External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis
Massimiliano Todisco , Giuseppe Cosentino Enrico Alfonsi
Abstract Multiple system atrophy is a sporadic, progressive, adult-onset, neurodegenerative disorder characterized by autonomic dysfunction symptoms, parkinsonian features, and cerebellar signs in various combinations. An early diagnosis of multiple system atrophy is of utmost importance for the proper prevention and management of its potentially fatal complications leading to the poor prognosis of these patients. The current diagnostic criteria incorporate several clinical red flags and magnetic resonance imaging markers supporting diagnosis of multiple system atrophy. Nonetheless, especially in the early disease stage, it can be challenging to differentiate multiple system atrophy from mimic disorders, in particular Parkinson’s disease. Electromyography of the external anal sphincter represents a useful neurophysiological tool for differential diagnosis since it can provide indirect evidence of Onuf’s nucleus degeneration, which is a pathological hallmark of multiple system atrophy. However, the diagnostic value of external anal sphincter electromyography has been a matter of debate for three decades due to controversial reports in the literature. In this review, after a brief overview of the electrophysiological methodology, we first aimed to critically analyze the available knowledge on the diagnostic role of external anal sphincter electromyography. We discussed the conflicting evidence on the clinical correlations of neurogenic abnormalities found at external anal sphincter electromyography. Finally, we reported recent prognostic findings of a novel classification of electromyography patterns of the external anal sphincter that could pave the way toward the implementation of this neurophysiological technique for survival prediction in patients with multiple system atrophy.
Key Words: bowel dysfunction; differential diagnosis; dysautonomia; electrophysiology; multiple system atrophy; Onuf’s nucleus degeneration; parkinsonism; Parkinson’s disease; prognostic prediction; urogenital symptoms 1Translational Neurophysiology Research Unit, IRCCS Mondino Foundation, Pavia, Italy; 2Movement Disorders Research Center, IRCCS Mondino Foundation, Pavia, Italy; 3Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
Introduction
Multiple system atrophy (MSA) is a severe α-synucleinopathy clinically featured by autonomic failure associated with poorly levodopa-responsive parkinsonism and/or cerebellar syndrome (Fanciulli and Wenning, 2015). Anatomical sites and load of the insoluble a-synuclein aggregates, forming glial cytoplasmic inclusions in the oligodendrocytes, determine the type and severity of autonomic disturbances along with the predominant motor phenotype (Goedert et al., 2013). Urogenital dysfunction is a common manifestation of dysautonomia in MSA and often appears earlier than cardiovascular abnormalities, such as orthostatic hypotension (Kirchhof et al., 2003). Urinary symptoms of MSA patients stem from detrusor hyperreflexia and urethral sphincter weakness, which cause urgency and incontinence, or from impaired detrusor contraction and detrusor-sphincter dyssynergia, which result in increased post-void residual volume (Beck et al., 1994). Widespread neurodegeneration of areas subserving autonomic control accounts for the multifaceted pathophysiology of urogenital dysfunction (Fowler et al., 2008). Neuronal loss in the basal ganglia, which physiologically sends inhibitory input to the pontine micturition center, leads to detrusor hyperreflexia. Loss of preganglionic parasympathetic neurons in the intermediolateral cell columns of the sacral spinal cord in combination with degeneration of the dorsal pons and lateral medullary reticular formation is responsible for the inability to voluntarily initiate voiding and incomplete bladder emptying due to detrusor failure. Loss of anterior horn cells in Onuf’s nucleus, which is located between the second and fourth sacral segments of the spinal cord, causes denervation of the pelvic floor muscles, in particular the striated muscles of urethral and anal sphincters, resulting in sphincter weakness and consequent urinary incontinence (Mannen et al., 1982).
An early MSA diagnosis is critical to prevent potentially life-threatening complications, to allow the possible recruitment of patients in diseasemodifying clinical trials, and to avoid futile urological surgical interventions. For instance, it was previously found that approximately 40% of men underwent prostatic or bladder neck surgery for outflow obstruction symptoms before being diagnosed with MSA (Beck et al., 1994). However, especially in the early disease stage, the differential diagnosis can be tricky (Palma et al., 2018; Kauppila et al., 2022), as shown by the evidence that movement disorders specialists perform a correct antemortem clinical diagnosis in approximately 50% of pathologically proven MSA patients (Litvan et al., 1997). In particular, the differentiation of MSA from Parkinson’s disease (PD) is hampered by the occurrence of autonomic dysfunction symptoms in both conditions, although with different levels of severity.
Neurophysiological assessment has proven to be of great relevance in exploring distinctive clinical features of MSA patients (Alfonsi et al., 2016; Todisco et al., 2020, 2022). Notably, electromyography (EMG) of the external anal sphincter (EAS) was proposed as an ancillary investigation in the past diagnostic flow chart of MSA, since it can provide useful clues for the differential diagnosis (Quinn et al., 1994). Indeed, bearing in mind that EAS is a striated muscle of the pelvic floor that is innervated by motor neurons of Onuf’s nucleus via the pudendal nerve, the EMG observation of EAS denervation and reinnervation may indirectly corroborate the histopathological findings of Onuf’s nucleus degeneration, therefore supporting MSA diagnosis (Figure 1). Controversies in the literature on the criteria for establishing electrophysiological abnormalities and on the real diagnostic value of EAS EMG have been dragging on for years. Discrepancies among authors derive from the lack of a standardized electrophysiological methodology, from differences in sample size, age, and disease duration of MSA patients and control subjects, and from the absence of healthy controls in several studies.

Figure 1|Examples of neurogenic abnormalities found at EAS EMG as electrophysiological correlates of Onuf’s nucleus degeneration in MSA.
In this review, we summarized the electrophysiological methodology and discussed the available knowledge on the role of EAS EMG for diagnostic characterization, clinical correlations, and prognostic stratification of MSA patients. We left out the corresponding evidence on urethral sphincter EMG, considering that, when compared to EAS EMG, it is technically more complex, more uncomfortable for the patient, and provides similar findings in MSA (Eardly et al., 1989; Beck et al., 1994; Stocchi et al., 1997; Giladi et al., 2000). Nonetheless, urethral sphincter EMG is a viable alternative option when EAS EMG is contraindicated, for example in the event of severe hemorrhoids or perianal infections.
Search Strategy and Selection Criteria
We searched the literature for peer-reviewed articles published by August 2022, written in English, and indexed in the National Library of Medicine (PubMed/MEDLINE) database. We used the following search terms: “external anal sphincter” AND “electromyography” AND “multiple system atrophy”. We initially identified 36 papers, then we excluded six review articles or opinion papers, and one letter to the editors on patients finally diagnosed with PD. We finally selected 30 studies reporting original data on MSA cohorts (Additional Table 1), after including an additional article that was retrieved by searching among references of the screened papers. The steps for the literature search strategy are summarized in Figure 2.
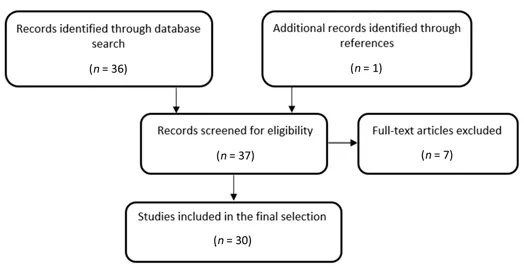
Figure 2|Flow diagram summarizing the search strategy for literature selection.
Methodology of External Anal Sphincter Electromyography: Acquisition and Analysis
The electrophysiological procedure requires that the patient assumes the lateral position on the bed examination, keeping hips and knees flexed. A concentric needle electrode is inserted into the four quadrants of the EAS under audio guidance, while the ground electrode is placed on a limb, typically the thigh contralateral to the side-lying position. Given that the recording site and type of muscle activation influence motor unit action potential (MUAP) parameters of the EAS, both the superficial and deep muscle layers as well as both the tonic and phasic EAS activity should be explored to achieve a comprehensive MUAP characterization. Indeed, MUAPs recorded from the superficial layer of the EAS and low-threshold MUAPs, constituting its basal activity, have a lower amplitude and shorter duration as compared to MUAPs from the deep layer and high-threshold MUAPs recruited after voluntary or reflex activation (Podnar and Vodusek, 1999). The superficial layer is assessed using perpendicular insertion of the needle electrode, 1 cm laterally to the anal orifice at a depth of 3–6 mm, whereas the deep layer is reached at the anal orifice by inserting the needle electrode at an angle of 30° to a depth of 15–25 mm (Podnar and Vodusek, 1999). The basal tonic activity is observed at rest with the subject fully relaxed, otherwise from the voluntary or reflex phasic activation, which is recorded by asking the patient to squeeze glutes or to cough, respectively. It is noteworthy that at least ten MUAPs from different EAS sites should be assessed to draw appropriate conclusions from the electrophysiological examination since denervation and reinnervation can affect only a proportion of MUAPs (Beck et al., 1994).
Several EMG findings can be evaluated to identify the presence and severity of neurogenic damage of the EAS. Pathological spontaneous activity (i.e., fibrillation potentials, positive sharp waves, or complex repetitive discharges) can be challenging to discriminate from EAS basal tonic activity, thus it is often not taken into account (Libelius and Johansson, 2000). Nevertheless, a careful analysis of the characteristic shape and firing rate enables the detection of pathological spontaneous activity that may represent a valuable electrophysiological finding, especially when not associated with abnormalities of MUAP parameters, therefore suggesting an initial disease stage of ongoing active denervation without significant chronic reinnervation (Todisco et al., 2022).
Rather than the amplitude, the percentage of polyphasic potentials and mainly duration were found to represent the most reliable MUAP parameters to discriminate neurogenic abnormalities in MSA patients (Libelius and Johansson, 2000; Tison et al., 2000; Yamamoto et al., 2012; Cao et al., 2020). Measurement of MUAP parameters greatly depends on the method of acquisition and analysis. On the one hand, the automated techniques (i.e., manual-MUAP and multi-MUAP analysis) allow a quick and thorough collection of a sizeable sample of EAS MUAPs, but fail to detect unstable complex MUAPs and do not include satellite potentials, if not followed by manual revision (Libelius and Johansson, 2000; Podnar et al., 2000). Available studies on MSA patients did not always include satellite potentials when calculating MUAP duration and this evidence may be a relevant source of heterogeneity. Although there is no consensus on this matter, several authors argued that EAS EMG examination should encompass these late components of MUAPs, as satellite potentials may be an expression of neurogenic damage per se, deriving from increased temporal dispersion in collateral branches of regenerating axons. On the other hand, single-MUAP analysis is timeconsuming, examiner-dependent, and biased towards the highest-amplitude MUAPs using a ‘trigger and delay line’. However, the single-MUAP technique requires a manual revision to ensure the correct placement of markers and thereby enables an accurate calculation of MUAP duration, including satellite potentials (Libelius and Johansson, 2000; Todisco et al., 2022). Despite these methodological discrepancies, which can explain some disagreement among studies, the different approaches to the acquisition and analysis of MUAPs were shown to detect EAS neuropathic changes at similar rates (Podnar et al., 2002). Whatever the technique for MUAP analysis used, each EMG laboratory must collect its data from healthy controls or, at least, considers normative values reported in the literature accurately replicating the electrophysiological methodology (Podnar et al., 2000).
There is a variety of assessment methods for MUAP recruitment, although only a few authors investigated this electrophysiological parameter in MSA patients. The subjective grading of interference pattern was replaced over time by the calculation of the ratio of simple phase and simple-mix phase or by the measurement of amplitude and phase pattern during maximal voluntary contraction (Qiu et al., 2019; Miao et al., 2020; Niu et al., 2022). Alternatively, the mean number of MUAPs per insertion site, alone or together with other quantitative parameters, represents another objective evaluation of recruitment that was explored in MSA cohorts (Gilad et al., 2001; Todisco et al., 2022).
An appropriate clinical setting is crucial for a proper interpretation of the electrophysiological findings. Indeed, when EAS EMG abnormalities are detected, the diagnostic accuracy of Onuf’s nucleus degeneration increases after excluding alternative causes of neurogenic damage, such as pudendal nerve entrapment or cauda equina syndrome (Todisco et al., 2022). However, in the case of comorbidities potentially associated with neurogenic alterations (e.g., history of pelvic surgery or diabetes mellitus), EAS EMG is of value when providing normal electrophysiological findings, since it makes MSA diagnosis less likely (Colosimo et al., 2000).
Some of the skepticism towards a systematic application of EAS EMG in MSA derives from the belief that this investigation may require particular expertise and could be poorly tolerated by some individuals (Schwarz et al., 1997; Tison et al., 2000). In keeping with other authors, we believe, however, that EAS EMG can be easily performed in any neurophysiology laboratory without causing patients too much discomfort.
External Anal Sphincter Electromyography for Differential Diagnosis of Multiple System Atrophy
Usefulness of EAS EMG for MSA diagnosis
It is well known that MSA patients show a high prevalence of EAS EMG abnormalities, which range from 62% to 93% in studies assessing MSA cohorts without any control group (Kirchhof et al., 2003; Pellegrinetti et al., 2003; Yamamoto et al., 2005, 2019). Similar findings were also reported in comparison with healthy subjects (Jian et al., 2015; Libelius and Johansson, 2000; Niu et al., 2022; Todisco et al., 2022). Whether this instrumental assessment can help in the differential diagnosis of MSA is instead a controversial topic. Nonetheless, the majority of authors share the view that EAS EMG can be of value for MSA diagnosis, although there is debate about which electrophysiological parameter should preferably be used (Additional Table 1). The evidence of prolonged values of duration and increased rates of polyphasic potentials in MSA allowed its discrimination from other causes of cardiovascular dysfunction or cerebellar ataxia, such as diabetes mellitus and spinocerebellar ataxia (Jian et al., 2015; Miao et al., 2020), and mostly from PD (Beck et al., 1994; Valldeoriola et al., 1995; Stocchi et al., 1997; Libelius and Johansson, 2000; Tison et al., 2000; Winge et al., 2010; Yamamoto et al., 2012; Qiu et al., 2019; Cao et al., 2020; Niu et al., 2022; Todisco et al., 2022). By contrast, two studies found that pathological spontaneous activity or reduced recruitment pattern was the only electrophysiological parameters to ensure an MSA diagnosis (Schwarz et al., 1997; Gilad et al., 2001).
There are inconsistencies among definitions of neurogenic abnormalities and cut-off values of MUAP parameters to discriminate MSA from mimic disorders, above all PD. A mean MUAP duration > 10 ms is the most common parameter supporting MSA diagnosis (Stocchi et al., 1997; Libelius and Johansson, 2000; Kirchhof et al., 2003; Yamamoto et al., 2005, 2012; Niu et al., 2022). However, other authors established different or supplementary criteria for neurogenic damage: more than 20% of MUAPs with duration > 10 ms (Beck et al., 1994; Yamamoto et al., 2005, 2012; Niu et al., 2022), more than 30% of MUAPs with duration > 10 ms and mean number of MUAP phases > four (Libelius and Johansson, 2000), mean MUAP duration > 9 ms (Beck et al., 1994), more than 40% of polyphasic potentials (Libelius and Johansson, 2000; Niu et al., 2022), average amplitude > 1 mV or more than 27% of polyphasic potentials (Stocchi et al., 1997), or proportion of satellite potentials > 13% (Niu et al., 2022). Valldeoriola et al. (1995) defined EAS EMG abnormalities in the presence of more than 50% of MUAP with duration > 12 ms and more than five phases. Recently, we identified four EAS EMG patterns of increasing severity based on duration and spatial recruitment of MUAPs with or without pathological spontaneous activity (Todisco et al., 2022).
Several studies showed high accuracy of MUAP parameters in the differential diagnosis of MSA. MUAP duration was found to differentiate MSA from PD with sensitivity ranging from 71% to 93% and with specificity varying from 65% to 100% (Tison et al., 2000; Winge et al., 2010; Qiu et al., 2019; Cao et al., 2020). Cut-off values of MUAP duration having the best diagnostic accuracy differ among studies since they vary from 10.9 ms to 14 ms (Tison et al., 2000; Winge et al., 2010; Qiu et al., 2019; Cao et al., 2020). Some authors noted that a higher threshold for MUAP duration raises the specificity but lowers its sensitivity substantially. For instance, Tison et al. (2000) found a specificity of 100% but a sensitivity of 55% with MUAP duration > 16 ms, implying that this finding is exclusively observed in MSA patients, although almost half of MSA cases are missed. In parallel, a sensitivity of 100% corresponded to a threshold value of 12 ms, consequently, no MSA patients had a mean MUAP duration below 12 ms. Differently from other authors, Yamamoto et al. (2012) reported that MUAP duration > 10 ms allowed the identification of MSA with a lower sensitivity of 35%, but a comparable specificity of 90%. Of note, the percentage of more than 20% of MUAPs with duration > 10 ms was not a significant criterion for the differential diagnosis (Yamamoto et al., 2012). The rate of satellite potentials, number of phases, and prevalence of polyphasic potentials were observed to be other useful EMG parameters for the differential diagnosis between MSA and PD, with sensitivity of 73–80% and specificity of 65–93% (Cao et al., 2020). Studies that defined neurogenic damage taking into account simultaneously different electrophysiological features confirmed the diagnostic value of EAS EMG, finding a sensitivity of 86–96% and a specificity of 67–99% (Todisco et al., 2022).
The notion that EAS EMG would be highly specific but poorly sensitive also arises from reports supporting the time dependency of Onuf’s nucleus involvement in MSA. Indeed, Yamamoto et al. (2012) argued that the lower sensitivity values described in their study were probably due to the shorter disease duration of the recruited patients. In the early disease stage, a normal EAS EMG would not rule out an MSA diagnosis, since neurogenic changes were detected in 52% of MSA patients one year after symptom onset, and then increased to 83% by the fifth year (Yamamoto et al., 2005). Similarly, Stocchi et al. (1997) showed normal EAS EMG findings in individuals with less severity and shorter duration of disease, but those same subjects developed electrophysiological abnormalities within two years after the baseline evaluation. Accordingly, it was proposed that an abnormal EAS EMG in the early disease stage firmly supports an MSA diagnosis, while alternative diagnoses should be considered in the event of a normal neurophysiological evaluation more than five years after symptom onset. Although the absence of EAS EMG abnormalities in the advanced disease stage is very unlikely in pathologically proven MSA patients, this eventuality cannot be ruled out considering pathology data of Onuf’s nucleus preservation in a few cases (Wenning et al., 1994). In addition, the observation of EAS EMG alterations in a long-standing disease complicates the diagnostic interpretation, due to an increase in MUAP duration and a higher rate of neurogenic abnormalities in the mid- and late-stage of PD (Libelius and Johansson, 2000; Niu et al., 2022). In contrast, other authors showed the lack of correlation between neurophysiological data and MSA stage, therefore supporting the diagnostic value of EAS EMG regardless of disease duration (Tison et al., 2000; Pavior et al., 2005; Winge et al., 2010; Todisco et al., 2022).
Evidence against the diagnostic value of EAS EMG in MSA
The diagnostic criticism of EAS EMG is mainly fueled by the above-mentioned reports of non-negligible rates of false negative and false positive patients, contributing to assigning a marginal role to this instrumental technique. The suggestion of EAS EMG abnormalities in patients with alternative diagnoses was also corroborated by the pathology evidence of Onuf’s nucleus degeneration in a few cases of PD and progressive supranuclear palsy (PSP) (Scaravilli et al., 2000). Still, some authors found that PD patients could be misdiagnosed as MSA in the event of neurogenic changes at EAS EMG, supporting its negative rather than positive predictive value for differential diagnosis (Colosimo et al., 2000). The prevailing dissenting opinions, therefore, cast doubt on whether the observation of EAS EMG findings discriminates MSA from other degenerative parkinsonisms. In this regard, a large overlap of MUAP parameters was shown between MSA and PD groups (Schwarz et al., 1997; Giladi et al., 2000; Linder et al., 2012). At first glance, these findings could result from the recruitment of patients in the advanced disease stage and from the exclusion of satellite potentials for the calculation of MUAP duration causing an underestimation of neurogenic abnormalities (Schwarz et al., 1997; Giladi et al., 2000). However, similar results were also found in the early disease stage and after the inclusion of satellite potentials for MUAP analysis (Linder et al., 2012). Moreover, some authors described that EAS EMG failed to discriminate between MSA and PSP since both patients’ cohorts showed neurogenic changes to a similar extent (Valldeoriola et al., 1995; Winge et al., 2010; Linder et al., 2012). The study by Gilad et al. (2001) even questioned the presence of EMG abnormalities in MSA because of the lack of significant differences in MUAP parameters between MSA patients and healthy controls.
Clinical Correlations of External Anal Sphincter Electromyography Findings in Multiple System Atrophy
Even though motor neurons innervating urethral and anal sphincters have a different location within Onuf’s nucleus, both cell groups are involved throughout the disease course (Konno et al., 1986). Therefore, the detection of neurogenic changes at EAS EMG may be a neurophysiological correlate of several symptoms of both bladder and bowel dysfunction in MSA patients. Nonetheless, the lack of correlation of urinary symptoms with the presence and severity of EAS electrophysiological abnormalities would not be surprising, given that Onuf’s nucleus degeneration is only one of the underlying multiple pathophysiological mechanisms in MSA. For example, a combination of detrusor overactivity and urethral sphincter denervation accounts for urinary incontinence in MSA patients, thus sphincter etiology may be a mere contributory factor (Valldeoriola et al., 1995). The notion that sphincter denervation does not univocally lead to urinary symptoms was suggested by some authors, who described MSA patients with abnormal EAS EMG but no urinary symptoms. Stocchi et al. (1997) found that, in the early disease stage, only 77% of MSA patients with urinary urgency and abnormal urodynamic examination showed neurogenic changes at EAS EMG. However, all remaining patients complaining of urinary symptoms with a normal electrophysiological evaluation developed neurogenic abnormalities within two years from the baseline evaluation. Tison et al. (2000) reported a slight, but not significant increase in the mean MUAP duration with longer duration of urinary symptoms. Similar assumptions could be made for bowel disturbances. Indeed, fecal incontinence is reported in MSA patients regardless of the observation of marked neurogenic changes in EAS MUAPs (Wenning et al., 1994). A recent study showed that rates of EAS neurogenic abnormalities did not depend on the presence of defecation disorders as well (Niu et al., 2022). The argument that Onuf’s nucleus degeneration is not necessarily associated with bladder and bowel disturbances was also supported in PSP patients, who denied urinary or fecal incontinence despite having EAS neurogenic impairment in some cases (Valldeoriola et al., 1995). Conversely, PD patients can manifest urinary incontinence even without showing sphincter denervation, due to the involvement of a different pathway of the autonomic nervous system (Valldeoriola et al., 1995).
In contrast with the aforementioned line of thought, several authors substantiated the hypothesis of a correlation of bladder and bowel dysfunction with EAS EMG alterations in MSA. Indeed, sphincter denervation may directly determine urinary incontinence in patients without urodynamic evidence of overactivity or low compliance of the detrusor muscle and could be linked to a decrease in anal squeeze pressure, resulting in fecal incontinence (Yamamoto et al., 2005). Some studies showed that virtually all MSA patients with genitourinary symptoms had EAS denervation and reinnervation (Beck et al., 1994; Kirchhof et al., 2003). Yamamoto et al. (2005) also noted a higher incontinence severity in the presence of EAS abnormalities. Moreover, it was argued that the correlation between the degree of neurogenic changes and the extent of post-void residual volume could be a non-causal finding, reflecting a parallel degeneration of sacral parasympathetic neurons and Onuf’s nucleus (Yamamoto et al., 2005, 2019). More recently, we found that EAS EMG patterns of increasing severity were not only associated with the prevalence of urogenital symptoms and fecal incontinence, but also with symptom type at disease onset (Todisco et al., 2022).
EAS neurogenic alterations were observed irrespective of the detection of postural hypotension (Yamamoto et al., 2005; Niu et al., 2022). Likewise, several studies did not report parallelism between EAS neurogenic damage in MSA patients and motor impairment. In particular, motor symptoms were assessed utilizing the Unified Parkinson’s Disease Rating Scale, the Hoehn and Yahr Scale, the International Cooperative Ataxia Rating Scale, or the Unified Multiple System Atrophy Rating Scale (Winge et al., 2010; Yamamoto et al., 2019; Todisco et al., 2022). Conversely, in a single study, the prevalence of EAS neurogenic abnormalities was found to increase with the severity of posture and gait disturbances, which were evaluated using the corresponding item of the International Cooperative Ataxia Rating Scale (Yamamoto et al., 2005). According to most literature, it could be therefore speculated that the degenerative process in MSA may involve several neuronal areas to varying degrees or at different disease stages, leading to a heterogeneous progression of symptoms.
External Anal Sphincter Electromyography for Prognostic Prediction in Multiple System Atrophy
Recently, we explored whether a novel pattern classification based on the presence and severity of EAS neurogenic abnormalities could be of value in the survival prediction of MSA patients (Todisco et al., 2022). We indeed showed that the stepwise electrophysiological impairment was associated with a progressive worsening of prognosis. Of interest, the uncommon observation of a normal EAS EMG allowed the identification of a small MSA cohort characterized by an unusually prolonged survival. That said, survival of more than 15 years was reported in a few pathologically confirmed MSA patients. In particular, subjects with the long-standing disease showed a late onset of cardiovascular and urinary autonomic dysfunction, in keeping with the association between early autonomic failure and short survival (Watanabe et al., 2002; Tada et al., 2007; Calandra-Buonaura et al., 2013).
Conclusions and Future Perspectives
The prevailing opinion in the literature supports the value of EAS EMG for the differential diagnosis of MSA. The most common caveats include the inclusion of satellite potentials for MUAP analysis and the exclusion of other potential causes of EAS neurogenic changes before speculating on Onuf’s nucleus degeneration. In the appropriate clinical setting, MSA diagnosis is bolstered by the evidence of neurogenic abnormalities, namely pathological spontaneous activity, reduced MUAP recruitment, increased rate of polyphasic potentials, and, particularly, prolonged MUAP duration. The diagnostic accuracy of EAS EMG is, however, limited by the observation that neurogenic findings could not be detected in the early stage of MSA, while they may be reported in the advanced stage of PD. This electrophysiological examination can therefore be implemented in clinical practice to rule out mimic disorders, such as PD, especially in the early disease stage when the clinical picture is unclear. A recent classification of EAS EMG patterns can help to disentangle MSA from PD and may provide useful insights into the prognosis of MSA patients. The development of advanced algorithms for a precise MUAP analysis could assist the clinician in the diagnostic workup. Future prospective studies with longer clinical and electrophysiological follow-up evaluations, larger patients’ cohorts, and pathological confirmation are desirable to strengthen the current diagnostic evidence, to deeply explore clinical correlations using specific instrumental examinations, and to confirm the recent prognostic findings in MSA.
Author contributions:MT conceived and wrote the first draft of the manuscript. GC and EA reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:Additional Table 1:Studies assessing external anal sphincter EMG in MSA patients.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system

