New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system
Ning He , Xing-Jia Mao , Yue-Min Ding, Tong Zuo, Ying-Ying Chen, Lin-Lin Wang
Abstract Traumatic injuries in the central nervous system, such as traumatic brain injury and spinal cord injury, are associated with tissue inflammation and the infiltration of immune cells, which simultaneously affect the self-renewal and differentiation of neural stem cells. However, the tissue repair process instigated by endogenous neural stem cells is incapable of restoring central nervous system injuries without external intervention. Recently, resident/peripheral immune cells have been demonstrated to exert significant effects on neural stem cells. Thus, the restoration of traumatic injuries in the central nervous system by the immune intervention in neural stem cells represents a potential therapeutic method. In this review, we discuss the roles and possible mechanisms of immune cells on the selfrenewal and differentiation of neural stem cells along with the prognosis of central nervous system injuries based on immune intervention. Finally, we discuss remaining research challenges that need to be considered in the future. Further elucidation of these challenges will facilitate the successful application of neural stem cells in central nervous system injuries.
Key Words: B cells; central nervous system injury; macrophages; microglia; neural stem cells; spinal cord injury; T cells; traumatic brain injury
Introduction
Post-traumatic injury of the central nervous system (CNS) mainly includes traumatic brain injury (TBI) and spinal cord injury (SCI) caused by external physical insults (Wu et al., 2022). Both injuries are increasingly considered to be important global health priorities because they not only cause health defects for individuals; they also create a significant economic burden (GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, 2019). CNS injury is a complex process; many factors are involved in such injuries, including the death of neurons and oligodendrocytes, neuronal demyelination, gliosis, inflammation, apoptosis, and necrosis, thus resulting in a complex repair process (Zhang et al., 2019). There is a critical need for improved treatment options for both individuals and society. At present, the treatment of post-traumatic injury in the CNS mainly focuses on spinal fixation/stabilization, high-dose methylprednisolone, and neurotrophic factors (Zhang et al., 2019; Huang et al., 2020; Luo et al., 2021). Previous research has shown that original neural stem cells (NSCs) give rise to all neural cells in the CNS, thus providing a rationale for utilizing NSCs to regenerate the damaged CNS (Okano, 2010). Researchers and clinicians have paid significant attention to the interaction of immune cells and NSCs (Miron et al., 2013; Xiong et al., 2016). Traumatic injury causes disruption of the blood-brain barrier (BBB) (Takase and Regenhardt, 2021), neurological deficits, and the migration of resident/peripheral immune cells to the lesion site, including microglia/macrophages, T cells, and B cells (Monje et al., 2002; Ziv et al., 2006; Ortega et al., 2020; Weissleder et al., 2021). Furthermore, endogenous NSCs are activated to rescue and repair the damaged tissue. However, previous reports showed that endogenous NSCs have poor survival rates after injury due to the surrounding hostile niche of NSCs (Ming and Song, 2005). Endogenous NSCs are unable to form a sufficient number of neurons and oligodendrocytes to promote neurological recovery after injury (Gregoire et al., 2015; Jure et al., 2022).
Many studies have reported that specific subtypes of immune cells could rescue a region of damage and recover neurological function (Ziv et al., 2006; Chen and Trapp, 2016; Xiong et al., 2016). These phenomena offer new insight in that we should focus on how immune cells affect the self-renew and differentiation of NSCs during post-injury regeneration. Here, we summarize the divergent roles of immune cells on the self-renewal and differentiation of NSCs after traumatic injury in the CNS and discuss the prognosis of CNS injuries based on the intervention of immune cells. Furthermore, we consider the remaining research challenges and future direction. Defining the effects of immune cells on NSCsin vivoandin vitrowill shed light on the therapeutic possibility of using NSCs to treat CNS injuries.
Retrieval Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published up to July 31, 2022. A combination of the following keywords (MeSH terms) was used to maximize search specificity and sensitivity: “neural stem cells”; “spinal cord injury”; “traumatic brain injury”; “microglia”; “macrophages”; “T cells”; and “B cells”. The articles identified were further screened by title and abstract, and only those studies exploring the relationship between immune cells and NSCs in the CNS were included to investigate the specific effects of immune cells on traumatic injury in the CNS. No language or study type restrictions were applied.
Features and Functions of Neural Stem Cells in Post-Traumatic Central Nervous System Injury
NSCs reside in the CNS and are characterized by their capacities for selfrenewal, maintaining an adequate pool of NSCs and their differentiation into neural progenitor cells (NPCs) and oligodendrocytic progenitor cells (OPCs), thus forming neurons, astrocytes, and oligodendrocytes (Willis et al., 2020). The process underlying the differentiation of NSCs is regulated by a range of molecules or signaling pathways, including Wnt, Notch, Neurog2, Tbr2, Prox1, NFIX, Tlx, CcnD2, and Ascl1 (Vieira et al., 2018). Several microRNAs have also been shown to play a role in self-renewal and differentiation, including miR-124-3p and miR-127-3p (Vieira et al., 2018). In addition, the differentiation of NSCs is dependent on the microenvironment. In normal and physiological conditions, the surrounding microenvironment of NSCs (i.e., the NSC niche), which mainly includes mesenchymal cells and immune cells, regulates the capacity and velocity of NSC proliferation and differentiation. Terminally differentiated NSCs are also capable of integrating efficiently into the existing nervous tissue to maintain homeostasis in the CNS (Aimone et al., 2014). NSCs can also release microvesicles, exosomes, mitochondria, and a series of growth/trophic factors to promote the regeneration process and synaptogenesis in post-traumatic CNS injury (Figure 1; Sharma et al., 2019; Willis et al., 2020; Yuan et al., 2021).
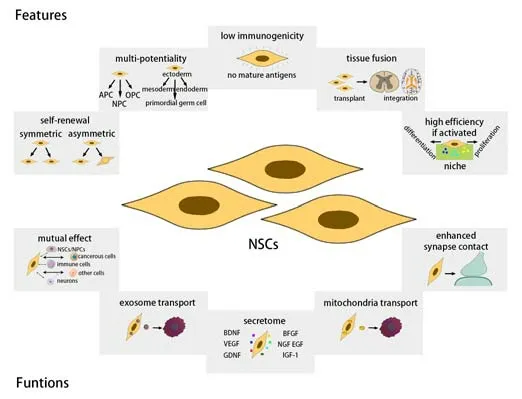
Figure 1|The features and functions of NSCs.
Resident and Infiltrating Immune Cells in Post-Traumatic Central Nervous System Injury
The idea of the CNS exhibiting immune privilege remains controversial (Engelhardt et al., 2017; Xu et al., 2021) although recent research has found that there is a functional lymphatic system within the meninges (Louveau et al., 2015). In fact, the CNS is closely monitored by the immune system. Resident immune cells are mainly composed of microglia and special macrophages outside the parenchyma and are known as border associated macrophages which reside in the perivascular space, meninges, and choroid plexus (Mrdjen et al., 2018; Van Hove et al., 2019). Microglia are the main immune cells in the spinal cord (approximately 78% of the total population of immune cells); macrophages are the second most common form of immune cells (approximately 7% of the total number of immune cells) (Wang et al., 2022). Microglia and border associated macrophages both express CD11b, CD45, ionized calcium binding adaptor molecule 1, and CX3C motif chemokine receptor 1; these are the major immune cells of the CNS in physiological states and usually remain quiescent, performing the function of immune surveillance across the CNS (Chen and Trapp, 2016; David et al., 2018). Microglia/macrophages are involved in various morphological and histological changes and contribute to the establishment of complex connection networks that are necessary for development.
When the integrity of the BBB is impaired, peripheral immune cells proceed to infiltrate the damaged area of the CNS (Zhang et al., 2011; Waisman et al., 2015; Prinz and Priller, 2017). Our previous single-cell RNA sequencing data showed that macrophages, neutrophils, mast cells, monocytes, monocytederived cells, dendritic cells, natural killer (NK) cells, NK T cells, T cells, and B cells were present in the spinal cord after SCI (Wang et al., 2022). For example, in the acute stage at 3 days post-injury (dpi), myeloid cells, and particularly macrophages and neutrophils, were found to infiltrate the spinal cord. Macrophages showed significant infiltration of the injury site at 3 dpi although this was much reduced at 14 dpi. Lymphocytes showed significant infiltration of the injury site at 14 dpi. These cells were restricted to the core of the injury site. The main subpopulation of lymphocytes was T cells at 3 and 14 dpi. In addition, NK cells and NK T cells were first detected at 14 dpi (Wang et al., 2022).
Resident and peripheral immune cells work together to participate in the pathophysiological process after SCI, thus regulating the immune inflammatory microenvironment and influencing the prognosis (Alizadeh et al., 2019). In the next section, we focus on the roles of microglia, macrophages, T cells, and B cells in post-traumatic CNS injury, especially with regards to the regulation of NSCs.
Divergent Roles of Immune Cells in Neural Stem Cells
The roles of microglia/macrophages on NSCs
Under pathological states, microglia/macrophages can be activated and polarized by adequate stimulation and demonstrate either pro- or antiinflammatory properties (Alizadeh et al., 2019). Activated microglia/macrophages can be divided into two major subtypes, an M1/proinflammatory phenotype (iNOS+/CD68+) induced by lipopolysaccharide (LPS) and an M2/anti-inflammatory phenotype (Arg1+/CD68+) induced by interleukin-4 (IL-4); these both perform different roles in post-traumatic CNS injury (Jiang et al., 2020). The M1/pro-inflammatory phenotype inhibits the proliferation and differentiation of NSCs while the M2/anti-inflammatory phenotype promotes the proliferation and differentiation of NSCs (Figure 2).
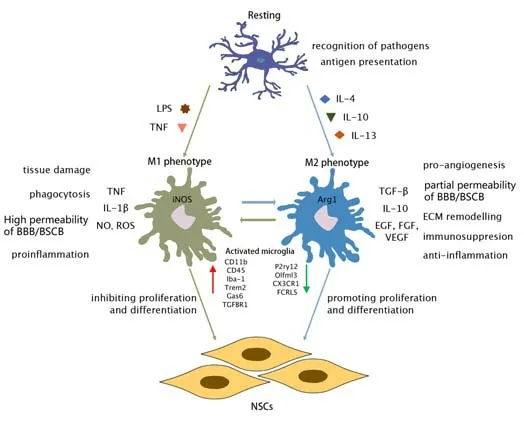
Figure 2|Resting and activating microglia/macrophages.
The pro-inflammatory phenotype of microglia/macrophages inhibits neurogenesis
Oxidative stress is an essential aspect of secondary injury in the CNS. Cytotoxic reactive oxygen species (ROS) react with polyunsaturated fatty acids to cause lipid oxidation and degradation; these processes affect the fluidity and permeability of cell membranes and hinder cell metabolism and ion-channel exchange (Zhang et al., 2019; An et al., 2021). M1 macrophages produce numerous inflammatory substances, such as ROS and reactive nitrogen species (RNS) through oxidative reactions, thus leading to further oxidative stress and the exacerbation of SCI (Wynn et al., 2013; An et al., 2021). M1 microglia/macrophages, as a pro-inflammatory phenotype, can produce cytokines, such as tumor necrosis factor (TNF), express nitric oxide (NO) and induce high levels of permeability in the BBB, further facilitating the infiltration of peripheral immune cells following CNS injury (Kostianovsky et al., 2008; Patel et al., 2013; Yu et al., 2022). These cells are located close to the NSCs and influence their proliferation and differentiation (Ziv et al., 2006). Monje et al. (2003) previously reported that exposure to LPS significantly increased the number of activated M1 microglia in the dentate gyrus and reduced the density of proliferative neurons, as indicated by bromodeoxyuridine (BrdU, a marker of proliferation) and doublecortin (Dcx, a marker of early neurons). Furthermore, these authors blocked the inflammatory response with non-steroidal anti-inflammatory drugs (NSAID) and that this treatment completely blocked the effect of peripheral LPS on neurogenesis. The authors obtained the same results in rats following X-ray irradiation injury. Their results suggested that neuroinflammationin vivoled to the inhibition of neurogenesis and that this form of neuroinflammation was mediated by M1 microglia (Ekdahl et al., 2003).
Subsequent exploration ofin vitromechanisms found that the extent of neurogenesis was reduced in co-culture with LPS-activated M1 microglia (Monje et al., 2003; Osman et al., 2019). Monje et al. (2003) further reported that the mechanism by which M1 microglia inhibited the proliferation and differentiation of NSCs was primarily mediated through the soluble inflammatory factors interleukin-6 (IL-6) and TNF. Osman et al. (2019) and Butovsky et al. (2006a) used the samein vitroLPS-activated microglia model and reached the same conclusion. Over time, the local microglia and newly recruited M2 phenotype macrophages were gradually transformed into the M1 phenotype in the peri-infarct regions (Xiong et al., 2016). Interestingly, compared to the consistently inhibitory effect of M1 microglia on neurogenesisin vivoandin vitrofollowing LPS treatment, the effect on neurogenesis in interferon (IFN)-γ-activated microgliain vivoandin vitroshowed the opposite effect. Rather, IFN -γ-activated microgliain vitropromoted the differentiation of NSCs into neurons; in other words, this enhancement was observed in the neurogenesis process (Zhang et al., 2020). However,in vivocerebral ventricle injections of IFN -γ revealed that microglia significantly inhibited neurogenesis in the hippocampus due to a rise in M1 microglia, thus resulting in cognitive deficit behaviors (Butovsky et al., 2006a; Zhang et al., 2020).
M1 macrophage/microglia-mediated inflammatory response is detrimental to the repair process after SCI (Fan et al., 2016). Quercetin has long been used as an antioxidant and anti-inflammatory agent in traditional Chinese medicine (Abarikwu et al., 2012) and has been confirmed to exert neuroprotective effects on SCI by inhibiting inflammatory responses (Zhang et al., 2015). Fan et al. (2019) previously found that quercetin alleviated necroptosis in oligodendrocytes by inhibiting M1 macrophage/microglia polarization via inhibition of the STAT1 and nuclear factor -κB (NF-κB) pathways after SCI. Moreover, tight junctions between endothelial cells are an essential part of maintaining normal barrier function in the blood-spinal cord barrier (BSCB) (Kumar et al., 2017). Persistent leakage of the BSCB can inhibit the effects of tissue restoration (Beck et al., 2010). Luo et al. (2022) confirmed that the tight junctions between vascular endothelial cells were impaired by M1 macrophages in the injury core, thus resulting in continuous leakage from the BSCB after SCI. Preventing M1 polarization of macrophages may promote restoration of the BSCB, thus accelerating functional recovery after SCI. However, it must be considered thatin vitromicroglia are unlikely to reflect thein vivophenotype of microglia biology.
The M1/M2 phenotype of microglia can be easily inducedin vitro, although this may not have much functional significancein vivo. The polarization of M1 and M2 polarizationin vivois not as pronounced asin vitropolarization; furthermore, there are multiple intermediate or transitional phenotypes with both pro- and anti-inflammatory properties (Zhang et al., 2020). Thein vivointeraction of microglia and NSCs might be influenced by other cellular components in the niche, such as changes in the endothelial cells and astrocytes. In general, these phenomena provide us with a clue that activated M1 microglia could inhibit the proliferation and differentiation of NSCs into neurons post-injury bothin vivoandin vitro, even though moderate inflammation and debris clearance are the important prerequisites for neurogenesis (Xiong et al., 2016). However, despite this breakthrough, the effect of direct cell contact between microglia and NSCs cannot be excluded and requires further research.
The anti-inflammatory phenotype of microglia/macrophages promotes neurogenesis
M2 microglia/macrophages generate different types of cytokines, such as IL-10 and transforming growth factor β, and can antagonize cytotoxic effects and exert anti-inflammatory, immunosuppressive and pro-angiogenic properties (Boche et al., 2013; Marina et al., 2019). Compared to the effects of M1 microglia on NSCs, many studies have shown that the M2 phenotype can promote the proliferation and differentiation of NSCsin vitro(Butovsky et al., 2006a; Matsui and Mori, 2018; Osman et al., 2019). Jin et al. (2014) and Yang et al. (2019) used animal models of stroke and TBI, respectively, and reported that M2 microglia/macrophages exerted neuroprotective effects by enhancing the proliferation of NSCs and the process of neurogenesis. It is possible that the pro-neurogenic processes involving M2 microglia/macrophages may involve the reduction of TNF, interleukin-1β (IL-1β), and IL-6 levels and an increase in the levels of IL-4, IL-10, and transforming growth factor β.
With regards to SCI, studies have demonstrated the pro-neurogenetic role of M2 microglia/macrophages after injuries by detecting elevated levels of IL-10, oncomodulin, brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and nerve growth factor (NGF) (Shechter et al., 2009). Microglia-derived BDNF does not appear to support neurogenesis in the hippocampus. The selective knockdown of microglia-derived BDNF did not inhibit neurogenesis but rather increased the new generation of neurons under physiological and inflammatory conditions (e.g., LPS-induced inflammation and TBI) (Harley et al., 2021). It has been hypothesized that the pro-neurogenic effects of microglia lacking BDNF are attributed to alterations in the phenotype of microglia themselves rather than from the loss of BDNF, as subsequent experiments revealed that the absence of these microglia did not alter the normal rate of neurogenesis.
The pro-inflammatory phenotype of microglia/macrophages inhibits oligodendrogenesis
Microglia also affect post-injury remyelination via the NSCs and OPCs. Microglia serve as a significant cause of remyelination, namely the regeneration of the myelin sheath by newly differentiated oligodendrocytes. It was reported that microglia could exert regenerative characteristics by phagocytizing the remains of myelin (Kotter et al., 2006) and by secreting both growth and neurotrophic factors (Kotter et al., 2005). Previousin vitrostudies reported that LPS-activated M1 microglia were able to inhibit oligodendrogenesis via the production of TNF and IL-1β (Butovsky et al., 2006a; Osman et al., 2019); however, the effect on NSCs or OPCs was not explained. With regards toin vivoexperiments, M1 microglia/macrophages also play an important role in the process of remyelination; because the processes of phagocytosis/decomposition after the injury are mainly mediated by the M1 phenotype. Moreover, it was determined that the death of M1 microglia/macrophages is an essential prerequisite for effective remyelination (Miron et al., 2013). These authors showed that resting microglia and infiltrated macrophages were activated in abundance and polarized into the M1 phenotype following demyelination injury. These M1 microglia/macrophages then phagocytized cellular debris and the remains of myelin in the NSCs niche, thus initiating programmed death, and creating an adequate space and beneficial cellular microenvironment for subsequent NSCs/OPCs-mediated oligodendrogenesis (Miron et al., 2013). However, to date, few studies have investigated the interaction between M1 phenotype and oligodendrogenesisin vivo; this represents a potential research priority for future demyelination therapy.
The anti-inflammatory phenotype of microglia/macrophages promotes oligodendrogenesis
Unlike M1 microglia/macrophages, the effect of the M2 phenotype on oligodendrogenesis is more distinct. Several previous reports have demonstrated that M2 microglia/macrophages promoted oligodendrogenesis bothin vitroandin vivoafter demyelination injury (Butovsky et al., 2006a, b; Miron et al., 2013; Lloyd et al., 2019). Butovsky et al. (2006a) found that IL-4-activated M2 microglia could promote the differentiation of NSCs/OPCs into oligodendrocytesin vitro, thus enhancing oligodendrogenesis. Subsequently, these authors found that in line with theirin vitroresults, the activation of microglia with IL-4 pretreatment improved the prognosis of demyelinating injury through the oligodendrogenesis process and that insulin-like growth factor 1(IGF-1) might be involved in this process (Butovsky et al., 2006b). A few years later, Miron et al. (2013) showed that M2 microglia/macrophages contributed to oligodendrogenesis. These authors found that with the onset of remyelination, microglia and peripheral macrophages underwent a transformation from an M1-dominated phenotype to an M2-dominated phenotype. Furthermore, differentiation into oligodendrocytes was enhancedin vitroin M2 conditioned medium. After the depletion of M2 microglia/macrophages in demyelinating lesions, the differentiation of oligodendrocytes was then impairedin vivo; the demyelinating lesions with a high density of the M2 phenotype usually exhibited better remyelination via oligodendrogenesis. Moreover, these authors showed that blocking activin-A (transforming growth factor β superfamily member) derived from M2 microglia/macrophages inhibited the differentiation of oligodendrocytes during remyelination (Miron et al., 2013). Therefore, activin-A might be the key factor that mediates M2 microglia/macrophages to promote oligodendrogenesis after demyelination injury. However, this raises the question of which mechanisms regulate the transformation of M1-dominated microglia/macrophages to the M2-dominated phenotype after demyelination injury, thus promoting the oligodendrogenesis process. This question was answered by (Lloyd et al., 2019) some years later. These authors found that the transformation from pro-inflammatory to anti-inflammatory/pro-regenerative stages required the necroptosis (a form of programmed death) of M1 microglia/macrophages; this was positively related to effective oligodendrogenesis and remyelination after demyelination injury. The activation of M2 microglia could be blocked once the death of M1 macrophages was impaired. Lloyd et al. (2019) used RNA sequencing to analyze genetic changes in M1 and M2 microglia during necroptosis and found that during the M1–M2 microglia transition, type-1 IFN signaling was involved in mediating oligodendrogenesis and remyelination by promoting the repopulation of M2 microglia.
Recently, a new subtype of M2 microglia (M2c) was found to enhance oligodendrogenesis and promote neural regeneration after demyelination injury by upregulating the Wnt7a signaling pathway (Mecha et al., 2020). With regards to other nerve injury models, such as SCI and sciatic nerve injury, the M2 phenotype still promoted oligodendrogenesis and regeneration via IL-10 and oncomodulin (Shechter et al., 2009; Kwon et al., 2013). In general, these phenomena indicate that M2 microglia can promote the differentiation of NSCs/OPCs into oligodendrocytes after demyelination injury, bothin vivoandin vitro. The effect of M2 microglia/macrophages on oligodendrogenesis is relatively more consistent when compared to the heterogeneous phenotype of M2 microglia on neurogenesis.
The role of T cells on NSCs
Only a small number of T cells reside in the cerebrospinal fluid under physiological states. However, when CNS injuries occur, peripheral T cells will infiltrate into the injured site and arrive at the NSC niche via integral BBB damage (Prinz and Priller, 2017). Therefore, the number of T cells rise significantly at the site of injury (Vindegaard et al., 2017). T cells exert immunoregulation properties during neuroinflammation and also play a critical role in the proliferation and differentiation of NSCs (Ransohoff et al., 2003; Reboldi et al., 2009). However, T cells exhibit a wide range of subtypes, mainly including Th1, Th2, CD4+, regulatory T cells (Tregs), and CD8+T cells; the functions of these cells differ while partially overlapping. This diversity means that the functionality of T cells is quite intricate. In the next section, we discuss the different subtypes of T cells that have been reported to influence neurogenesis.
The role of CD4+ T cells on NSCs
CD4+T cells are the most varied population of cells in the immune system, including Th1, Th2, Th9, Th17, Th22, Tfh, and Tregs (Zhou et al., 2009). When the CNS is injured, peripheral CD4+T cells infiltrate into the injury site to mediate the immune response. These cells also play a critical role in aggravating inflammation and repairing damage by direct combination with self-antigens (Shaked et al., 2004; Saino et al., 2010). However, compared with microglia polarization, the cellular heterogeneity of CD4+T cells after exposure to different antigens is known to be higher after various CNS injuries. Furthermore, compared with the number of microglia, the effect of the relatively inadequate number of CD4+T cells may possibly be obscured by microglia in the inflammatory response after injury. Therefore, adoptive transfer may represent a feasible method to test the role of CD4+T cells after injury.
Based on the adoptive transfer of CD4+T cells activated by myelin basic protein (MBP), Moalem et al. (2000) used a rat model of optic nerve injury to investigate the effect of TMBPcells (T cells activated by the autoantigen MBP) on neurogenesis. These authors found that CD4+TMBPcells could protect injured neurons from secondary degeneration after injury via the production of NGF, BDNF, neurotrophin-3, and neurotrophin4/5. Furthermore, analysis of the expression profiles of cytokines and neurotrophins showed that CD4+TMBPcells represented the phenotypes of Th1 and Th2 subtypes. Interestingly, although Moalem et al. (2000) did not directly demonstrate that CD4+T cells promoted neurogenesis in optic nerve injury, BDNF and neurotrophin-3 have been reported to be associated with neurogenesis after injury (Ockel et al., 1996; Ziv et al., 2006). Ziv et al. (2006) further confirmed the role of CD4+T cells in maintaining neurogenesis and promoting learning capacity. These authors also found that adult neurogenesis and learning activity require the maintenance of CD4+TMBPcells. These CD4+TMBPcells move to the NSC niche in the hippocampus and shape the behavior of microglia in a manner that promotes the survival and regeneration of neurons; this means that the roles of T cells and resident microglia/macrophages in neurogenesis may potentially be interconnected (Ziv et al., 2006). In another study, Wolf et al. (2009) showed that endogenous neurogenesis in the hippocampus was partially attributed to CD4+T cells. Moreover, Wolf et al. (2009) excluded the role of CD8+T cells and B cells, thus identifying a regulatory role for CD4+T cells in hippocampal neurogenesis. The findings of Ziv et al. (2006) and Wolf et al. (2009) indicated that the mechanism that enhances neurogenesis might involve CD4+T cell-derived BDNF instead of NGF. However, in terms of the source of BDNF, Ziv et al. (2006) believed that neurogenesis in the hippocampus mainly involved CD4+TMBPcells mediated by CNS-specific antigens. According to Wolf et al. (2009), this process was maintained by CD4+T cells that were mediated by non-specific antigens.
Because the multiple subtypes and different/overlapping functions of CD4+T cells, it is the predominant subtypes, such as Th1, Th2, and Tregs, that determine the precise effects, which may differ widely owing to different experimental conditions and parameters. For example, Saino and colleagues obtained very different results from Ziv’s results. Saino et al. (2010) found that the depletion of CD4+T cells, but including neuroprotective Tregs, could significantly promote neurogenesis by reducing the apoptosis of NSCs and by enhancing neurogenesis after stroke instead of a physiological status. This is most likely due to the fact that the CD4+T cells infiltrating from the peripheral circulation after stroke were largely activated and polarized to the Th1 subtype by damaging substances within the inflammatory microenvironment, thereby enhancing the release of pro-inflammatory factors and inhibiting the pro-neurogenic processes. Another study showed that when compared with CD4+Th1 T cells that produce proinflammatory factors, the adoptive transfer of the IL-4-producing Th2 subtype significantly attenuated the proinflammatory properties of meningeal myeloid cells and improved the learning and cognitive activities mediated by endogenous neurogenesis. This effect of the Th2 subtype might also be regulated by BDNF as proposed by Derecki et al. (2010). Although the authors did not use the same model of stroke as described by Saino et al. (2010), the function of CD4+cell polarization was clearly enhanced.
CD4+T cells are known to have a potential stimulatory effect on oligodendrogenesis after demyelination. For example, Hvilsted et al. (2011) used a model of multiple sclerosis (MS) with demyelination injury and found that T cells activated by myelin proteolipid protein (PLP) of the CNS-specific antigen (namely CD4+TPLPcells) could enhance the proliferation of OPC and oligodendrogenesis at the side of injury in the dentate gyrus axons in the hippocampus. The authors found that CD4+TPLPand TMBPcells had the same ability to promote the clearance of myelin debris; however, the adoptive transfer of TPLPcells exhibited a different inflammatory profile. During the regeneration of remyelination, TPLPcells were shown to increase and maintain the levels of TNF level for a long period of time but did not significantly affect the levels of IGF-1 and BDNF (Hvilsted Nielsen et al., 2011). Because T cells, microglia, and macrophages produce TNF after demyelination injury, the precise role of CD4+TPLPcells in promoting oligodendrogenesis has yet to be fully elucidated. Therefore, the precise mechanism responsible for how CD4+T cells promote adult neurogenesis and oligodendrogenesis still needs further exploration in the future. In particular, identifying the function of CD4+T cellsin vivowill facilitate the improvement of CNS injuries by regulating T cells in the future.
The role of CD8+ T cells on NSCs
CD8+T cells, also referred to as cytotoxic T lymphocytes, can destroy target cells infected with pathogens, tumor cells, and other antigen materials, thus protecting normal functionality and resulting in an inflammatory response (Haring et al., 2006). CD8+T cells exert a similar inhibitive effect on postinjury neurogenesis and oligodendrogenesis when compared to highly heterogeneous CD4+T cells. CD8+T cells have been previously reported to inhibit neurogenesis and oligodendrogenesis not only in mice with CNS injuries but also in aging mice (Wang et al., 2010; Bond and Song, 2019; Daglas et al., 2019; Dulken et al., 2019). Daglas et al. (2019) used a mouse model of TBI and found that CD8+T cells were present in the brain for a long period after injury and gradually lead to the deterioration of neurological/motor function via chronic inflammation in late SCI. Although no significant improvement in cognitive function was detected at 8 weeks, these authors could not exclude earlier cognitive changes. Daglas et al. (2019) further confirmed that the depletion of CD8+T cells improved the prognosis of TBI by initiating a Th2 T cell-mediated immune response and by minimizing the production of granzyme B, thus implying that CD8+T cells were responsible for the long-term neurological damage after TBI and this effect may be related to the inhibition of neurogenesis and/or oligodendrogenesis.
Wang et al. (2010) also provided evidence to support the fact that the infiltration of CD8+T cells inhibited the proliferation of NSCs and blocked neurogenesis via granzyme B. In addition to TBI, the inhibition of neurogenesis by CD8+T cells was still detected in a model of stroke. Although CD8+T cells and NK cells, which both produce perforin after injury, infiltrated the ischemic brain, only brain-infiltrating CD8+T cells (and not NK cells) were more active than their splenic counterparts. Following stroke, the depletion of CD8+T cells reduced infarct volume and improved behavioral defects, while the depletion of NK cells had no such effect. Researchers also found that the deleterious effect of CD8+T cells was mediated by the production of perforin and inhibited neurogenesis (Mracsko et al., 2014).
CD8+T cells, which are similar to CD4+T cells, require specific antigens for activation before they exert functional actions. Therefore, in models of various injuries, CD8+T cells can be stimulated by specific antigens, become activated, and exert heterogeneity, at least to some extent. However, at present, research appears to indicate that the use of CD8+T cells for motor function recovery are negative, and this effect is possibly mediated by influencing the survival of existing neurons, along with the proliferation and differentiation of NSCs (Balood et al., 2022). Because few CD8+T cells reside in the CNS, it is speculated that the effect of CD8+T cells on neurogenesis and oligodendrogenesis may be mediated by other cells, such as microglia expressing major histocompatibility complex class I. Therefore, the specific effect of CD8+T cells on NSCs needs to be investigated further.
The role of regulatory T cells on NSCs
Regulatory T cells (e.g., Treg cells and CD4+CD25+Foxp3+cells) are subtypes of CD4+T cells. Tregs are functionally adapted for different physiological or pathological conditions and exert a great immunosuppressive effect on other lymphocytes whose functions could result in a severe immune response (Josefowicz et al., 2012). Many reports of CNS injury have shown that Tregs exhibit neuroprotective effects. In acute experimental TBI, Krämer et al. (2019) found that the depletion of Treg cells increased T cell brain infiltration, reactive astrogliosis, IFN-γ gene expression, and transient motor deficits. During the process of post-injury inflammation resolution, the expression of IL-10 in the brain reduced the overexpression of various pro-inflammatory cytokines, thus preventing the development of secondary injury (Liu et al., 2018). These data suggested that IL-10 might play a key role in neurological recovery after injury by influencing the proliferation and differentiation of NSCs.
Wang et al. (2015) used a mouse model of stroke and found that the number of NSCs in subventricular zone proliferated significantly in mice treated with activated Treg cells. These NSCs expressed Mash1 (a neuroblast marker), thus indicating an enhancement in neurogenesis. Interestingly, when IL-10 was knocked out, the number of neuroblasts was reduced; however, the number of NSCs increased; these data suggest that Treg cells may play a role in regulating the level of oligodendrogenesis and/or reactive gliosis. Other studies have shown that Treg cells could enhance post-stroke neurogenesis via direct or indirect actions (Ishibashi et al., 2009; Saino et al., 2010; Poittevin et al., 2013). However, some researchers offered a different view in that although Treg cells could improve the prognosis of stroke via the production of IL-10, this mechanism was unrelated to neurogenesis. Brea et al. (2014) used a rat model of stroke and showed that the adoptive transfer of Treg cells into ischemic rats 3–28 days after injury and the stimulation of endogenous T cell proliferation with a CD28 super-agonist could reduce infarct size. However, Treg cell therapy did not alter the proliferation rate of cells that were positive for neuronal nuclei (NeuN, a marker of mature neurons) and neural cell adhesion molecule (NCAM, a marker of immature neurons), thus excluding a protective effect on neurogenesis (Brea et al., 2014).
It is important to consider that the same model can produce radically different results; this involves a diverse range of factors. The results obtained by Brea et al. were based on flow cytometry, which means that the tissue was extracted from sites that included not only the core injury area but also the peripheral injury area and part of the undamaged area (Brea et al., 2014). This situation resulted in a lower number of NeuN+BrdU+and NCAM+BrdU+neuronal cells in the injury area. Furthermore, the activation time of endogenous Treg cells and the time of detection for NeuN+BrdU+cells and NCAM+BrdU+cells also differed from previous studies (Liu et al., 2018). Finally, Ortega et al. (2020) demonstrated the possible involvement of B cells in the promotion of neurogenesis in the brain after stroke injury, both on the injury side and the contralateral side.
Other than rodents as animal models, new evidence has arisen from other species that has suggested a pro-neurogenetic role for Treg cells in the repair and regeneration of nervous tissue. In zebrafish, Treg cells expressing homologous forkhead box p3a (Foxp3a), equivalent to mammalian Foxp3, were shown to infiltrate into the injured spinal cord and retina to stimulate the proliferation of NPCs by releasing two specific pro-regenerative factors, NT-3 and IGF-1, respectively (Hui et al., 2017; Sharma and Rudra, 2018). Therefore, it is assumed that Treg cells provide NSCs with neurogenic signals, thus promoting post-injury neurogenesis.
Treg cells can also exert influence on oligodendrogenesis. Dombrowski et al. (2017) found that Treg cells were needed during post-stroke remyelination for the efficient differentiation of OPCs to oligodendrocytes; this effect was demonstrated bothin vitroandin vivo. In addition, proteomic analysis revealed that cellular communication network factor 3 played a crucial role in mediating Treg cells to promote oligodendrocyte differentiation and myelination. Subsequently, Shi et al. (2021) used transcriptomic analysis to show that brain infiltrating Treg cells were reprogrammed after stroke, thus facilitating the mobilization and activation of phagocytes. For example, the interaction of Treg cells with microglia generated an osteopontin-rich microenvironment to polarize microglia from the M1 to the M2 phenotype, thus promoting oligodendrogenesis and facilitating white matter repair and regeneration in the chronic phase of ischemic stroke. Therefore, the importance of Treg cell/microglia interaction during oligodendrocyte replacement and white matter repair after stroke was highlighted.
Much of the existing literature demonstrates that Treg cells play a beneficial role in both neurogenesis and oligodendrogenesis after injury. Researchers have also found that this beneficial effect on post-injury neurogenesis may be generated via indirect effects on NSCs, such as inhibiting the activation of other inflammatory cells and transforming the microglial phenotype. In contrast, only a few reports have focused on the direct effects of Treg cells on NSCs in animal models of injury; thus, future research should investigate the direct mechanism involved.
The role of B cells on NSCs
In previous studies, the activation or inhibition of microglia/macrophages and T cells has been reported to result in differing outcomes in different models of CNS injuries. This is because microglia possess M1/M2 phenotypes and T cells predominantly possess Th1/Th2/Th17 phenotypes; these cells produce very different effects at different time points. Although much research has focused on microglia/macrophages and T cells, it is important to also consider the critical role of B cells in the regulation of NSCs. It is possible that B cells could also exert neuroprotective effects on NSCs after injury (Ankeny and Popovich, 2010). Tanabe and Yamashita (2018) showed that B-1a cells, as a subtype of B cells, are present in abundance in the brains of newborn mice. Furthermore, B cells in the peripheral circulation could differentiate into the B-1a phenotype following infiltration into the CNS. Other than their immune role, B-1a cells could accelerate the proliferation of OPCs. If B-1a cells were eliminated from the process of CNS development, the numbers of OPCs and mature oligodendrocytes would decrease dramatically. In addition, the authors showed that IgM-Fcα/µR signaling mediated the contribution of B-1a cells to oligodendrogenesis and myelination by facilitating the proliferation of OPCs.
It is possible that during injury, B cells have the potential capacity to repair nerves by regulating OPCs and by attenuating inflammation. This hypothesis was supported by the enhanced immune response caused by B cell deficiency (Daglas et al., 2019). A recent study by Ortega et al. (2020) found that neuroinflammation occurs immediately after stroke in remote areas (outside of the area of cerebral infarction). These authors quantified the number of B cells after stroke and confirmed that B cells infiltrated bilaterally into the infarcted and contralateral regions, thus enhancing neurogenesis to promote the neurological recovery of locomotor and cognitive functions. The depletion of B cells after stroke also confirms their neuroprotective role in neurogenesis and the recovery of spatial learning and kinematic compatibility. One mechanism for promoting post-stroke neurogenesis, as with Treg cells, could be the production of IL-10, which is known to be independent of immunoregulation (Ortega et al., 2020). It has been established that B cells play a crucial role in neurogenesis during cerebral development and postinjury repair. However, the detailed mechanisms involved have yet to be elucidated.
In summary, different immune cells play different roles in NSCs, as well as in different stages of self-renewal and the differentiation of NPCs and OPCs (Figure 3 and Table 1).

Table 1 |The effects of immune cells on NSCs and OPCs
Intervention of Immune Cells in Post-Traumatic Central Nervous System Injury
Because of the close relationship between immune cells and the immune inflammatory response in post-traumatic CNS injury, researchers have tried to investigate appropriate new treatment strategies. First, it may be possible to switch the M1 phenotype of microglia/macrophages to the M2 phenotype by physical, chemical, or biological interventions; this might represent a promising therapeutic strategy for regeneration. Peripheral macrophagederived exosomes, M2 macrophage-derived exosomes, MSCs-derived small extracellular vesicles, olfactory unsheathing cells-derived exosomes, and the over-expression of IL-10 in MSCs have all been demonstrated to switch the phenotype of macrophages/microglia and promote M2 macrophage polarization (Peng et al., 2021; Zhang et al., 2021). Second, it may be possible to transplant beneficial immune cells. Selective M2 microglial transplantation, and adoptive transfer of Th1-conditioned cells have both shown potential as a new therapeutic strategy for recovery after SCI (Kobashi et al., 2020). Third, we could deplete harmful immune cells. There have been some interesting attempts to deplete microglia using the animal models of TBI and SCI. In the early phase after SCI, Jakovčevski et al. (2021) showed that the population of microglia/macrophages was reduced to approximately 23% when compared to the control group, thus leading to improved locomotor recovery. In another study, six weeks of microglial depletion after SCI reduce was shown to reduce chronic inflammation and neurodegeneration in the brain and improved neurological recovery in male mice (Li et al., 2020). During the chronic phase of experimental TBI, microglial depletion markedly reduced chronic neuroinflammation and associated neurodegeneration, as well as related motor and cognitive deficits (Henry et al., 2020). Moreover, in the late stage of SCI, the depletion of macrophages (Zhu et al., 2015; Lee et al., 2018), neutrophils (Kenne et al., 2012), and monocytes (Makinde et al., 2017, 2018) has been shown to attenuate lesion size and promote recovery after traumatic injury. However, we also noted that in some studies, the impact on NSC activity was not mentioned. We believe that these interventions would inevitably affect the functionality of endogenous NSCs and should be investigated further.
Conclusion and Future Perspectives
It is important to target immune cells in CNS injury based on the relationship between immune cells and NSCs. There are several challenges that need to be overcome. First, we need to define the complex cellular and molecular changes in the NSC niche as a whole; there are no relevant mapping studies at present. Secondly, we need to ensure the effective proliferation of NSCs and encourage them to selectively differentiate into neurons and oligodendrocytes instead of astrocytes without oncogenesis. Third, promoting the differentiation of NSCs with immune cellsin vitrobefore transplanting or directly transplanting NSCs and adoptively transferring immune cells to injured sites could result in a more favorable prognosis following CNS injury. Fourth, we need to investigate the effects of NSCs during the acute and chronic phases after the activation of specific immune cells. Finally, we need to perform more clinical studies. Collectively, these strategies will help to accelerate the application of NSCs and immune cells as therapeutic strategies. Given existing data, the use of immune cells as an intervention could represent a potential solution for overcoming the difficulty associated with the treatment of CNS injuries. Our suggestions for future research are as follows. Firstly, it is important to identify a strategy to reduce the amount or inhibit the function of endogenous immune cells that have a negative effect on NSCs. Secondly, we need to develop a strategy to increase the amount or promote the function of endogenous immune cells that have a positive effect on NSCs. Third, we need to transplant exogenous immune cells into the injured spinal cord and investigate their positive effects on NSCs (Figure 4). Furthermore, there are no authoritative clinical guidelines relating to the treatment of CNS injuries with NSCs. Therefore, it is vital that further clinical research is undertaken. In addition, biomaterials are also widely used in SCI regeneration and repair research (Zhang et al., 2019). Bioactive scaffolds not only act as vehicles for the delivery of cells to alter the microenvironment; they also exert influence on nerve repair. Furthermore, biomaterials can be altered to perform anti-inflammatory roles by regulating polarization (Li et al., 2022). Thus, selecting or designing a biomaterial that provides a good platform for immune cells and NSCs may become a potential strategy for SCI repair.In summary, approaches for nerve regeneration by modulating NSCs in combination with specific immune cells are drawing significant attention from researchers and clinicians because these methods are safer and more effective than existing strategies. The fact that we can control and regulate the activity of NSCs to achieve this goal with immune cells represents a significant foundation for future research.
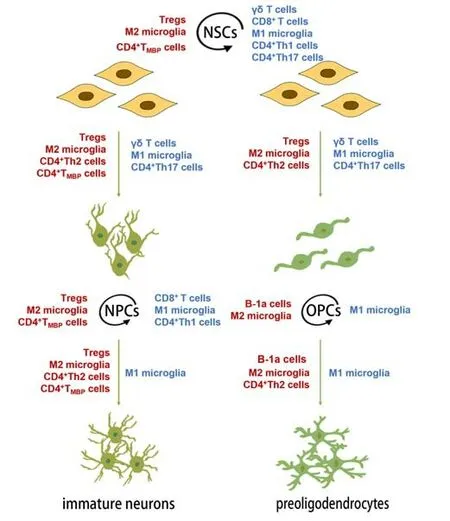
Figure 3|Immune cells affect the self-renewal and differentiation of NSCs, NPCs and OPCs in the CNS.
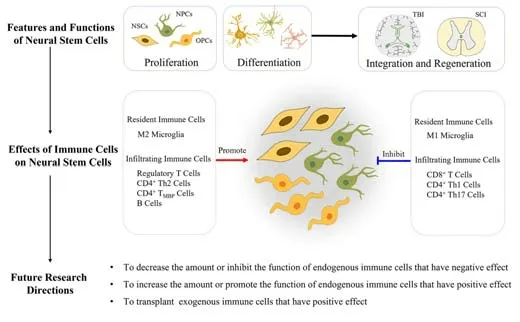
Figure 4|Biological roles of resident and infiltrated immune cells on neural stem cells in the post-traumatic CNS injury.
Author contributions:NH and XJM wrote the manuscript, YMD, TZ and YYC reviewed literature, and LLW critically revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest:The authors declare no conflict of interest in the publication of this article.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

