Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
Andreu Matamoros-Angles , Behnam Mohammadi , Feizhi Song Mohsin Shafiq Santra Brenna, Berta Puig, Markus Glatzel Hermann C. Altmeppen
Abstract In the last decades, the role of the prion protein (PrP) in neurodegenerative diseases has been intensively investigated, initially in prion diseases of humans (e.g., Creutzfeldt-Jakob disease) and animals (e.g., scrapie in sheep, chronic wasting disease in deer and elk, or “mad cow disease” in cattle). Templated misfolding of physiological cellular prion protein (PrPC) into an aggregation-prone isoform (termed PrP “Scrapie” (PrPSc)), self-replication and spreading of the latter inside the brain and to peripheral tissues, and the associated formation of infectious proteopathic seeds (termed “prions”) are among the essential pathogenic mechanisms underlying this group of fatal and transmissible spongiform encephalopathies. Later, key roles of the correctly folded PrPC were identified in more common human brain diseases (such as Alzheimer’s disease or Parkinson’s disease) associated with the misfolding and/or accumulation of other proteins (such as amyloid-β, tau or α-synuclein, respectively). PrPC has also been linked with neuroprotective and regenerative functions, for instance in hypoxic/ischemic conditions such as stroke. However, despite a mixed “bouquet” of suggested functions, our understanding of pathological and, especially, physiological roles played by PrPC in the brain and beyond is certainly incomplete. Interactions with various other proteins at the cell surface or within intracellular compartments may account for the functional diversity linked with PrPC. Moreover, conserved endogenous proteolytic processing of PrPC generates several defined PrPC fragments, possibly holding intrinsic functions in physiological and pathological conditions, thus making the “true and complete biology” of this protein more complicated to be elucidated. Here, we focus on one of those released PrPC fragments, namely shed PrP (sPrP), generated by a membrane-proximate ADAM10-mediated cleavage event at the cell surface. Similar to other soluble PrPC fragments (such as the N1 fragment representing PrP’s released N-terminal tail upon the major α-cleavage event) or experimentally employed recombinant PrP, sPrP is being suggested to act neuroprotective in Alzheimer’s disease and other protein misfolding diseases. Several lines of evidence on extracellular PrPC (fragments) suggest that induction of PrPC release could be a future therapeutic option in various brain disorders. Our recent identification of a substrate-specific approach to stimulate the shedding by ADAM10, based on ligands binding to cell surface PrPC, may further set the stage for research into this direction.
Key Words: ADAM10; aggregation; Alzheimer’s disease; amyloid; antibodies; Creutzfeldt-Jakob disease; enzymatic cleavage; extracellular vesicles; neurodegeneration; neurotoxicity; proteolytic processing; stroke; transmissible spongiform encephalopathies
Introduction
The cellular prion protein (PrPC) is a ~30 kDa glycosylphosphatidylinositol (GPI)-anchored glycoprotein highly expressed in the nervous system, yet also found in most other tissues (Stahl et al., 1987; Barmada et al., 2004; Adle-Biassette et al., 2006). In prion diseases (or “transmissible spongiform encephalopathies”, short: TSEs), which consist of sporadic (“idiopathic”), genetic (“familial”), and transmitted (including “iatrogenic”) forms, such as sporadic Creutzfeldt-Jakob disease, fatal familial insomnia and variant Creutzfeldt-Jakob disease, respectively, PrPCis transformed into a β-sheet-enriched fibrillizing isoform (PrPSc) (Prusiner et al., 1982; Aguzzi, 2001; Spagnolli et al., 2019; Kamali-Jamil et al., 2021; Kraus et al., 2021; Hallinan et al., 2022; Hoyt et al., 2022; Telling, 2022) causing toxicity and death in cells expressing PrPC, particularly in neurons (Brandner et al., 1996; Lakkaraju et al., 2022).
In the last decade, a dual (i.e., detrimentalversusprotective) role of PrPChas also been described in Alzheimer’s disease (AD). On one hand, full-length (fl) membrane-bound PrPCmediates toxic signaling as a high-affinity receptor for extracellular amyloid-β (Aβ) and other harmful protein/peptide assemblies associated with neurodegenerative diseases, such as p-Tau and α-synuclein (Lauren et al., 2009; Resenberger et al., 2011; Ferreira et al., 2017; Hu et al., 2018; Ondrejcak et al., 2018; Younan et al., 2018; Gomes et al., 2019; Corbett et al., 2020). On the other hand, PrPCwas shown to reduce BACE1-mediated β-amyloid precursor protein cleavage, thus reducing the formation of Aβ (Griffiths et al., 2011). PrPChas also been implicated in the downregulation, phosphorylation, and processing of Tau protein (Vergara et al., 2015).
In line with a beneficial role, PrPCis enriched on many types of extracellular vesicles (EVs; i.e., membrane-enclosed particles released by most cells in the body), where it may sequester toxic Aβ oligomers into larger and possibly less harmful Aβ fibrils, thereby reducing toxicity (Wik et al., 2012; An et al., 2013; Falker et al., 2016). Further neuroprotective roles for PrPChave been described in other pathological conditions, such as oxidative stress (Watt et al., 2005; Mitteregger et al., 2007; Haigh et al., 2009), epilepsy (Walz et al., 1999; Carulla et al., 2011), ischemia and stroke (Shyu et al., 2005; Doeppner et al., 2015; Guitart et al., 2016; Beraldo et al., 2018; Brenna et al., 2020).
This neuroprotection against excitotoxic processes may be performed, at least in part, by PrP’s regulation of cellular Ca2+homeostasis, protecting the system from intracellular Ca2+overload (Khosravani et al., 2008; De Mario et al., 2019). Furthermore, depending on cell type and context, surface PrPCmay be relevant for the uptake or phagocytosis of toxic protein aggregates, resulting in either harmful cell-to-cell spread of pathology or increased degradation of those conformers, thus highlighting again the potential dual role of this protein (reviewed in Legname and Scialò (2020)).
PrPChas also been related to several physiological functions in the nervous system, such as neuronal differentiation and proliferation (Steele et al., 2006), neuritogenesis, and axonal growth/guidance (Santuccione et al., 2005; Loubet et al., 2011; Amin et al., 2016), myelin maintenance in the peripheral nervous system (Bremer et al., 2010; Kuffer et al., 2016), synapse regulation and long-term potentiation (Maglio et al., 2006; Khosravani et al., 2008), memory acquisition and behavior (Coitinho et al., 2003; Schmitz et al., 2014; Matamoros-Angles et al., 2022), and homeostasis of copper and other divalent metal ions (Brown et al., 1997; Gasperini et al., 2015). Among the many suggested functions attributed to PrPC, only a few have been unambiguously confirmed by several independent groups, yet even in these cases the underlying mechanisms are mostly still not fully understood (Kovač and Čurin Šerbec, 2022). It appears not only conceivable but even likely that some of these roles are mediated by certain constitutively generated PrPCfragments and/or extracellular forms, and an increasing amount of studies are currently supporting this concept.
PrPCundergoes complex posttranslational modifications, including GPI-anchor attachment and N-glycosylation (Puig et al., 2011), and can be subjected to at least four conserved proteolytic processing events (termed α-, β-, γ-cleavage and C-terminal shedding; an overview is exhibited in Figure 1), which lead to the generation of different membrane-bound or released PrPCfragments (reviewed in Linsenmeier et al. (2017)). As mentioned earlier, GPI-anchored PrPC(either fl-PrPCor its C1 fragment resulting from the α-cleavage in the middle of PrPC) can also be released by cells in association with EVs. Recent studies pointed out that this EV-PrPChas an essential regulatory influence on the uptake of EVs by recipient cells (Brenna et al., 2020; D’Arrigo et al., 2021), Aβ fibrillization (Falker et al., 2016), and neuritogenesisin vitro(Gonias et al., 2022).
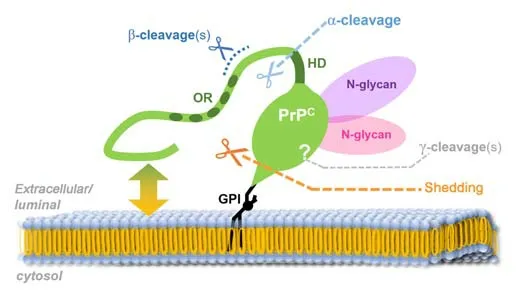
Figure 1|The prion protein (PrPC; green) and its conserved processing.
The multifunctional character of PrPC, especially its alleged dual role in brain diseases, is likely not restricted to the membrane-anchored full-length form of PrPC, on which the vast majority of research in the prion field has focused so far, but presumably involves proteolytically generated PrPCfragments and/or PrPCforms on the surface of EVs. We will here focus on one of these fragments, termed shed PrP (sPrP), resulting from the membrane-proximate release via the metalloprotease ADAM10 (Taylor et al., 2009; Altmeppen et al., 2011; Figure 1), and discuss new findings and concepts as to its role in neurodegeneration as well as neuroregeneration, and how its production may be stimulated in a substrate-specific manner and despite structural constraints.
Search Strategy and Selection Criteria
This review article was drafted based on relevant literature entries found after performing respective keyword searches (no further limits or filters applied) in PubMed®, NIH/NCBI (https://pubmed.ncbi.nlm.nih.gov) between February 2 and October 31, 2022. We apologize to all colleagues whose important contributions to aspects covered herein were not cited due to space limitations.
Lowering PrPC Expression and PrP-Targeting by Antibodies as Promising Strategies for Prion Disease Treatment and Beyond
Since the discovery of infectious prions (Prusiner et al., 1982), a wealth of studies using different approaches have been performed to cure the associated diseases, so far, unfortunately, without fundamental success. As demonstrated by convincing experimental evidence, prion disease incubation times inversely correlate with PrPCexpression levels (Sandberg et al., 2011). Earlier studies demonstrated that, while PrPCknockout (PrP-KO) mice are resistant to prion diseases, mice with heterozygous PrPCexpression (+/–) have a prolonged incubation time (Büeler et al., 1994) compared to wild-type controls (+/+), whereas tga20 mice with a severalfold overexpression of PrPCpresent a drastically shortened time to symptomatic disease and terminal stage (Fischer et al., 1996). In line with this, a conditional PrP-KO mouse model exhibited full recovery after depleting PrPCexpression in prion-infected preclinical animals (Mallucci et al., 2003). Moreover, while the presence and formation of PrPScare essential and defining for prion disease pathogenicity, net amounts of PrPScseem to be less determining for neurotoxicity and disease tempo than neuronal PrPCsurface levels (Altmeppen et al., 2015; Linsenmeier et al., 2021; Lakkaraju et al., 2022). Additionally, based on the knowledge obtained from studying PrP-KO mice (Steele et al., 2007) as well as some goats naturally devoid of PrPC(Benestad et al., 2012), lack of PrPCdoes not cause deleterious conditions as these animals experience rather normal life span with only subtle phenotypes if any. Thus, when it comes to treating patients with prion diseases or to preventing/delaying the establishment of the disease in confirmed carriers of respective PrP mutations (i.e., genetic cases) by lowering total PrP expression, potential side effects are most likely negligible in view of the anticipated gain. Therefore, reducing or even eliminating cell-associated PrPCis now considered to be one of the most promising therapeutic approaches (Tremblay et al., 1998; Minikel et al., 2020).The use of oligonucleotides targeting the mRNA to reducePrnpexpression has been studied for several years and this strategy is currently experiencing a straightforward revival and strong experimental support (White et al., 2008; Pulford et al., 2010; Friberg et al., 2012; Lehmann et al., 2014; Raymond et al., 2019; Minikel et al., 2020). For instance, antisense oligonucleotides (ASOs) designed against thePrnpmRNA were shown to not only significantly lower PrP expression, but also to prolong the incubation period for more than two months in prion-infected mice (Friberg et al., 2012; Raymond et al., 2019). In a comprehensive study, Minikel et al. (2020), by using a new set of more advanced ASOs, (i) first of all, confirmed that ASOs are effective in the reduction of PrPCexpression, and, more importantly, (ii) showed that a single dose administration of PrP-lowering ASOs in subclinical animals extends the survival time by 61–98%. Moreover, they (iii) highlighted that even neuroinflammatory and neuropathological markers (such as plasma neurofilament light chain levels) in these animals can be reversed, and also (iv) proved that –as expected for targeting PrP as the substrate for any prion conversion– the ASOs work against multiple prion strains (Minikel et al., 2020). These new findings may now pave the way for an effective and causative treatment against prion diseases.
In an alternative approach followed for more than two decades by now, different antibodies directed against PrP have been assessed as potential therapeutic agents against prion diseases (Enari et al., 2001; Peretz et al., 2001; Alexandrenne et al., 2009; Reilly et al., 2022). An early study using chronically prion-infected N2a cells showed that the monoclonal anti-PrP antibody 6H4 abrogated PrPScaccumulation, and a similar effect was also observed after phosphatidylinositol-specific phospholipase C treatment, which cleaves within the GPI anchor structure and releases PrP from the cell surface (Enari et al., 2001). Another study showed that antibodies against PrP inhibit prion propagation and efficiently clear cell cultures from prion infectivity in a dose-dependent manner (Peretz et al., 2001). Transgenic expression of anti-PrP antibodies even blocked prion infection in mice (Heppner et al., 2001). Strikingly, PrP-targeting antibodies are also protective against PrP-mediated neurotoxicity of Aβ oligomers (Lauren et al., 2009; Chung et al., 2010; Freir et al., 2011). For instance, the monoclonal antibodies 6D11 (Lauren et al., 2009) and ICSM35 (Freir et al., 2011), which bind to one of PrP’s Aβ-binding sites, were shown to efficiently block the Aβ-mediated inhibition of LTP. More recently, it was found that peripheral administration of a humanized anti-PrP antibody (PRN100) also interferes with the PrPC-Aβ interaction and prevents the otherwise harmful effects of Aβ derived from Alzheimer’s disease brain extracts when inoculated into rat brains (Klyubin et al., 2014).
Importantly, the same antibody was recently administered in a special treatment program to six Creutzfeldt-Jakob disease patients (Mead et al., 2022). Although all of the patients ultimately died of prion disease and their survival time and disease progression were not significantly different from historical controls, this important study revealed that administration of anti-PrP antibodies might be a suitable future therapeutic option (if given earlier), as an effective concentration of the antibody could reach the brain. Notably, this study also demonstrated that certain (though certainly not any) antibodies against PrPCare safe, as no severe adverse effects were observed (Mead et al., 2022). Moreover, antibody-based inhibition of prion-associated neurotoxicity was also demonstrated in murine hippocampal primary neurons (Reilly et al., 2022). A recent study provided important and detailed structural insight into how certain (rationally designed) ligands may interfere with prion toxicity (Frontzek et al., 2022), yet an effect on shedding was not assessed in that work. Several reports suggesting protective effects of PrP-directed antibodies in neurodegenerative protein misfolding diseases have recently been summarized (Linsenmeier et al., 2021), and it certainly deserves attention that this, more and more, seems to be ageneralized feature not only restricted to prion and Alzheimer`s diseases (Corbett et al., 2020).
A Deeper Look into PrP-Directed Antibody Treatment Reveals Two Overlooked Mechanisms That May Contribute To Protective Effects
In our recent study on PrPCshedding, we further assessed the treatment effects of different anti-PrP antibodies (Linsenmeier et al., 2021). We observed that cell lines or murine brain slice cultures incubated with most of the antibodies and ligands tested (e.g., 6D11, POM1, but also single-chain POM1, single-chain POM2, or a bispecific immunotweezer (Polymenidou et al., 2008; Bardelli et al., 2018)) significantly increase the ADAM10-mediated shedding of PrPC, regardless of the location of their epitopes within PrP. As a consequence, a greater fraction of PrPCis cleaved off from the membrane, and levels of sPrP, which is thought to act protectively against toxic oligomers in the extracellular space, are significantly increased. The exact mechanism of this antibody-mediated and, thus, substrate-specific stimulation of PrPCshedding remains largely unclear and crosslinking does not appear to be a prerequisite. One possible explanation, at least supported by small angle X-ray scattering measurement performed for one of the antibodies (6D11), could be that the flexible N-terminal tail of PrPCsomehow “shields” the C-terminal part by spatiotemporally restricting the access of ADAM10 to the cleavage site (yet the complete absence of the N-terminal tail is also disfavored as discussed below). The binding of any of the above-mentioned antibodies to PrPCmay slightly modify the “structure” and movements of the N-terminal tail (in relation to the C-terminal domain) to render the cleavage site more accessible for ADAM10, at least transiently. In stark contrast to the majority of antibodies that stimulated shedding, POM2, an antibody with four repetitive epitopes in PrP’s octameric repeat region localized within the flexible N-terminal tail, does not lead to more shedding but, instead, to a strong clustering of PrPCmolecules at the cell surface (most likely due to multimolecular crosslinking creating a dense meshwork of PrPCand antibody molecules), which subsequently leads to fast internalization and lysosomal degradation (Linsenmeier et al., 2021). While a degradation-promoting effect towards PrP has been shown earlier for other PrP-directed antibodies (Gilch et al., 2003; Perrier et al., 2004), this fast and remarkable reduction in total PrPCupon treatment with POM2 IgG has not been described before and might contribute to the protective effects attributed to this antibody (Sonati et al., 2013).
Last but not least, it should also be noted that several studies raised awareness that the administration of certain PrP-directed antibodies can also have relevant adverse effects, including severe neuronal loss and tissue damage (Solforosi et al., 2004; Lefebvre-Roque et al., 2007; Sonati et al., 2013; Herrmann et al., 2015; Reimann et al., 2016). From a mechanistic perspective, such harmful effects may involve (but are presumably not restricted to) epitope-dependent structural alterations and subsequent channel-forming insertion of several PrPs’ N-termini causing membrane perturbations (Sonati et al., 2013; Wu et al., 2017; Schilling et al., 2020), crosslinking-induced signaling cascades, impaired physiological trafficking and processing, shifted membrane localization, and/or blockage of PrP’s homeostatic interaction with important physiological binding partners. In conclusion, any therapeutic approaches involving PrP-directed antibodies (and possibly other ligands, too) therefore require careful consideration and experimental testing.
ADAM10-Mediated Shedding of PrPC: Sterical Considerations and Possible Regulatory Roles of Transmembrane Interactors
Several intuitively expectable structural constraints associated with PrPC, surprisingly, do not actually represent sterical hindrance for the access of and cleavage by ADAM10 (Figure 2A). Quite the contrary: the presence of the ever-moving and twisting, flexible, and readily membrane-interacting N-terminal tail (described to be a bona fide intrinsically disordered domain), which creates a spatiotemporal “cloud” or “shield” around PrP’s GPIanchored and structured C-terminal half (Sonati et al., 2013; Carter et al., 2015; Cheng et al., 2017; Wu et al., 2017; Linsenmeier et al., 2021), seems to be preferred by the protease, since fl-PrPCis more efficiently shed than its N-terminally truncated C1 fragment generated by the physiological α-cleavage (Linsenmeier et al., 2018). Moreover, despite the considerable size of the (up to two) complex-type N-glycans attached to PrPC(Rudd et al., 1999; Demarco and Daggett, 2009; Puig et al., 2011; Yi et al., 2018; Nakić et al., 2021), diglycosylated PrPCis clearly favored by ADAM10 over mono- and nonglycosylated PrPC(Linsenmeier et al., 2018). Lastly, as if these unexpected and somewhat counterintuitive preferences were not enough, our recent study discussed above further revealed that large ligands, such as IgGs (~150 kD), binding to the rather small PrPC(~30 kDa) even stimulate its ADAM10-mediated shedding in an efficient and substrate-specific manner (Linsenmeier et al., 2021). An overview of approximate size relations regarding the aforementioned aspects is depicted in Figure 2A. Increased shedding for other ADAM10 substrates had previously been shown upon dimerization or antibody-mediated crosslinking (Shi et al., 2001; Schelter et al., 2010; Hartmann et al., 2015), yet our study showed that dimerization of PrPCat the cell surface does not seem to be a prerequisite for its stimulated shedding, as single-chain antibodies targeting PrPCalso caused this effect. It will now be interesting to design and investigate smaller and therapeutically possibly more favorable ligands of PrPC, such as aptamers (Corda et al., 2018; Murakami et al., 2022) or nanobodies (Abskharon et al., 2019), to stimulate the ADAM10-mediated shedding.
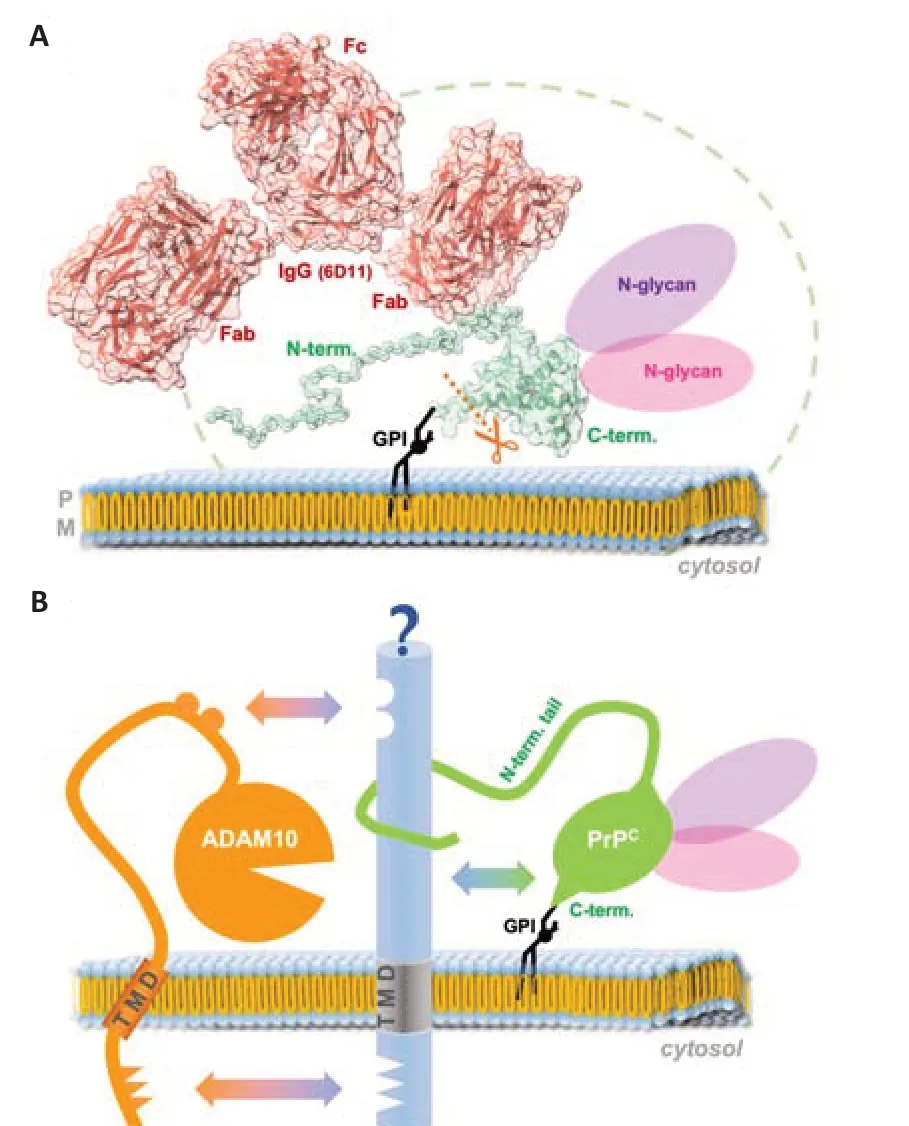
Figure 2|Size relation of modifications supporting the shedding of PrPC and potential influence of binding partners.
At second glance, however, a possible explanation for why the presence of the N-terminal tail and the N-glycans could be supportive of the shedding process may lie in a putative (and as yet clearly understudied) role of interaction partners. While the importance of N-glycans in protein-protein interactions (Wu et al., 2018) is a well-established biological feature (e.g., in cell adhesion (Zhao et al., 2008) or binding of viral surface proteins to host cell receptors (Cao et al., 2021; Hoffmann et al., 2021)), PrP’s flexible N-terminal tail is a particularly interesting major interaction hub allowing for the binding of various partner molecules (Beland and Roucou, 2012). By physically bridging protease and substrate, certain interactors may indeed warrant the transient yet close encounter between ADAM10 and PrP required for the cleavage process (as suggested in Figure 2B). While, in the case of ADAM10, respective interaction sites may be positioned in both the extracellular part (Janes et al., 2005; Seegar et al., 2017) as well as in the transmembrane and cytosolic domains (Deng et al., 2014; Hitschler and Lang, 2022), the latter does obviously not apply to PrPCwith its GPI anchor only sticking into the outer membrane leaflet. The N-terminal tail, however, may well accomplish sufficient interaction with extracellular domains of such putative binding partners at the cell surface. Strikingly, several transmembrane proteins at the cell surface co-localize or even interact with PrPC, and some of those, such as neural cell adhesion molecule 1 (Schmitt-Ulms et al., 2001; Santuccione et al., 2005; Saftig and Lichtenthaler, 2015; Slapsak et al., 2016), lipoprotein receptor-related protein 1 (Taylor and Hooper, 2007; Parkyn et al., 2008; Liu et al., 2009; Shackleton et al., 2016), cadherins (Morel et al., 2004; Málaga-Trillo et al., 2009; Seipold et al., 2018; Sachs et al., 2021), amyloid precursor protein (Epis et al., 2010; Jorissen et al., 2010; Kuhn et al., 2010; Kaiser et al., 2012), are themselves suggested or proven substrates of ADAM10. This raises the question if shedding might be co-regulated or whether heterophilic interactions of these proteins with PrPCmay even provide the impetus for the shedding of both in a (transient) complex. Alternatively, these proteins could also compete with PrPCfor the shedding by ADAM10. In this case, exact localization and membrane organization (e.g., center or periphery of lipid rafts versus nonraft regions (Puig et al., 2019)), possibly organized by additional regulators of trafficking and membrane subdomain architecture, such as tetraspanins (Levy and Shoham, 2005; Saint-Pol et al., 2017; Seipold et al., 2018; Koo et al., 2020; Lipper et al., 2022), may determine and modulate the shedding depending on (patho)physiological context and cellular state. In addition to protein-protein binding, interactions of PrP (and in particular its N-terminal tail; Figures 1 and 2A) with membrane lipids and associated effects on membrane subdomain regulation may directly affect its own proteolytic processing as well as effects caused by binding of its released fragments (e.g., N1 or shed PrP) to recipient cells.
As mentioned above, complex N-glycans also play a role in intermolecular crosstalk and this may account for the fact that fully glycosylated PrPCis preferred by ADAM10 over underglycosylated forms. Moreover, full glycan equipment may explain why sPrP seems to interfere with the misfolding process in prion diseases (Altmeppen et al., 2015; Linsenmeier et al., 2021; Mohammadi et al., 2022): Although the conversion of sPrP into an anchorless PrPScform seems possible (Aguilar-Calvo et al., 2020), the glycans may render sPrP much less prone for this pathogenic event (Winklhofer et al., 2003; Cheng et al., 2017; Camacho et al., 2019; Sevillano et al., 2020), thereby, at least relatively, hindering PrPScproduction [in stark contrast to underglycosylated anchorless PrP studied in respective transgenic mice (Chesebro et al., 2005; Race et al., 2017)].
Finally, when it comes to possible (patho)physiological functions of sPrP as a released factor in intercellular communication, both the N-terminal tail and the two glycans may be important features for putative ligand-receptor and other interactions. This should be taken into account when using any proxy, such as (non-glycosylated) recombinant PrP, in experiments aiming at investigating the “real” biological roles of bona fide shed PrP.
Protective Effects of Different PrP Forms and Fragments
While membrane-bound fl-PrPCas a cell surface receptor for toxic protein assemblies plays deleterious roles by inducing neurotoxicity in many neurodegenerative diseases (Lauren et al., 2009; Resenberger et al., 2011; Kostylev et al., 2015; Ferreira et al., 2017; Urrea et al., 2017; De Cecco and Legname, 2018; Corbett et al., 2020; Rösener et al., 2020), once it is cleaved by one of the naturally occurring proteolytic processing events [reviewed in (Altmeppen et al., 2013)], PrP’s neurotoxic potentials may turn into rather neuroprotective ones. For instance, previous studies have shown the protective effects of the soluble N1 fragment against the neurotoxicity of Aβ oligomers (Fluharty et al., 2013; Beland et al., 2014; Scott-McKean et al., 2016). A similar beneficial effect of N1 against toxic phosphorylated tau oligomers was also reported recently (Nieznanska et al., 2021). α-Synuclein oligomers were likewise shown to interact with the N-terminus of PrPC(Ferreira et al., 2017; Rösener et al., 2020) leading to the assumption of a more generalized protective role of N1 against different toxic oligomers. It is expected that other soluble fragments of PrPC(which carry the binding sites for toxic oligomers), such as sPrP, or PrPCforms decorating the surface of EVs, provide similar protective effects as N1.
Physiological roles of soluble PrP forms have been reported in different cellular processes (Martellucci et al., 2019; Gonias et al., 2022; Mantuano et al., 2022). For instance, recombinant PrP (to some extent recapitulating sPrP, yet lacking the important glycosylations) was shown to promote neurite outgrowth in PC12 and N2a cells via activation of N-methyl-D-aspartatereceptor and lipoprotein receptor-related protein 1. In Schwann cells, the interaction of recPrP with the lipoprotein receptor-related protein 1/N-methyl-D-aspartate-receptor complex activates ERK 1/2 and promotes cell migration (Mantuano et al., 2020). Interestingly, the same group later showed a similar effect of EV-associated PrPCusing extracellular vesicles derived from human plasma samples (Mantuano et al., 2022).
PrPCpromotes post-ischemic protection, (neuro-)regeneration, and angiogenesis (Doeppner et al., 2015), a phenomenon that might actually be mediated by released forms of PrPC. It was also shown that, after oxygenglucose deprivation (anin vitromodel of ischemic stroke), PrPClevels were increased in cultured astrocyte-derived EVs, which then had protective effects on oxygen-glucose deprivation-exposed neurons. This effect was seemingly dependent on EV-associated PrPC, as it was absent when the vesicles were isolated from PrP-KO cells (Guitart et al., 2016). Moreover, a smaller infarction area and faster tissue recovery after stroke was previously reported in PrPC-expressing compared to PrP-KO mice (Mclennan et al., 2004; Weise et al., 2006). A recent study further revealed that PrPCis essential for the regeneration of neurons, as its overexpression promotes early neurogenesis, whereas its absence delays neuronal regeneration after acute nasotoxic injury (Parrie et al., 2020). It certainly deserves additional studies to elucidate the relative contribution and involvement of fl-PrPCin comparison with its diverse fragments in these and other physiological processes linked with PrPC.
Concluding Remarks
The dual role attributed to PrP in neurodegeneration, especially in Alzheimer’s disease and prion diseases, may be explained by considering the conserved proteolytic processing of PrPC, resulting in the production of different fragments with supposedly overlapping but also distinct functions. While membrane-anchored PrPCtransduces toxicity of harmful protein assemblies, its extracellular forms (particularly N1, sPrP, and EV-PrP) seem to protect cells by trapping and possibly sequestrating these assemblies and reducing their toxicity. Therefore, any mechanisms capable of reducing fl-PrPClevels at the cell surface while increasing the release of protective PrPCforms into the extracellular space (such as the PrP-directed antibody treatment discussed herein) deserve to be considered and further investigated as potential treatment options against these currently incurable conditions. Moreover, the released PrPCderivatives likely hold functions as ligands in intercellular communication and may regulate diverse physiological processes. Further research into this direction is likewise certainly well justified, especially concerning regenerative processes in the brain.
Author contributions:AMA, BM, and HCA conceptualized and drafted the manuscript. All authors provided critical input and ideas and revised the text carefully. HCA and MS provided the illustrations. AMA, BM, and HCA finalized the manuscript. FS, SB, BP, and MG provided critical input and discussion and/or wrote short paragraphs of the text. All authors checked and approved the final version.
Conflicts of interest:The authors declare no conflicts of interest. No conflicts of interest exist between the Creutzfeldt-Jakob Disease Foundation, Inc. (USA) and publication of this paper.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis
- New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system

