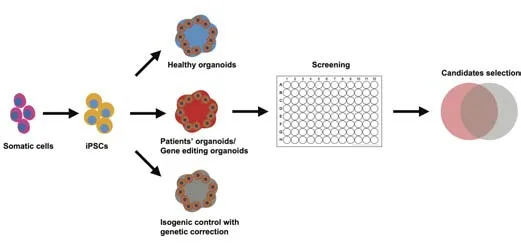
Figure 1|Modeling neurodegenerative and psychiatric diseases with brain organoids.
Screening of TCM compounds via the brain organoids model
TCM contains a huge resource of compounds with powerful neuroprotective effects (Wang et al., 2021c). Therefore, a disease model that offers highthroughput screening is essential to select the effective components from TCM for specific disorders. According to the development of high-content screening (HCS), the brain organoids disease model provides a promising tool for TCM drug screening. In HCS, the screening power of the system for a certain chemical library usually relies on the image feature of the subject (Rabal et al., 2010). Diverse HCS system instruments combining multiple laser channels, cell incubator environments, accelerated image-gaining speed, and analytical software packages have been developed, which are friendly for one-step screening (Wang et al., 2019). A recent study showed that HCS is applicable for screening TCM compounds on epithelial-mesenchymal transition, which is critical in metastasis and invasion during fibrotic diseases and cancer progression (Xu et al., 2022). The study identified five compounds with anti-epithelial mesenchymal transition activity (including camptothecin, dimethyl curcumin, artesunate, sinapine, and berberine) among 306 monomeric compounds from the MedChemExpress compound library (Monmouth Junction, NJ, USA) (Xu et al., 2022). To reflect the drug effects, an organoid-based high-throughput screening system requires confirmative phenotypic features that match the clinical symptoms and are easily detected. According to the phenotypic screening of intestinal organoids with libraries of identified targets (Lukonin et al., 2020), an applicable strategy for brain organoids is to first build the phenotypic landscape through the chemical library or genomic editing library (guide or small interfering RNA) with identified targets or biological effects (Kwon et al., 2013; Chen et al., 2015). Park et al. (2021) employed 1300 organoids from 11 participants to construct a HCS system and test blood-brain barrier-permeable U.S. Food and Drug Administration (FDA)-approved drugs, which provides a strategy for a precision medicine model using iPSC-derived cerebral organoids. Additionally, image analysis performed with a HCS system combined with the transcription profile of each feature could further represent the biological effects of different phenotypes. Based on the categorized phenotypic fingerprint, it is possible to use a TCM compounds library with unknown targets that would be well prepared for testing via matching with the biological effects in such a system (Figure 2). There are numerous chemical compounds available to establish the phenotypic deficits in organoids, including deficits in neurogenesis, neural survival, axonal growth, and calcium homeostasis (Figure 2). Table 1 summarizes the phenotypic features of the brain organoids (Zhu et al., 2017; Jacob et al., 2020; Marin Navarro et al., 2020; Ramani et al., 2020; James et al., 2022; Paulsen et al., 2022) or region-specific organoids (Gonzalez et al., 2018; Xu et al., 2019; Zhang et al., 2020; Kang et al., 2021; Tang et al., 2021; Urresti et al., 2021; Wegscheid et al., 2021) under different pathological conditions. Systematic image analysis for organoids could focus on marking neural stem cells, neurons, cell death biomarkers, oxidative stress, and calcium probes. Transcriptional profiles associated with the image features can provide supportive evidence to clarify the mechanisms for different drugs (Figure 3). Moreover, the application of single-cell sequencing can further deepen the understanding of cell-specific effects of certain drugs (Chang et al., 2020; Costamagna et al., 2021).
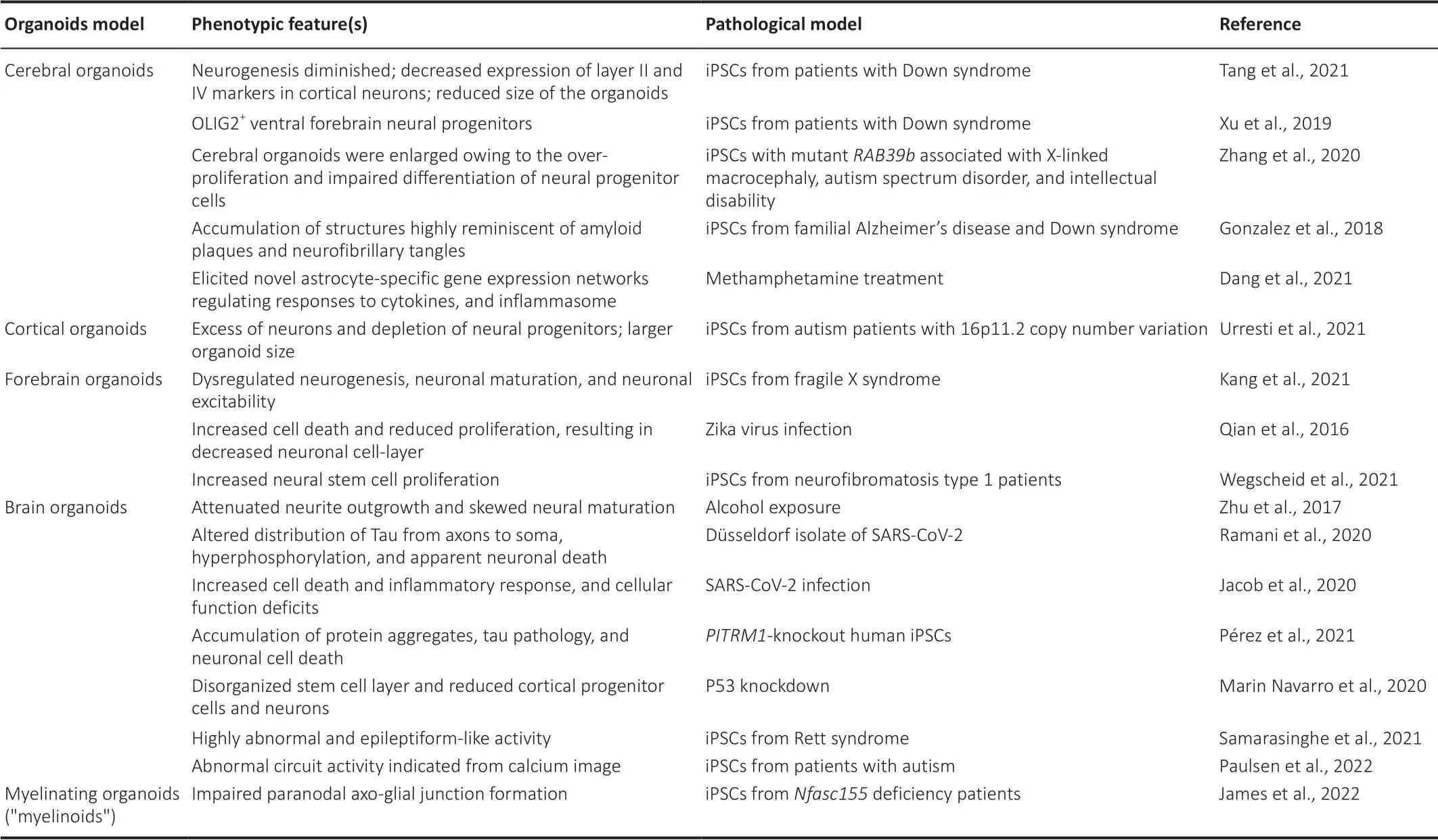
Table 1 |Phenotypic features of the brain organoids on different models
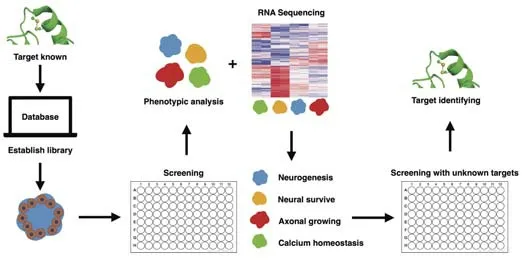
Figure 2|Phenotypic feature categorizing strategy for drug target identification.
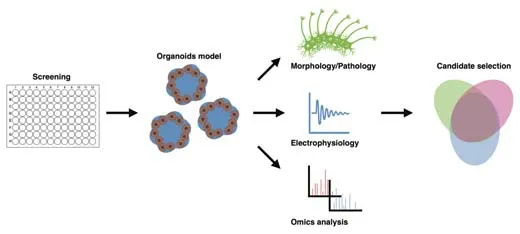
Figure 3|Multi-level screening of drugs with brain organoids.
Multiple components for network-target
The multiple bio-effects from the TCM formulas usually result from the synergetic effects of different compounds. However,in vitroscreening including the brain organoids platform is limited in administration with the TCM formulas directly because a considerable part of the effective compounds in TCM formulas is insoluble. Moreover, many compounds that perform indirect bioactive functions via systematic metabolic regulation or other organs or peripheral circulation could be missed in organoids screening. One strategy to overcome such issue is to select the compound candidates with network pharmacology, which could predict the biological targets for certain formulas (Zhang et al., 2019; Wang et al., 2021b). The concept of network pharmacology, which has been developed into a “network-target, multiple-component-therapeutics” model, could facilitate the establishment of a “compound-protein/gene-disease” network in a high-throughput manner (Zhang et al., 2019). For instance, a TCM systems pharmacology database and an analysis platform database were used to predict the human oral bioavailability, drug-likeness, Caco-2 permeability, ability to cross the bloodbrain barrier, half-life, targets, and relevant diseases of Danggui-shaoyaosan, which has been widely used to treat neurodegenerative diseases (Luo et al., 2016). Based on those factors, 52 effective compounds fromDangguishaoyao-sanwere predicted. The biological effects of such compounds library could also be further considered to confirmed in brain organoids with certain disease models. Additionally, the algorithms for predicting drugprotein/drug-disease networks such as Random Walk (Chen et al., 2012) and PRINCE (Vanunu et al., 2010) could suggest component candidates based on the disease targets, which could be further applied to drug screening with brain organoids. Combining the patient-specific brain organoids and their phenotypic fingerprints could allow drug screening of personalized and disease-specific TCM compounds based on appropriate formulas. Such principle could be further confirmed by the clinical practice in TCM diagnosis, which could form a loop for evidence-based personalized therapy.
Conclusion
TCM could provide a large number of resources for drug development. To enhance drug exploration efficiency in the future and identify the most effective compounds in the ocean of natural products, high-throughputin vitrodisease models with markable phenotypes are urgently needed. Brain organoids could provide a human-derivedin vitro3D “mini-brain” that contains various cell types and comparable morphologies as thein vivomodel. The updating of methods to stabilize the phenotypes of the organoids and the increasing discoveries about disease-specific phenotypes mean highthroughput screening with brain organoids would be increasingly recognized as the main strategy in drug discovery and TCM modernization. To date, only a few studies have demonstrated the application of brain organoids as a model for TCM screening. However, it is anticipated that brain organoids will be employed as a disease model for exploring TCM compounds in precise medicine after resolving the critical challenges, e.g., phenotypic landscape, culture condition controls, and evaluating standards. In the future, TCM compound libraries would be based on the traditional formula that has effects in Chinese medical clinics or would be based on biological functions including antioxidative effects, mitochondrial regulation, or synaptic plasticity functions.The therapeutic outcome of TCM drugs predominantly depends on their synergetic effects of multiple components on different targets in different cell types. Therefore, 3D human-derived organoids to mimic organogenesis can provide multiple cell types for expanding the horizon of the active TCM compounds in complex neurological or psychiatric disorders. However, in this review, we have only discussed the application of the brain organoids for discovering the effective TCM compounds that directly target the brain tissue. It is possible that the components act to improve the functions of other organs or systems. One possible strategy is to use a conditional medium of the organ-specific organoids or organoids chips of interest to connect the brain organoids with other organoids by microfluid. Furthermore, confirming the biological effects of candidate compounds on animal models should also be considered for further verifying the testing efficacy with organoids.
Culture of the organoids could gain enormous samples, which fulfills the demands of drug screening in a high-throughput manner. However, 2D cultural cells have advantages in screening systems compared with 3D organoids (Table 2). Thus, given the high number of resources of TCM compounds, the whole process of the drug screening could be separated into several steps including the target prediction for the first round, followed by the drug screening on 2D cell lines (SH-SY5Y, PC12, or SN4741), primary cultured neurons or induced functional neurons with iPSCs/ESCs for the second round. Screening on organoids could then be performed after narrowing the candidates of the effective drugs. Additionally, animal models could also be used to further confirm the drug effects on the whole-body level. In the future, it is highly expected that a library of TCM compounds will be developed for brain organoids screening based on a target disease approach or a TCM formula approach. To summarize, brain organoids could provide a cutting-edge tool for mimicking neurological or psychiatric diseases and performing TCM drug screening. However, the whole screening process still requires rigorous planning and reliable phenotypic monitoring. The limitation of this review is that there is insufficient evidence to demonstrate the applicable usage of brain organoids to mimic the key phenotypes closely related to the clinical biomarkers and simultaneously suited to high-content screening. Moreover, studies using human brain organoids in screening TCM compounds are also lacking.
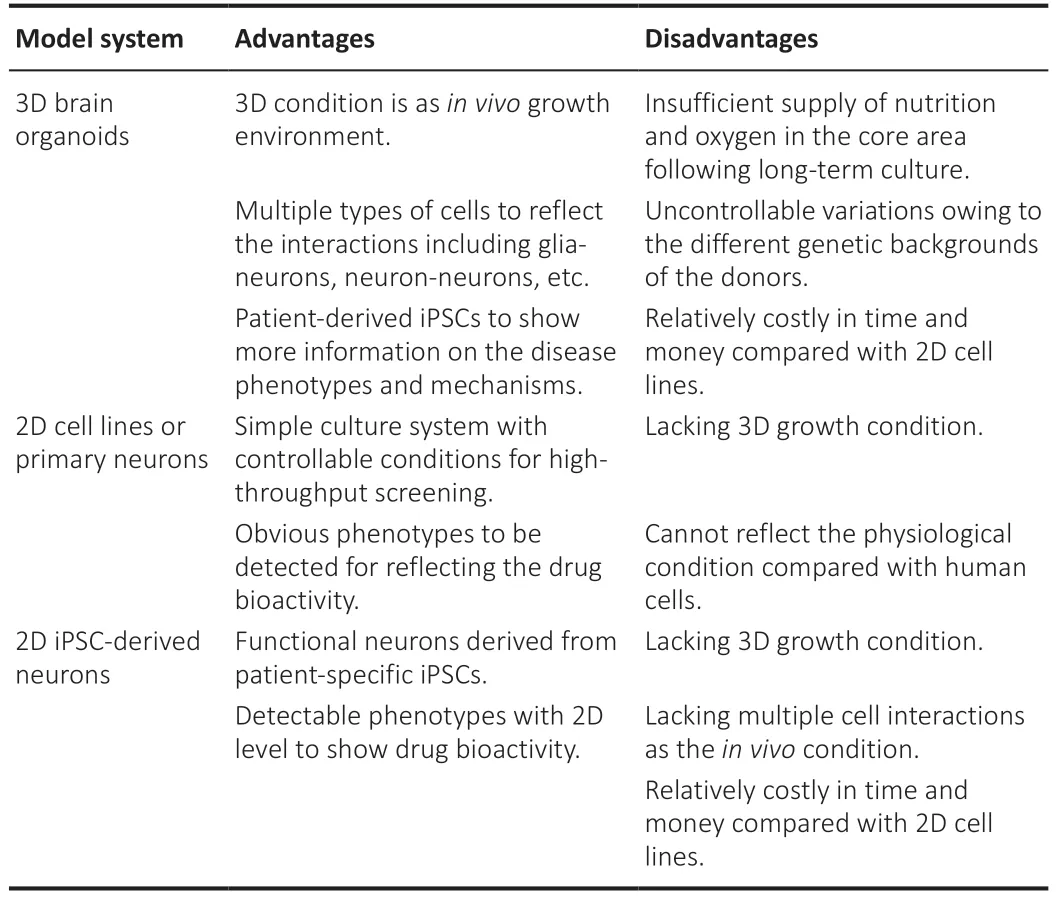
Table 2|Advantages and disadvantages of 2D cells versus 3D organoids
Acknowledgments:We would like to thank the support from Dr. Kai Lei in School of Life Science, Westlake University. Corresponding author worked as a postdoc on organoids study in Dr. Lei’s Lab.
Author contributions:Review design, supervision, and funding acquisition: CG; data collection, figure preparation, and manuscript revision: CTL, DHH, HDZ, YNX, ZTJ; manuscript draft: CG, JQZ, LHZ. All authors read and approved the final version of the manuscript.
Conflicts of interest:All listed authors in this article have no conflict of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Melissa Schepers, Hogeschool PXL, Belgium.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis
- New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system

