Injectable collagen scaffold with human umbilical cordderived mesenchymal stem cells promotes functional recovery in patients with spontaneous intracerebral hemorrhage: phase I clinical trial
Xiao-Yin Li , Wu-Sheng Deng , Zi-Qi Wang , Zheng-Chao Li Shu-Lian Chen Zhen Song Quan Zhang Jin Liang , Xu-Yi Chen
Abstract Animal experiments have shown that injectable collagen scaffold with human umbilical cord-derived mesenchymal stem cells can promote recovery from spinal cord injury. To investigate whether injectable collagen scaffold with human umbilical cord-derived mesenchymal stem cells can be used to treat spontaneous intracerebral hemorrhage, this non-randomized phase I clinical trial recruited patients who met the inclusion criteria and did not meet the exclusion criteria of spontaneous intracerebral hemorrhage treated in the Characteristic Medical Center of Chinese People’s Armed Police Force from May 2016 to December 2020. Patients were divided into three groups according to the clinical situation and patient benefit: control (n = 18), human umbilical cord-derived mesenchymal stem cells (n = 4), and combination (n = 8). The control group did not receive any transplantation. The human umbilical cord-derived mesenchymal stem cells group received human umbilical cord-derived mesenchymal stem cell transplantation. The combination group received injectable collagen scaffold with human umbilical cord-derived mesenchymal stem cells. Patients who received injectable collagen scaffold with human umbilical cord-derived mesenchymal stem cells had more remarkable improvements in activities of daily living and cognitive function and smaller foci of intracerebral hemorrhage-related encephalomalacia. Severe adverse events associated with cell transplantation were not observed. Injectable collagen scaffold with human umbilical cord-derived mesenchymal stem cells appears to have great potential treating spontaneous intracerebral hemorrhage.
Key Words: clinical trial; collagen scaffold; efficacy; human umbilical cord-derived mesenchymal stem cells; human, safe; neurological recovery; spontaneous intracerebral hemorrhage; transplantation
Introduction
Spontaneous intracerebral hemorrhage (ICH) leads to permanent impairment of sensation, movement, speech, and cognition (Hallevi et al., 2009). ICH not only affects quality of life, but also imposes a financial burden on affected patients, their families, and society (Hesami et al., 2015). Although current ICH therapeutic strategies such as neurotrophic factors, surgery, and rehabilitation can extend patient lifespan, treatments that promote neurological recovery are not available (Law et al., 2017; Cao et al., 2020). The primary brain injury caused by ICH is mechanical injury (Li et al., 2021). Additional secondary injury is caused by altered calcium homeostasis, blood cytotoxicity, neurotransmitter excitotoxicity, and inflammation (Qureshi et al., 2003; Aronowski and Zhao, 2011; Yip et al., 2021). The treatment of ICH is challenging because of the complexity of central nervous system pathophysiological processes and the difficulties with neural regeneration. However, transplantation of biomaterial scaffolds combined with stem cells provides a new potential method for regaining neural function after ICH. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) have several advantages over other stem cell types for treating ICH (Nakano and Fujimiya, 2021). They are less controversial than embryonic stem cells and can be readily acquired and used without concern for immunological rejection. Furthermore, hUC-MSCs exhibit rapid self-renewal characteristics (Bonaventura et al., 2020). MSC transplantation is safe and feasible and associated with low risk of malignant transformation (Karussis et al., 2010; Tsai et al., 2018; Bydon et al., 2020; Yang et al., 2020). After spinal cord injury, transplantation of hUC-MSCs can inhibit astrogliosis and microglial activation, secrete trophic factors, modulate the inflammatory response at the site of injury, and promote neural growth (Park et al., 2012b; Urdzíková et al., 2014; Zhou et al., 2015; Wang et al., 2016). Collagen scaffold (CS) can guide nerve growth along its fibers, connect the gaps of severely injured spinal cords, rebuild the microenvironment at the site of injury, and inhibit scar formation (Lin et al., 2006; Stokols and Tuszynski, 2006; Li et al., 2013; Liu et al., 2021). It also acts as a delivery carrier for trophic factors or stem cells to promote neural regeneration (Zhang et al., 2016). Because the pathological mechanisms of ICH are extremely complex, a single therapeutic approach is unlikely to achieve satisfactory outcomes. Previous studies in animal spinal cord injury models have shown that CS combined with hUCMSCs can reduce the lesion area, guide orderly regeneration of nerve fibers, and promote remarkable neurological recovery (Li et al., 2017; Wang et al., 2018). Therefore, this approach has promise for treatment of ICH. However, no relevant clinical studies have been conducted. This study aimed to assess the safety and efficacy of transplantation of injectable CS combined with hUCMSCs in patients with ICH.
Methods
CS preparation
The CS was prepared from bovine aponeurosis (Wanlifa, Beijing, China) as previously reported (Lin et al., 2006; Xiao et al., 2016). Briefly, fresh white aponeuroses were repeatedly rinsed with cold distilled water. The adjunctive tissues, including connective tissue, fat and residual muscles were resected. Then, the samples were repeatedly rinsed and freeze-dried. Standard CS was established at 4°C until use. Biological safety of the CS was evaluated by the National Institutes for Food and Drug Control according to Chinese criteria for medical devices (Xiao et al., 2018).
Isolation, culture, and identification of hUC-MSCs
hUC-MSCs were obtained from healthy newborn umbilical cord. Human umbilical cord research was approved by the Ethics Committee of the Characteristic Medical Center of People’s Armed Police Forces (approval No. 2017-0008; July 20, 2017). Written informed consent was obtained from the newborns’ parents. hUC-MSCs were isolated, cultured, and identified using previously reported methods (Tu et al., 2012; Zhao et al., 2017). Briefly, the blood vessels and amnion were resected from the umbilical cord. The remaining Wharton’s jelly was sliced into tissue pieces (1–2 cm2) and digested with collagenase (Solarbio Science & Technology Co., Ltd., Beijing, China) and 0.25% trypsin (Solarbio Science & Technology Co., Ltd.). Then, the undigested tissue was separated using a 100-µm filter. Cell suspension was seeded in Dulbecco’s modified Eagle’s medium/F12 (Thermo Fisher Scientific Co., Ltd., Shanghai, China) containing 20% fetal bovine serum (MRC Biotechnology Co., Ltd., Changzhou, China), 2 mM glutamine (Sigma-Aldrich, St. Louis, MO, USA), and 100 U penicillin (Solarbio Science & Technology Co., Ltd.) and streptomycin (Solarbio Science&Technology Co., Ltd.). hUC-MSCs were cultured in a cell incubator at 37°C and 5% CO2. The medium was replaced every 3 to 4 days. hUC-MSCs were passaged when they attained 80% confluence. The cell number for transplantation was 1 × 107cells/2 mL. The morphology of the cells was observed under an inverted fluorescence microscope (Leica DMI3000 B, Wetzlar, Germany). Cells were identified using flow cytometry (Beckman CytomicsTMFC 500, Brea, CA, USA) with antibodies against CD105, CD73, CD90, and human leukocyte antigen-antigen D (all from Abcam, Cambridge, UK). hUC-MSCs were also identified by immunofluorescence staining (CD73, CD90, and CD105; Abcam). Cells (1 × 106) were washed in phosphate buffered saline and incubated for 20 minutes at room temperature. Primary antibodies labeled with fluorescein isothiocyanate were used. Mouse isotype antibody was used as control (Tu et al., 2012). Expanded MSCs needed to meet the following conditions for transplantation: 1) ≥ 95% of expanded MSCs were positive for CD73, CD90, and CD105; and 2) < 3% were positive for HLA-DR. MSCs used for transplantation needed to express key MSCs markers (CD73, CD90, and CD105) without expressing the hematopoietic marker HLA-DR (Zhao et al., 2017).
Clinical trial section
Patient selection
The clinical prospective controlled trial protocol was approved by the Ethics Committee of the Characteristic Medical Center of People’s Armed Police Forces (approval No. 2017-0008; July 20, 2017) and conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants. Our reporting follows the guidelines of the Transparent Reporting of Evaluations with Nonrandomized Designs statement (Des Jarlais et al., 2004).
Inclusion criteria were as follows: 1) male or female, age 35 to 75 years; 2) surgery performed > 6 hours after symptom onset; 3) Glasgow Coma Scale (GCS) score 4–14 (Gladstone et al., 2019); and 4) computed tomography (CT) diagnosis of spontaneous ICH (hematoma volume, 20–40 mL). Patients with severe heart failure, severe lung disease, uremia, cirrhosis, end-stage cancer, coagulation disorders, stroke sequelae, and those who experienced asphyxia, cardiac arrest, or cardiopulmonary resuscitation before hospitalization were excluded. We also excluded pregnant or lactating women. Potential patients were assessed for study participation and the legal authorized person of the patient provided informed consent for clinical trial participation.
Thirty-four patients who met criteria were recruited between May 2016 and December 2020. Four were lost to follow-up (one in the control group, one in the hUC-MSCs group, and two in the combination group). Therefore, 30 patients were included for analysis. These patients were divided into three groups according to the clinical situation and patient benefit: control (n= 18), hUC-MSCs (n= 4), and combination (n= 8). In the hUC-MSCs group, 1 × 107hUC-MSCs were transplanted into the hematoma cavity. In the combination group, injectable CS with hUC-MSCs was implanted into the hematoma cavity. In the control group, all patients received conventional treatment. All patients in all groups received the same surgical intervention. The study flow chart is shown in Figure 1.
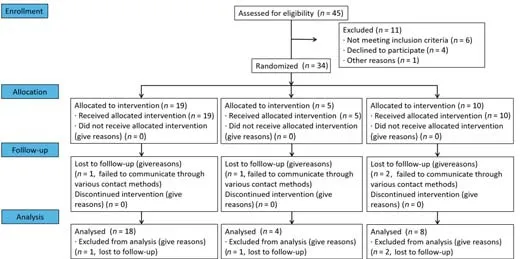
Figure 1|Study flow chart.
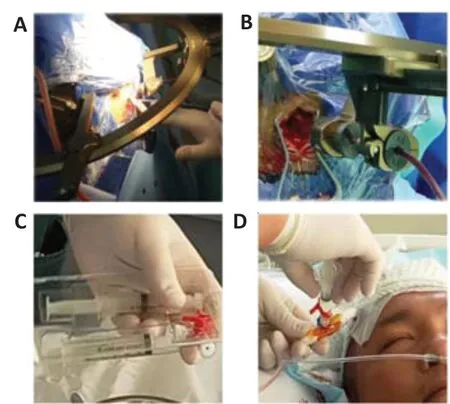
Figure 2|Transplantation procedure.
Surgical procedure and transplantation of hUC-MSCs or CS with hUC-MSCsThe patients underwent general anesthesia with endotracheal intubation. All surgical procedures were performed by the same experienced neurosurgeon. After stereotactic intracranial hematoma localization, a drainage tube was inserted (Figure 2A and B). Patients were monitored in the neurological intensive care unit (ICU) after surgery. The drainage tube was kept in place for 1 to 2 days until adequate drainage of the hematoma. Daily drainage volume was recorded. Before drainage tube removal, 2 mL of injectable gelatinous CS with 1 × 107/mL hUC-MSCs were transplanted into the hematoma cavity (Figure 2C and D) (Xiao et al., 2018). The syringe for loading hUC-MSCs was a conventional 2 mL syringe, which was diluted with 3 to 8 mL of normal saline after injection. Postoperative vital signs, complete blood count, blood electrolytes, blood glucose, hepatorenal function, coagulation function, and blood-gas analysis were monitored and recorded every 4 hours after admission for 7 days after surgery.
Rehabilitation program and nutritional measures
All patients experienced multisystem rehabilitation and exercise training to maximize recovery of muscle strength and sensation function. Each patient received a diet high in protein and fiber and low in carbohydrates and fat.
Neuroelectrophysiology studies
Somatosensory evoked potentials (SEPs) and electroencephalography (EEG) were performed before surgery and 1, 3, 6, 12, 18, and 24 months after. SEPs were performed using the Viking Quest system (Nicolet Biomedical, Madison, WI, USA). EEG was performed using the EEG-1200C (Nihon Kohden, Tomioka, Japan); scalp electrodes were placed using the International 10–20 system. Electrophysiological studies were made by the same neurologist.
For SEP studies of the upper extremities, electrodes were positioned at the wrist to stimulate the median nerve. Recording electrodes were placed on the contralateral C3’-Fz and Cz’-Fz, respectively. The stimulating intensity was enough to cause a visual contraction of the targeted muscles (time, 200 µs; intensity, 35–50 mA). SEP latency and amplitude were recorded.
EEG parameters were as follows: time constant, 0.3 seconds; and high frequency filter, 70 Hz. The criteria for abnormal EEG were as follows: (1) Mild abnormality-waveforms were mostly α or β activity with low to medium amplitude, scattered irregular θ activity in the medium amplitude; (2) moderate abnormality-waveforms were mostly irregular θ activity in the medium amplitude, α or β activity was weakened, and a small amount of δ activity appeared; and (3) marked abnormality-waveforms were mostly polymorphic δ activity or irregular θ activity with medium to high amplitude, α or β activity was obviously weakened, and occasional sharp and spinous waves.
Head CT
Head CT was performed before surgery and 1, 4, 7 days and 1, 3, 6, 12, 18, and 24 months post-surgery. Scans were performed using a 64-slice Magnetom Verio scanner (Siemens, Munich, Germany) with 5 mm slices. Hematoma volume on CT was determined using the Coniglobus formula. CT scans were performed by the same radiologist. CT results were determined by another radiologist.
Clinical assessments and postoperative follow-up
At 1, 3, 6, 12, 18, and 24 months post-surgery, clinical scores were recorded for the following scales: orientation, myodynamia, and walking ability (Table 1); visual analog scale (VAS) for neuropathic pain (Chang et al., 2021); Barthel index (a measure of activities of daily living); mini-mental state examination (MMSE); and the Brunnstrom scale (Staszewski et al., 2022). Patients were permitted to take analgesic medications according to their conditions. Clinical assessments were performed by the same neurologist to eliminate interrater variability.
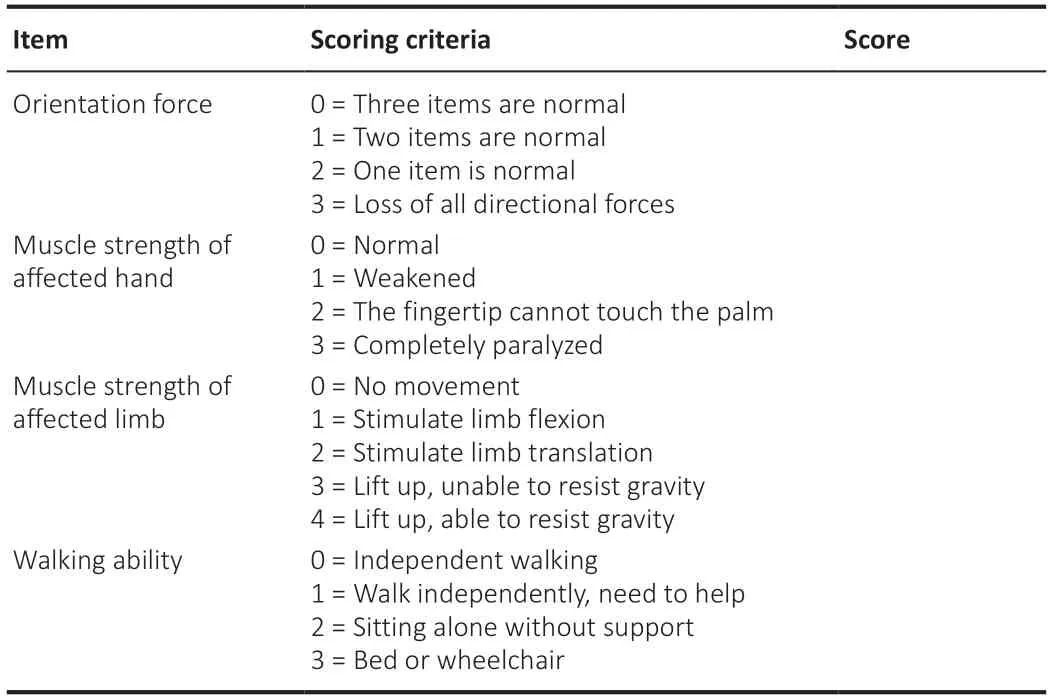
Table 1 |Evaluation Form of orientation force, muscle force and walking ability
Length of stay
Length of hospitalization and length of time in the ICU were recorded.
Adverse events
Complications and adverse reactions were recorded and handled in a timely manner.
Statistical analysis
Statistical analyses were performed using SPSS software version 15.0 (IBM Corp., Armonk, NY, USA). Data are presented as means ± standard deviation.P< 0.05 was considered significant. Categorical data were compared between groups using the chi-square test. Continuous data were compared using the Mann-WhitneyUtest. Multiple-group comparisons of time after ICH and GCS score were performed using one-way analysis of variance.
Results
Patient characteristics
Patient characteristics are summarized in Figure 3. Age, sex, time after ICH, hematoma volume, and GCS score did not significantly differ between groups.

Figure 3|Patient characteristics.
Orientation, myodynamia, and walking ability
Orientation, myodynamia, and walking ability scores according to group over time are shown in Figure 4A. Group scores did not significantly differ at any time point. At 24 months, the mean score was lower in the combination group than the control and hUC-MSCs groups; however, the difference was not significant.
Barthel index
Barthel index scores according to group over time are shown in Figure 4B. At 24 months, the mean score was significantly higher in the combination group than the control group (P< 0.05).
Brunnstrom scale
Brunnstrom scale scores according to group over time are shown in Figure 4C. Scores did not significantly differ at any time point.
VAS score
VAS pain scale scores according to group over time are shown in Figure 4D. Mean score was significantly lower in the combination group than the control group at 1 month (P< 0.01). Mean scores at other time points did not significantly differ between the groups.
MMSE scale
MMSE scores according to group over time are shown in Figure 4E. At 18 and 24 months, mean score was significantly higher in the combination group than the control group (P< 0.05). These results suggest that transplantation with CS and hUC-MSCs improved cognitive function.
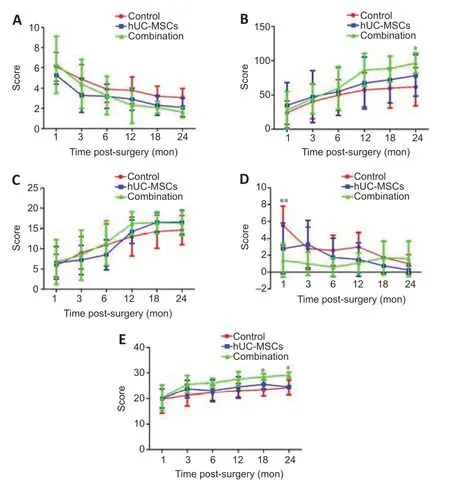
Figure 4|Functional outcome scores in the three experimental groups over time.
SEPs
Compared with the levels in the control group, SEP amplitude was higher and latency was shorter in the combination group at 24 months (Figure 5). These results suggest that transplantation of CS and hUC-MSCs accelerated recovery of locomotor function.
EEG
At 24 months, EEG detection showed occasional slow wave, α or β activity and θ activity in the control, hUC-MSCs and combination groups, but no spikes or sharp waves, or spike and wave discharges. The proportion of patients with α or β activity and θ activity in hUC-MSCs group and combination group was significantly higher than that in control group (P< 0.05; Figure 6). These findings suggest that transplantation of CS and hUC-MSCs did not increase the risk of epilepsy.
CT findings
In the control and hUC-MSCs groups, the post-ICH foci of encephalomalacia did not change much over follow-up (Figure 7A–J). Hematoma volume before surgery in the three groups is shown in Figure 3D. In contrast, the post-ICH foci of encephalomalacia in the combination group decreased in size or disappeared (Figure 7K–O). No sign of tumorigenesis was found at the implantation site. These results suggest that transplantation of CS and hUCMSCs accelerated central nervous system regeneration.
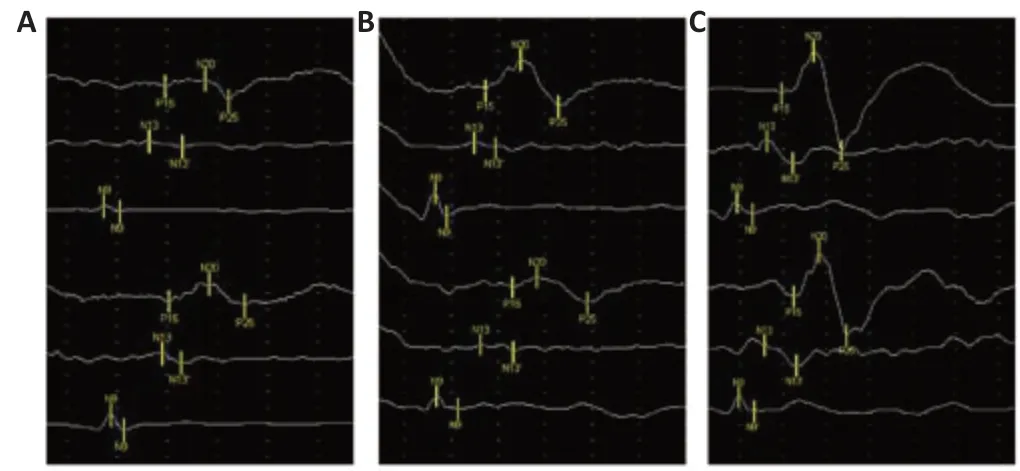
Figure 5|Representative SEP tracings.
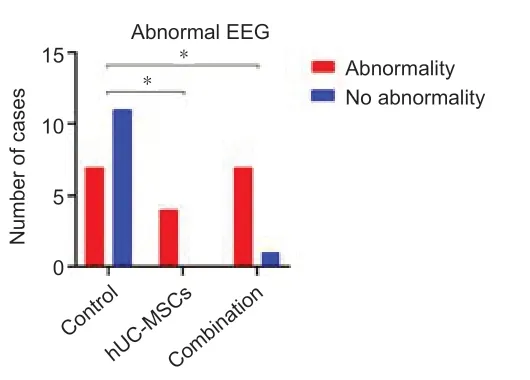
Figure 6|Comparison of the proportion of ICH patients with frequent α or β activities and θ active EEG among the three groups.
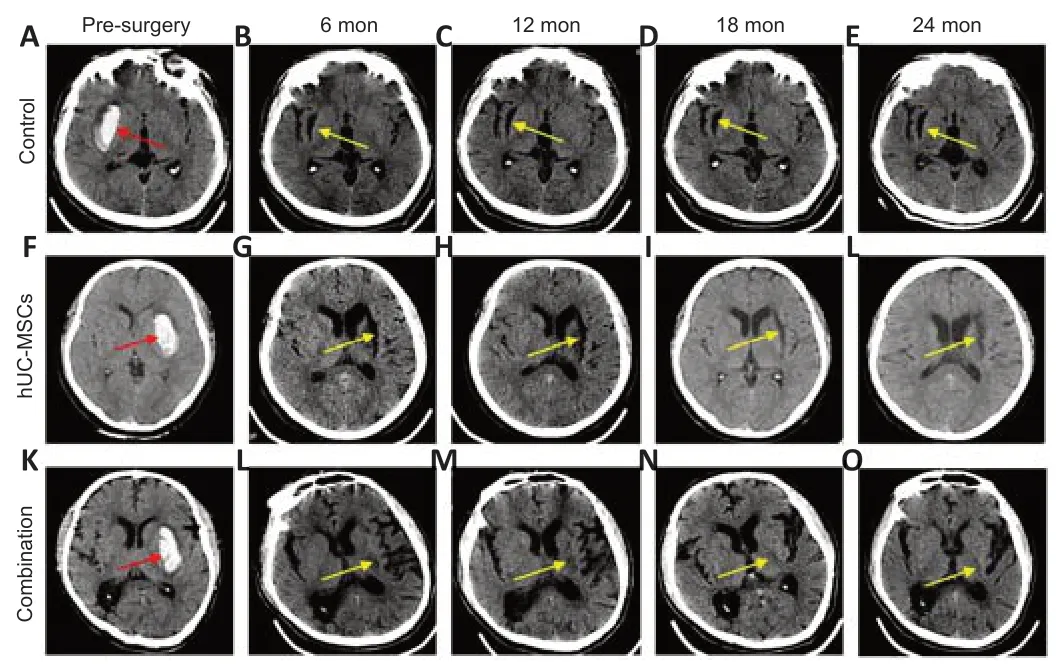
Figure 7|Computerized tomography (CT) imaging before and after surgery in the three groups.
Safety
Adverse events are shown in Table 2. Seizure, coagulation complications, intracranial infection, and cardiovascular complications did not occur. Nine patients experienced pneumonia. No adverse events were considered transplantation-related. Intracranial rebleeding, deep vein thrombosis, and pneumonia occurred in the control and hUC-MSCs groups. Length of hospitalization and length of ICU stay did not significantly differ between the combination group and the control group or hUC-MSCs group (Figure 8A and B).

Figure 8| Length of hospital stay and NICU stay according to group.
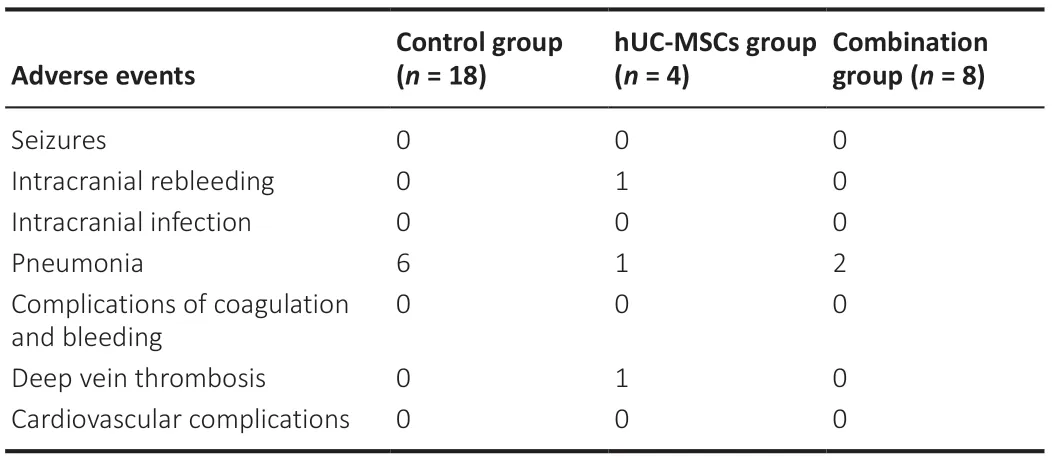
Table 2|Adverse events in control, hUC-MSCs and combination groups
Discussion
ICH can be neurologically devastating and can impose an economic burden on families and society. However, there is currently no effective treatment (Rouanet et al., 2017; Qian et al., 2021). In our study, transplantation of CS and hUC-MSCs resulted in higher Barthel index, VAS, and MMSE scores; increased frequency of α, β, and θ waves on EEG, and smaller foci of encephalomalacia on CT. No serious adverse reactions such as seizure, intracranial infection, myocardial infarction, or tumor were observed over 24 months of follow-up. These findings suggest that transplantation of CS and hUC-MSCs is safe and effective for treating patients with ICH.
Transplantation of CS and hUC-MSCs did not increase the length of hospital stay. However, several mild adverse events occurred, including pneumonia and urinary tract infection, which resolved after antibiotic administration. In the hUC-MSCs group, one patient developed deep venous thrombosis. These adverse events are unlikely to be directly related to transplantation.
Various types of biomaterial have been explored for brain injury repair in animal models, including chitosan, hyaluronic acid, and fibrous protein (Jiang et al., 2021). Collagen has been considered a suitable biomaterial because of its accessibility, biocompatibility, biodegradability and low immunogenicity (Rezvani Ghomi et al., 2021). Recent clinical trials examining transplantation of CS have demonstrated its safety (Xiao et al., 2016, 2018; Zhao et al., 2017). Similarly, transplantation of hUC-MSCs to treat ICH has been a recent area of focus. In our study, severe adverse events such as tumorigenesis and infection were not associated with transplantation. Zahra et al. (2020) reported that hUC-MSC transplantation is safe in patients with ICH. Larocca et al. (2017) reported that image-guided percutaneous intralesional administration of hUC-MSCs is safe in patients with spinal cord injury. The safety of hUC-MSC transplantation is primarily owed to advances in cellular harvesting, isolation, culturing and evaluation. To more thoroughly evaluate adverse events, studies with follow-up longer than 2 years are needed.
Activities of daily living were improved after implantation of CS with MSCs. Compared with the control group, ADL scores for getting dressed and moving significantly increased in the combination group at 24 months, which indicated that the treatment improved self-care ability. Barthel index scores were higher in the combination group than the control group, suggesting a positive effect on activities of daily living. Surprisingly, some patients showed improvement in Barthel index score without improvement in Brunnstrom scale score. This was likely related to long-term training. Long-term follow-up will be intriguing.
Electrophysiological examinations are useful for evaluating sensory function after ICH. They can provide a quantitative assessment of reticular circuitry changes and assist with comprehending restoration mechanisms, such as myelinization and synaptization (Lu et al., 2012; van Gorp et al., 2013; Rosenzweig et al., 2018). The mechanism of an intervention used for promoting recovery of neurological function may be embodied in changes in electrical signals. In our study, EEG examination 24 months after transplantation found that patients in the hUC-MSCs and combination groups showed greater α or β activities and θ activity compared with the control group; however, all patients were free of epileptic waves such as spike wave and spike slow wave. Alpha and β are medium and high frequency activities that represent processing of different information and are a sign of sensorimotor activity. Beta activity in particular is the main component of the excitation mechanism while θ is related to mental work (Müller-Putz, 2020).The increased frequency of these waveforms indicated that CS and hUC-MSCs transplantation increased the electrical activity of the brain. It may be that CS and hUC-MSCs transplantation promoted the remodeling of neural pathways or neural repair and regeneration. SEPs recovered 2 years after transplantation. Furthermore, some patients in the combination group showed positive changes in upper extremity SEPs. SEP amplitude was higher and latency was shorter in the combination group, suggesting that transplantation of CS combined with hUC-MSCs improves conductivity repair in ICH (Lu et al., 2012). Electrophysiological improvement after implantation may be attributed to several mechanisms including remyelination, reconnection of damaged nerve fibers, and/or new neural circuitry activity (Curt and Dietz, 1999; Xie and Boakye, 2008).
In addition to motor and sensory function, assessment of cognitive function is required to fully evaluate neurological recovery after ICH. Cognitive dysfunction is common after ICH and may be severe (Scopelliti et al., 2022). Furthermore, it responds poorly to conventional treatment. We observed that MMSE scores were significantly higher in the combination group than the control group. Similarly, Yip et al. (2021) reported mild improvement in cognitive function after hUC-MSC transplantation. Transplantation appears to be beneficial for improving cognitive function.
As stem cells secrete prostaglandins and cytokines, they can trigger nociceptors, regulate the expression of sensory neuron genes, and activate aberrant changes in central nervous system networks that lead to neuropathic pain, spasticity, or dystonia (Hofstetter et al., 2005; Macias et al., 2006). Furthermore, several studies have reported that stem cell transplantation increases the risk of neuropathic pain (Hofstetter et al., 2005). In our study, patients who underwent transplantation of CS with hUC-MSCs reported lower VAS pain scores 1 month after surgery than those in the other two groups. Furthermore, pain scores in these patients did not significantly increase later in the study period. Therefore, transplantation of CS with hUC-MSCs did not seem to increase neuropathic pain.
In our study, patients in the combination group showed a reduction or disappearance in the foci of ICH-related encephalomalacia. However, these foci persisted in the control and hUC-MSC group patients. Chen et al. (2013) reported CT results after hUC-MSC therapy in stroke patients that are similar to ours. The reduction or disappearance of the foci of encephalomalacia may reflect axonal regeneration. At present, brain injury researchers are focusing on diffusion tensor imaging (Douglas et al., 2018). Diffusion tensor tractography, which intuitively describes the distribution of white matter fiber bundles, can quantitatively evaluate white matter tracts by measuring diffusion of water molecules and accurately predict neurological recovery (Cho and Jang, 2021). Use of diffusion tensor tractography may increase the reliability of our results.
Cell delivery for transplantation in the central nervous system can be performed via intravenous, intrathecal, and intramedullary injections. Intravenous delivery is easiest but has unsatisfactory results because the transplanted cells must penetrate the blood-brain barrier (Pal et al., 2009; Mäkelä et al., 2015). Intrathecal injection is more conducive (Bakshi et al., 2004; Paul et al., 2009) but has several disadvantages. First, a large number of stem cells is required. Second, intramedullary or subarachnoid adhesions may block stem cells from migrating to the target. Third, therapeutic stem cell homing effects may not occur in ICH (McColgan et al., 2011; Saberi et al., 2011; Park et al., 2012a). Although many studies have reported the use of intramedullary injection for transplantation (Saberi et al., 2011; Park et al., 2012b; Jiang et al., 2013; Larocca et al., 2017), this strategy did not achieve significant effects in ICH patients. Cerebral edema, ischemia, free radical production, necrosis, and hemorrhage occur in the area of brain injury in these patients. Hematoma that cannot be removed impedes neural regeneration (Lukovic et al., 2015). Surgical hematoma removal is a direct approach for eliminating the impediment. We speculate that surgical removal potentially affects neurological improvement by improving the hematoma microenvironment.
In conclusion, our study has some encouraging results, which indicate that transplantation of hUC-MSCs and CS is safe and effective in ICH patients. However, it has several limitations, especially the number of patients. Initially, patients were included and excluded in strict accordance with the experimental protocol. However, some patients withdrew from the trial for various reasons. Considering experimental preciseness, it was difficult to recruit new volunteers because the trial’s uncertainty and the COVID-19 pandemic. We will make further improvements in future studies. We hope that this trial can provide the basis for a new method of ICH treatment in the future and that future studies will take the shortcomings of this trial into account, so as to obtain more complete and useful experimental results.
Acknowledgments:We thank Professor Sai Zhang (Yantai Zhenghai Biotechnology Co., Ltd.) for financial assistance, and thank to the team of Professor Jian-Wu Dai (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for technical support.
Author contributions:Study design: XYL, WSD, XYC; trial implementation: XYL, WSD, ZQW, ZCL, SLC, ZS, QZ, JL, XYC; trial: XYL, WSD, ZQW; material contribution and equipment coordination: JL, XYC; manuscript writing: XYL, WSD. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Nemil N. Bhatt, The University of Texas Medical Branch at Galveston, USA.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

