Photobiomodulation provides neuroprotection through regulating mitochondrial fission imbalance in the subacute phase of spinal cord injury
Xin Li , Xuan-Kang Wang , Zhi-Jie Zhu , Zhuo-Wen Liang Peng-Hui Li Yang-Guang Ma Tan Ding Kun Li Xiao-Shuang Zuo Cheng Ju Zhi-Hao Zhang Zhi-Wen Song Hui-Lin Quan Jia-Wei Zhang Liang Luo Zhe Wang , Xue-Yu Hu
Abstract Increasing evidence indicates that mitochondrial fission imbalance plays an important role in delayed neuronal cell death. Our previous study found that photobiomodulation improved the motor function of rats with spinal cord injury. However, the precise mechanism remains unclear. To investigate the effect of photobiomodulation on mitochondrial fission imbalance after spinal cord injury, in this study, we treated rat models of spinal cord injury with 60-minute photobiomodulation (810 nm, 150 mW) every day for 14 consecutive days. Transmission electron microscopy results confirmed the swollen and fragmented alterations of mitochondrial morphology in neurons in acute (1 day) and subacute (7 and 14 days) phases. Photobiomodulation alleviated mitochondrial fission imbalance in spinal cord tissue in the subacute phase, reduced neuronal cell death, and improved rat posterior limb motor function in a time-dependent manner. These findings suggest that photobiomodulation targets neuronal mitochondria, alleviates mitochondrial fission imbalance-induced neuronal apoptosis, and thereby promotes the motor function recovery of rats with spinal cord injury.
Key Words: low-level laser therapy; mitochondria; mitochondrial dynamics; mitochondrial fission imbalance; neuron; photobiomodulation; secondary injury; spinal cord injury 1Department of Orthopedics, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi Province, China; 2967 Hospital of People’s Liberation Army Joint Logistic Support Force, Dalian, Liaoning Province, China
Introduction
Spinal cord injury (SCI), resulting from sudden or sustained trauma, can result in neurological cell death that contributes to long-term disability and sensory deficits (Jazayeri et al., 2015; Hachem et al., 2017; Donovan and Kirshblum, 2018). Mechanistically, the majority of neuronal cell death originates from a phase of delayed secondary injury with progressive cascades, such as oxidative stress (Fatima et al., 2015), inflammation (Qiao et al., 2010, 2015) and mitochondrial disruption (Fatima et al., 2015; Scholpa and Schnellmann, 2017), rather than from the primary injury, referring to the immediate mechanical trauma.
Mitochondria modulate free radical formation, buffer calcium, and generate energy, and thereby affect immune response, autophagy and cell survival (Grohm et al., 2012; Tait and Green, 2012; Gollihue and Rabchevsky, 2017; Ozgen et al., 2022; Rey et al., 2022). Among the mechanisms of secondary injury progressive cascades, mitochondrial disruptions, including morphological alterations, mitophagy, adenosine triphosphate (ATP) loss, mitochondrialmediated apoptosis and mitochondrial oxidative stress, play a particularly critical role in neuronal death cascades in neurodegenerative diseases and central nervous system injuries (Calkins et al., 2011; Liu et al., 2016; Suárez-Rivero et al., 2016; Ottolini et al., 2017; Chen et al., 2018; Zhang et al., 2021). Restoration of mitochondrial disruptions has been shown to be a potentially effective therapeutic strategy for secondary injury after SCI, with strategies including mitochondrial permeability transition pore inhibitors (McEwen et al., 2007; Ravikumar et al., 2007), biofuels (Ravikumar et al., 2007; Patel et al., 2010), antioxidants (Hall, 2011; Bains and Hall, 2012; Chen et al., 2018) and mitochondrial division inhibitors (Li et al., 2015; Liu et al., 2015).
Mitochondria exist in dynamic networks that continuously undergo fusion and fission. The number and morphology of mitochondria within cells are closely related to mitochondrial function. Under pathological conditions, mitochondria morphology and number vary with the demand for mitochondrial homeostasis (Hoppins et al., 2007; Youle and van der Bliek, 2012; Meyer et al., 2017). Research in the past decade has shown that an imbalance of mitochondrial dynamics, which shifts the balance toward fission, may produce an accumulation of mitochondrial DNA defects, ATP depletion, and impaired regulation of oxidative stress, thereby promoting neuronal death (Grohm et al., 2010, 2012; Lu et al., 2017). These studies suggested that during neuronal cell death, dynamic tubulation of mitochondria was fragmented into smaller and dysfunctional organelles. However, it remains unknown whether mitochondrial fission imbalance regulates neuronal cell death in subacute SCI. If so, as reported in previous studies (Grohm et al., 2012; Li et al., 2015; Liu et al., 2015), restoration of mitochondrial dynamics might be an effective therapeutic strategy to ameliorate delayed neuronal cell death.In recent years, medical application of low-energy lasers with light in the red to near-infrared range (630–1000 nm) has become known as low-level laser therapy or photobiomodulation (PBM), and its use has broadened to central nervous system diseases and injuries (Ying et al., 2008; Huisa et al., 2013; Xuan et al., 2014; Micheli et al., 2017, 2019). There is growing evidence indicating that PBM, especially the wavelength of 810 nm, acts on cells through a primary photoacceptor, which has been identified as cytochrome C oxidase, part of mitochondrial complex IV, to convert light energy into chemical energy. Activation of cytochrome C oxidase then initiates related changes in mitochondria and downstream expression of many protective gene products with anti-apoptotic, anti-oxidant, and pro-proliferation effects (Karu et al., 1994, 2003, 2004, 2005; Morimoto et al., 1994; Pastore et al., 2000; Eells et al., 2004; Liang et al., 2006; Yeager et al., 2006; Karu, 2008). In a beta amyloid-induced Alzheimer’s disease rat model, PBM ameliorated mitochondrial fragmentations to reduce oxidative stress and neuronal damage (Lu et al., 2017). Mitochondria might evolve from photosynthetic organisms (Cavalier-Smith, 2006), and a low-energy laser was shown to increase ATP production and mitochondrial DNA replication (Vacca et al., 1993). Thus, it can be hypothesized that an 810 nm laser may improve the mitochondrial response to SCI to enhance the therapeutic effects.
On the basis of the above studies, we postulated that impaired modulation of mitochondrial dynamics toward fission during secondary injury results in delayed neuronal cell death in SCI rats, and that PBM might have a positive effect on neural protection. In the present study, transmission electron microscopy (TEM) was used to detect mitochondrial morphology in neurons after SCI. We also investigated the effects of PBM on self-repair of mitochondrial fragmentation and neuronal cell deathin vivo. This study is the first to examine whether PBM has a therapeutic effect for SCI via ameliorating mitochondrial fission imbalance in the subacute phase of SCI.
Methods
Crush injury model in rat and laser treatment
This research project was approved by the Welfare and Ethics Committee of Experimental Animal Center of Fourth Military Medical University (approval No. 20210452) on April 1, 2021. All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Adult male Sprague-Dawley rats (8 weeks old and weighing 250–280 g) were purchased from Experimental Animal Center of Fourth Military Medical University (license No. SYXK (Shaan) 2019-001). A total of 162 rats were randomly divided into Sham, SCI, and PBM treatment groups (Additional Figure 1).
The SCI model was established with the bilateral spinal cord compression method, as performed in a previous study (Song et al., 2017) with some modifications. Rats were anesthetized with an intraperitoneal injection of 1% pentobarbital (50 mg/kg, Huimei Biotechnology Co., Ltd, Xi’an, Shaanxi Province, China). Then, a midline dorsal thoracic incision was made above the T8–T12 vertebrae to expose the vertebral column. A laminectomy was performed with rongeurs at the T11 level, exposing the spinal cord. Forceps with metal spacers were used to laterally compress the spinal cord to a fixed thickness of 0.5 mm, and the compression was held for 40 seconds. Both hind limbs twitching involuntarily and the tail twisting indicated the successful completion of the SCI model.
In the PBM treatment rats, the head and tail of the light-emitting area of the optical fiber (Yazetech Medical Technology Co., Ltd, Xi’an, Shaanxi Province, China) were sutured to the paravertebral soft tissues of the T10 and T12 vertebrae after SCI, respectively. Immediately after SCI, 810-nm infrared light with an average radiant power of 150 mW (Lei Shi Optoelectronics Co., Ltd, Changchun, Jilin Province, China) was used for the treatment, 60 minutes each time, once a day. In the sham operation animals, the T8–T12 spinal cord received the identical surgical procedure but with no crush injury or laser irradiation; these were regarded as the control groups. After operation, an intramuscular injection of penicillin G (70 mg/kg) was administered to protect the rats from infection once per day for 7 days, and manual bladder expression was performed twice per day until the animals regained bladder function. The rats were sacrificed 12 hours after PBM treatment for subsequent analyses. SCI was divided into acute (at 1 day after SCI) and subacute stages (at 7 and 14 days after SCI) according to the duration of the injury. The detailed animal experimental design for the study was illustrated in Additional Figure 1.
Transmission electron microscopy
To detect mitochondrial morphology and number in neurons, spinal cord sample preparations were conducted for TEM at 1, 7 and 14 days after SCI. Rats were sacrificed after anesthesia as described above and perfused with 4% glutaraldehyde. The tissue centered on the injured area was cut perpendicular to the long axis into blocks 2 mm wide and postfixed with 4% glutaraldehyde overnight. The tissue was then fixed with 2.5% glutaraldehyde for 2 hours, postfixed with 2% osmium tetroxide, dehydrated with a graded ethanol series, embedded in resin, sliced with an ultramicrotome (Leica, Wetzlar, Germany), stained and viewed on a Tecnai G2 TEM (Thermo Fisher, Waltham, MA, USA) at 80 kV. Elongated mitochondria were defined as mitochondria with a ratio between the major and minor axis greater than 2 (Calkins et al., 2011). Mitochondrial area was defined as the cross-sectional area. Feret’s diameter was calculated as longest distance between any two points of the mitochondrial external perimeter. Mitochondrial area, diameter and number were measured by FIJI analysis software (Fiji-64bit, National Institutes of Health, Bethesda, MD, USA; Schindelin et al., 2012).
Western blot analysis
Mitochondrial fractions were prepared using a kit (C3606, Beyotime Institute of Biotechnology, Nantong, Jiangsu Province, China). Rats were deeply anesthetized and sacrificed at 7 and 14 days postoperation, and a 0.5-cm segment of spinal cord centered on the injury site was rapidly stripped. Fresh tissue was homogenized in ice-cold mitochondria isolation reagent A containing 1 mM phenylmethylsulfonyl fluoride and centrifuged at 600 ×gfor 10 minutes at 4°C. The supernatants were transferred to another tube and centrifuged at 11 000 ×g, 4°C for 10 minutes to precipitate the mitochondria. The isolated mitochondria were digested by cold mitochondrial lysis buffer and then subjected to western blotting analysis.
The mitochondrial proteins (10 µg) were loaded onto 4–20% Bis-Tris gels (M00656, GenScript, Nanjing, Jiangsu Province, China), transferred onto a nitrocellulose membrane (EMD Millipore Corp, Burlington, MA, USA), blocked and then incubated with primary antibody to mitochondrial fission 1 (Fis1; rabbit, 1:1000, Proteintech Group, Wuhan, Hubei Province, China, Cat# 10956-1-AP, RRID: AB_2102532), mitochondrial fission factor (Mff; rabbit, 1:1000, Proteintech Group, Cat# 17090-1-AP, RRID: AB_2142463), dynamin-related protein 1 (Drp1; rabbit, 1:2000, Cell Signaling Technology, Danvers, MA, USA, Cat# 8570S, RRID: AB_10950498), or heat shock protein 60 (rabbit, 1:3000, Servicebio, Wuhan, Hubei Province, China, Cat# GB11243) overnight at 4°C in blocking buffer. After incubation with goat anti-rabbit horseradish peroxidase antibody (1:1000, InCellGene, San Antonio, TX, USA, Cat# SA-10011 or goat anti-mouse horseradish peroxidase antibody (1:1000, InCellGene, Cat# SA-10010) at 25°C for 1 hour, visualization was detected by chemiluminescence (Amersham Imager 600, General Electric, Boston, MA, USA). Optical density was performed using FIJI analysis software.
Malondialdehyde, 3-nitrotyrosine, superoxide dismutase and total antioxidant capacity assay
Oxidative stress and antioxidative parameters were measured from spinal cord homogenates at 1, 7 and 14 days after SCI. Lipid Peroxidation MDA Assay Kit (Cat# S0131M, Beyotime Institute of Biotechnology) and Nitrotyrosine ELISA Kit (Cat# MM-70408R1, Jiangsu Meimian Industrial Co., Ltd, Suining, Jiangsu Province, China) were used to measure oxidative stress markers specific to lipid oxidation (malondialdehyde, MDA) and protein oxidation (3-nitrotyrosine, 3-NT), respectively. Superoxide Dismutase Assay Kit (Cat# S0101M, Beyotime Institute of Biotechnology) and Total Antioxidant Capacity Assay Kit (Cat# S0116, Beyotime Institute of Biotechnology) were used to measure antioxidative parameters superoxide dismutase (SOD) and total antioxidant capacity (TAOC), respectively. The detailed experimental manipulations were performed according to the manufacturer’s instructions. The absorbances were detected using a microplate reader (SYNERGY H1, BioTek, Winooski, VT, USA) at 532, 450, 450, and 593 nm for MDA, 3-NT, SOD and TAOC, respectively.
Immunofluorescence staining
Rats were deeply anesthetized as described above and sacrificed for collection of frozen sections in gray matter of the injured spinal cord, according our previous method (Wang et al., 2021). Approximately 10-µm-thick serial sagittal sections were sliced and then placed on slides. Frozen sections from rats at 1, 7 and 14 days post-SCI were washed three times with phosphate buffer saline, permeabilized with 1% Triton X-100, and blocked with 5% bovine serum albumin and normal goat serum for 1 hour at 25°C. After blocking, sections were incubated with primary antibody of microtubuleassociated protein 2 (MAP2; rabbit, 1:200, Cell Signaling Technology, Cat# 8707T, RRID: AB_2722660) at 4°C overnight, which is a sensitive indicator for the assessment of neuronal injury. The sections were then incubated with secondary antibody (Cy3-conjugated donkey anti-rabbit IgG, 1:200, Jackson Immuno Research, West Grove, PA, USA, Cat# 159918, RRID: AB_2307443) for 1 hour at 25°C at room temperature and stained with the 4′,6-diamidino-2-phenylindole (C0065, Solarbio, Beijing, China) to detect the nucleus. Immunostaining images of MAP2 dispersion were observed with confocal microscopy (FV3000, Olympus Corporation, Tokyo, Japan), and dispersion was calculated as the area of pore unstained with MAP2 or 4′,6-diamidino-2-phenylindole divided by the total area.
TdT-mediated dUTP nick end labeling staining
To assess the level of spinal cord tissue apoptosis at 14 days post-SCI, the one step TdT-mediated dUTP nick end labeling (TUNEL) apoptosis assay kit (Cat# C1090, Beyotime Institute of Biotechnology) was used, according to our previous method (Wang et al., 2021). Fragmented DNA of apoptotic cells was stained red by TUNEL, and images were obtained from a fluorescence microscope (Olympus Corporation, BX51).
Basso, Beattie, and Bresnahan score and footprint analysis
After SCI, the Basso, Beattie, and Bresnahan (BBB) locomotor scale (Basso et al., 1995) was used to assess behavioral function every day until rats were sacrificed. The observers were blind to experimental groups and familiar with the scoring criteria. BBB is a 22-point scale that indicates hindlimb functional recovery, where a score of 0 indicates complete paralysis and a score of 21 indicates normal walking.
Footprint analysis was performed to assess motor coordination at 14 days post-surgery, as described in a previous study (de Medinaceli et al., 1982). The back and feet of the hindlimbs were stained with red ink and blue ink, respectively. Stride length and stride width of the footprints were obtained from papers covering a narrow runway of 1 m length and 7 cm width. Stride length was determined as the distance between the central pads of two consecutive prints and stride width was measured as the distance between the central pads of the hindpaws.
Statistical analysis
The differences in BBB scores were evaluated by Scheirer-Ray-Hare test in SPSS 25.0 (IBM Corp., Armonk, NY, USA) (Guan et al., 2021). GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used to carry out other statistical analyses. One-way analysis of variance with least significant differencepost hocanalysis was used for multiple comparisons. For all analyses, the significance of statistical analyses was set at a level of 0.05. All data are presented as mean ± standard error of the mean (SEM).
Results
PBM enhances the self-repair of mitochondrial dynamics in vivo
To explore the effects of PBM on the dynamic tubulation of mitochondria, TEM was used to examine mitochondrial morphology in individual neurons from rats at 1, 7 and 14 days postsurgery (Figure 1). Representative TEM images showed that mitochondria were present in dynamic networks in control rats (Figure 1A). At 1 day, mitochondrial alterations in the neurons from SCI rats were characterized by decreased mitochondrial number, increased mitochondrial area and cristae loss, and the percentage of swollen mitochondria in SCI rats was higher than that in the control animals. Treatment with PBM restored the alterations in mitochondrial number, mitochondrial volume, cristae loss and the percentage of swollen mitochondria to some extent, though the measures did not reach the levels of the control group (Figure 1A–E and Additional Figure 2). At 7 and 14 days, the absolute number of mitochondria per neuron in SCI rats was significantly higher than that in control rats, and the majority of mitochondria exhibited short and round appearance (Figure 1A, F and G). PBM treatment attenuated the decrease in the percentage of elongated mitochondria (Figure 1F) and the increase in mitochondria number (Figure 1G) at 7 and 14 days. Interestingly, SCI rats at 14 days post-surgery had a higher percentage of elongated mitochondria (Figure 1F) and a lower number of mitochondria (Figure 1G) than those at 7 days, and PBM treatment for 14 consecutive days had a larger effect than treatment for 7 consecutive days (Figure 1F and G). Data of the PBM treatment groups did not reach the control group levels except for mitochondria number at 14 days post-surgery (Figure 1F and G).
PBM enhances the self-repair of mitochondrial targeting fission proteins in vivo
We next investigated the effects of PBM on mitochondrial targeting fission proteins after SCI. Western blot analysis showed that the expression levels of fission-related proteins (Drp1, Fis1 and Mff) in the SCI rats were higher than those in the control rats at 7 (Figure 2A) and 14 days (Figure 2B) after SCI, and administration of PBM decreased the expression levels of fissionrelated proteins compared with those from SCI rats at 7 and 14 days. The expression levels of fission-related proteins in the PBM rats were higher than those in the control rats at 7 days, except for Fis1 protein (Figure 2A). There were no significant differences in the fission-related protein expression levels between the control and PBM groups at 14 days, except for Mff protein (Figure 2B). In addition, the SCI rats at 14 days had lower levels of fission-related proteins than those at 7 days (Figure 2C), and the SCI rats with PBM for 14 consecutive days showed lower levels of fission-related proteins than those for 7 consecutive days, except for Fis1 protein (Figure 2D), indicating that PBM enhanced the self-repair of mitochondrial targeting fission proteins in a time-dependent manner.
PBM enhances the self-repair of mitochondrial oxidative stress in vivo
To explore the effects of PBM on oxidative stress, oxidative stress marker s specific to protein and lipid oxidation, 3-NT and MDA, and anti-oxidant parameters, SOD and TAOC, were detected in spinal cord tissue homogenates at 1, 7 and 14 days postsurgery. In the acute phase (1 day postsurgery), the SCI group had a marked increase in 3-NT level, but not MDA level compared with the control group (Figure 3A and B). Anti-oxidant markers were significantly reduced in SCI groups compared with control rats (Figure 3C and D). Compared with the SCI group at 1 day, the PBM treatment group had no significant difference in the levels of 3-NT, MDA and SOD (Figure 3A–D). At 7 days, SCI rats had the highest levels of oxidative stress markers (Figure 3A and B) and the lowest levels of anti-oxidant markers (Figure 3C and D). Interestingly, rats with PBM treatment at 7 days had higher levels of oxidative stress markers (Figure 3A and B) and lower levels of anti-oxidant markers (Figure 3C and D) than those at 1 day.
Similar to the trend of mitochondrial morphological alterations in the subacute phase, SCI rats at 7 and 14 days post-surgery had higher oxidative stress markers (Figure 3A and B) and lower anti-oxidant parameters (Figure 3C and D) than the controls, and PBM attenuated the increase of oxidative stress markers and the decrease of anti-oxidant parameters to some extent. The SCI rats with PBM for 14 consecutive days showed lower levels of oxidative stress markers (Figure 3A and B) and higher levels of anti-oxidant parameters (Figure 3C and D) than those with PBM for 7 consecutive days, suggesting that longer PBM treatment had a greater effect on self-repair of oxidative stress.
PBM improves neuronal damage and locomotor function after SCI
To assess the impact of PBM on neuronal injury, MAP2 dispersion was examined to assess the degree of neuronal injury. Confocal fluorescence images indicated that the neurons in the gray matter of the normal ventral spinal cord were tightly arranged with low MAP2 dispersion (Figure 4A–C). SCI significantly increased MAP2 dispersion at 1 (Figure 4A), 7 (Figure 4B) and 14 (Figure 4C) days after injury. SCI rats at 7 days had a higher level of MAP2 dispersion than those at 1 day (Figure 4D). PBM did not improve MAP2 dispersion at 1 day (Figure 4A), and PBM treatment for 7 and 14 consecutive days decreased MAP2 dispersion, but MAP2 dispersion did not reach the levels of the controls (Figure 4D). TUNEL staining confirmed that PBM improved apoptosis at 14 days after SCI (Additional Figure 3). Rats with PBM treatment at 7 days had a higher level of MAP2 dispersion compared with rats with PBM treatment at 1 day (Figure 4D). In addition, MAP2 dispersion was lower in the SCI rats at 14 days after operation than that at 7 days, suggesting self-repair of neuronal injury (Figure 4D). The neurons of ventral spinal cord around the injured area from rats with 14-day PBM had substantially lower MAP2 dispersion than those with 7-day PBM (Figure 4D).
BBB test and footprint analysis were performed for behavioral assessments of functional recovery. The highest BBB score of 21 was observed in the control group over the entire time range (Figure 5A), indicating that the sham surgical operation did not damage the spinal cord. Within the first 3 days after surgery, there was no significant difference in BBB score between the SCI and PBM groups. However, BBB score of PBM rats was higher than that of SCI rats from the 4thday after surgery, and the BBB score of the PBM group was rapidly restored from an average value of 5 to 11.6 on the 8th to 11th days, respectively, suggesting the therapeutic effect of PBM increased over time (Figure 5A). Finally, footprint analyses at 14 days after injury for the SCI group showed a reduction in stride lengths, an increase in stride widths and obvious instep dragging (Figure 5B–D). The PBM group showed a better restoration of stride length and stride width and clearer plantar stepping with less toe dragging compared with the SCI group, suggesting restoration of the base of support and motor function.

Figure 1|Effects of PBM on mitochondrial dynamics within neurons in spinal cord at 1, 7, and 14 days after SCI.
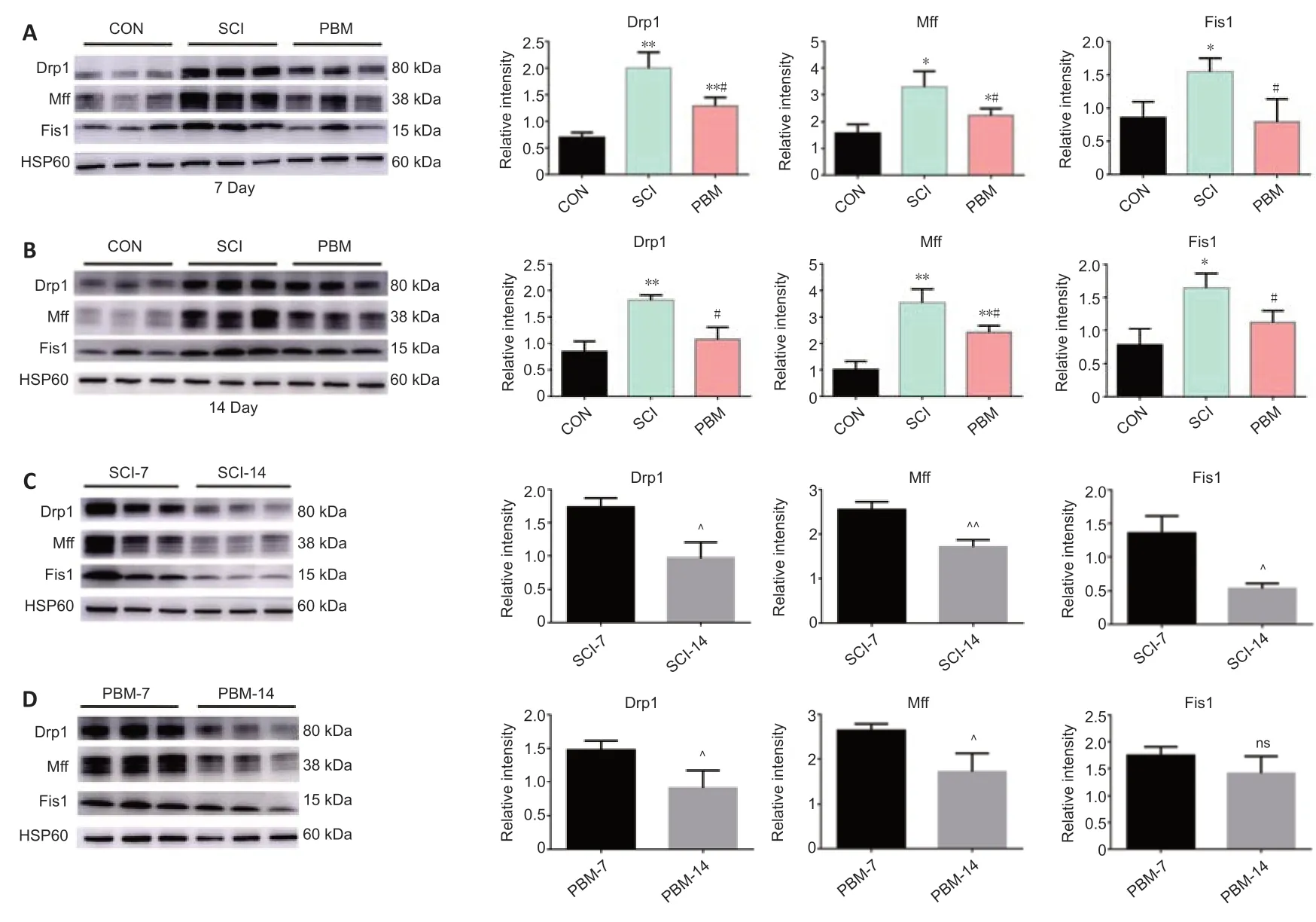
Figure 2|Effects of PBM on mitochondrial fission-related proteins at 7 and 14 days after SCI.
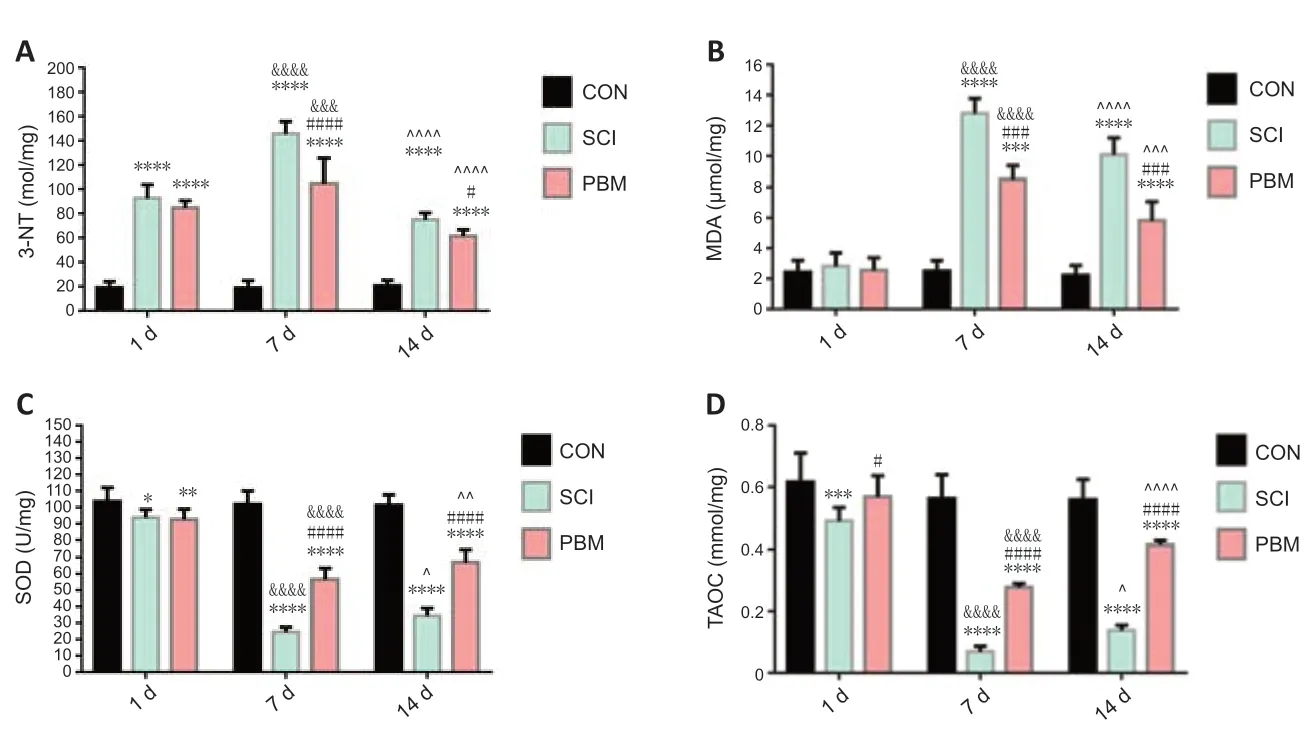
Figure 3|Effects of PBM on mitochondrial oxidative stress at 1, 7 and 14 days after SCI.
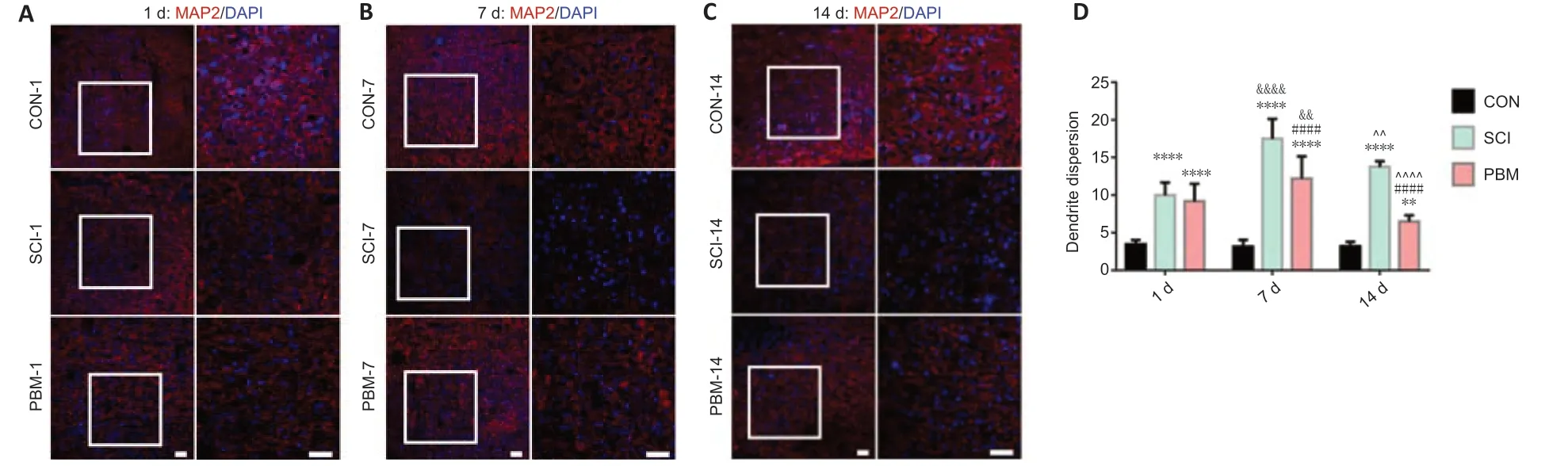
Figure 4|Effects of PBM on neuronal injury at 1, 7 and 14 days after SCI.
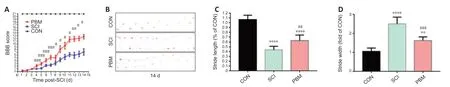
Figure 5|Effects of PBM on motor function recovery after SCI.
Discussion
Previous studies have elucidated that the number and morphology of mitochondria play an indispensable role in neuronal function and cell death, and have shown that restoration of mitochondrial fission imbalance is an effective therapeutic strategy for neuronal damage (Grohm et al., 2012; Li et al., 2015; Liu et al., 2015). Using a rat model of spinal cord crush injury, the present study was the first to explore the potential role and underlying mechanisms of PBM to alleviate mitochondrial dynamics defects in neurons after SCI.
Our data confirmed that mechanical damage resulted in a decrease in the number of mitochondria, swelling, and reduction or even disappearance of cristae in the acute phase after SCI. Mitochondrial alterations in the acute phase likely led to oxidative stress and rapid neuronal death. Our findings suggest that as injury progressed, mitochondrial dynamics transitioned to an imbalance of fission and contributed to worsened outcomes in oxidative stress and neuronal damage. Although PBM ameliorated mitochondrial changes in the acute phase to some extent, it did not improve oxidative stress and neuronal damage. Interestingly, the results suggest that PBM restored oxidative stress and neuronal damage by ameliorating the mitochondrial fission imbalance in the subacute phase in a time-dependent manner. Our findings provide evidence that PBM might be a promising therapeutic strategy for SCI.Research in recent years has demonstrated that impaired mitochondrial fission balance is commonly observed after SCI (Cao et al., 2013; Li et al., 2015; Liu et al., 2015; Jia et al., 2016) and is intimately related to mitochondrial dysfunction resulting in neuronal death. Following SCI in rats, mitochondrial fragmentation was detected at 24 hours (Cao et al., 2013; Jia et al., 2016), and whether mitochondrial fragmentation persists long term has not yet been reported. Mitochondrial division inhibitor-1, a selective Drp1 inhibitor, blocked mitochondrial fragmentation to improve ATP generation, eliminate excessive reactive oxygen species, and contribute to functional neuroprotection (Li et al., 2015; Liu et al., 2015). PBM was demonstrated as an effective physical therapy to restore the imbalance of mitochondrial division (Lu et al., 2017). Our previous research showed that PBM with anin vivooptical fiber implantation may be safe and stable and could be used to directly project light onto the spinal cord surface (Liang et al., 2020).
In the present study, we found that the morphology and number of mitochondria in neurons varied with the prolonged injury time. Compared with the control group, the absolute number of mitochondria per area in SCI group was significantly lower and the majority of mitochondria in SCI group exhibited swollen appearance in acute phase of SCI. However, the absolute number of mitochondria per neuron was significantly higher in SCI group and the majority of mitochondria in SCI group were fragmented in the subacute phase of SCI, suggesting a crucial role of mitochondrial fission imbalance in the subacute phase. Delayed secondary injury usually begins within minutes to hours after SCI and can last for weeks to months, depending on the extent of the injury (Qiao et al., 2010, 2015; Scholpa and Schnellmann, 2017). Consistent with the mitochondrial changes that were observed, there were significant differences between SCI rats in the acute and subacute phases for mitochondrial oxidative stress and neuronal damage, suggesting that mitochondrial fission imbalance worsened outcomes in oxidative stress and neuronal damage of SCI rats in the subacute phase. Our results are the first to provide evidence that mitochondrial fission imbalance in the subacute phase worsens delayed secondary injury in SCI.It has been suggested that Drp1 drives abnormal mitochondrial fission through binding to the outer membrane adaptors Fis1 (Frank et al., 2001; Yu et al., 2013; Hu et al., 2017) and MFF (Otera et al., 2010; Otera and Mihara, 2011), which the aggravates mitochondrial dysfunction, such as energy depletion, oxidative stress, and cell apoptosis, and leads to the loss of function. Drp1-dependent mitochondrial fragmentation has been shown to promote delayed neuronal cell death according to the colocalization of proapoptotic proteins, such as Bax and Bcl-2 antagonist/killer (Arnoult et al., 2005; Karbowski et al., 2006; Montessuit et al., 2010; Landes and Martinou, 2011). In the present study, we demonstrated that the effects of PBM on mitochondrial-related fission proteins, oxidative stress, neuronal injury and locomotor function recovery varied with mitochondrial changes in number and morphology in the subacute phase. Thus, the findings suggest self-repair of the mitochondrial fission imbalance and that the treatment effect of PBM on mitochondrial fragmentation improved with time.
Swollen mitochondria were observed at 1 day after injury, indicating that the mechanical trauma induced immediate mitochondrial damage. PBM partly restored the swollen mitochondrial damage in the acute phase, but it did not improve oxidative stress and neuronal damage, or prevent the deterioration of oxidative stress and neuronal damage, suggesting that PBM could not prevent secondary damage from progressing via restoring the swollen mitochondria in the acute phase. Under physiological conditions, approximately 90% of reactive oxygen species are generated as by-products of mitochondrial metabolism. In the present study, protein oxidation was increased, but not lipid oxidation, suggesting that protein oxidation is a key target for SCI in the acute phase.
There are some limitations to the present study that need to be addressed. Assessment of the behavioral response in the present study was limited to motor function, though previous studies have reported that PBM is also effective against neuropathic pain (Micheli et al., 2017, 2019). The key molecular and signaling mechanisms of PBM in improving neuropathic pain remain unknown. Additionally, this study focused only on the subacute phase after SCI. It remains unknown whether continuous PBM treatment in the chronic phase after SCI would further promote functional recovery and whether the recovery would be maintained after the cessation of PBM therapy. The key molecular and signaling mechanisms of PBM in regulating mitochondrial fission imbalance remain unknown. The issues mentioned above need attention and further investigation.
In conclusion, our findings support that mitochondrial fission imbalance is related to secondary injury progression in the subacute phase of SCI and ultimately to neuronal apoptosis. The findings suggest that PBM provided an effective protective mechanism to prevent neuronal damage and dysfunction caused by mitochondrial fragmentation after SCI in the subacute phase. Thus, the present research suggests that PBM may be a potential and safe interventional strategy for SCI.
Author contributions:Study conception and design: ZW, XL, XYH, XKW; experimental implementation: XL, ZJZ, XSZ, ZWL, TD, KL, XKW; data analysis: XL, ZJZ, YGM, PHL, CJ, ZHZ, ZWS, HLQ, JWZ, LL; manuscript draft: ZW, XL, XYH. All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Lorenzo Di Cesare Mannelli, University of Florence, Italy.
Additional files:
Additional Figure 1:Animal experimental design and PBM administration.
Additional Figure 2:Effects of PBM on mitochondrial morphology at 1 day after SCI.
Additional Figure 3:Effects of PBM on apoptosis at 14 days after SCI.
Additional file 1:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

