Serum response factor promotes axon regeneration following spinal cord transection injury
Guo-Ying Feng , Nai-Li Zhang , Xiao-Wei Liu , Ling-Xi Tong Chun-Lei Zhang Shuai Zhou Lu-Ping Zhang Fei Huang
Abstract Studies have shown that serum response factor is beneficial for axonal regeneration of peripheral nerves. However, its role after central nervous system injury remains unclear. In this study, we established a rat model of T9–T10 spinal cord transection injury. We found that the expression of serum response factor in injured spinal cord gray matter neurons gradually increased with time, reached its peak on the 7th day, and then gradually decreased. To investigate the role of serum response factor, we used lentivirus vectors to overexpress and silence serum response factor in spinal cord tissue. We found that overexpression of serum response factor promoted motor function recovery in rats with spinal cord injury. Qualitative observation of biotinylated dextran amine anterograde tracing showed that overexpression of serum response factor increased nerve fibers in the injured spinal cord. Additionally, transmission electron microscopy showed that axon and myelin sheath morphology was restored. Silencing serum response factor had the opposite effects of overexpression. These findings suggest that serum response factor plays a role in the recovery of motor function after spinal cord injury. The underlying mechanism may be related to the regulation of axonal regeneration.
Key Words: axon; growth associated protein 43; motor function; myelin sheath; neuron; regeneration; serum response factor; spinal cord; spinal cord transection
Introduction
The transcription factor serum response factor (SRF), widely expressed in organisms, performs numerous functions, and is vital for cell survival and differentiation, such as regulating the differentiation of skeletal muscle, smooth muscle, and the maturation of cardiomyocytes, and the metastasis and progression of cancer (Kim et al., 2009; Horita et al., 2016; Guo et al., 2018; Kwon et al., 2021; Onuh and Qiu, 2021). In general, SRF performs biological activities by directly binding to the serum response element at the promoter of the target gene. SRF binds to the skeletal α-actin promoter to initiate the differentiation of skeletal muscle (Kim et al., 2009). In the nervous system, SRF also exhibits important effects, especially in promoting axonal growth. Stern et al. (2013) confirmed that SRF depletion in the peripheral nervous system reduced regeneration of neuronal axons and growth cones. SRF does not act as a transcription factor, but needs translocation from the nucleus to the cytoplasm (Stern et al., 2013). Although there is evidence that SRF contributes to axonal generation in the peripheral nervous system, its role and mechanism in the repair of spinal cord injury (SCI) still needs to be explored.
In the present study, we examined the changes in SRF expression in spinal cord tissue of rats with SCI. Furthermore, we investigated the role of SRF by constructing a lentivirus, which was injected locally into the injured spinal cord with overexpressed or silenced SRF. Behavior, morphology, and molecular biological methods were used to understand the role of SRF in rats with SCI.
Methods
Animals
The long urethra of male rats is not conducive to urination after SCI, so only female rats were used in this study. Specific-pathogen-free level 2-monthold female Sprague-Dawley rats weighing 200 ± 20 g were purchased from Pengyue Experimental Animal Breeding Co., Ltd (Jinan, Shandong, China; license No. SCXK (Lu) 2019-0003) and raised in Experimental Animal Center of Luye Pharmaceutical Group (Yantai, Shandong, China). This study was approved by the Institutional Animal Care and Use Committee of Binzhou Medical University (approval No. 2016-168) on October 20, 2016. Rats were raised in a dry and clean environment (temperature 22 ± 2°C and relative humidity 45 ± 10%) under a 12-hour light/dark cycle. Food and water were availablead libitumat all times. All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Establishment of a SCI model
Fifty-four rats were randomly divided into two groups: normal (n= 5) and SCI groups (n= 45). Rats with SCI were anesthetized with 1% pentobarbital sodium (40 mg/kg body weight, Sigma, St. Louis, MO, USA) through intraperitoneal injection and kept in a prone position to prepare for surgery. A 1.5-cm midline skin incision under antiseptic condition was made, and paravertebral muscle was dissected bluntly to expose the spinal lamina and spinous process. Subsequently, laminectomy was performed between T9 and T10 to fully expose the spinal cord approximately 1 cm in length. Complete transection of the spinal cord was performed using microscissors between T9 and T10. The cut end of spinal cord was lifted by small forceps to ensure the completeness of the transection. At this time, the muscle tension of the lower limbs and the tail completely disappeared, accompanied by arch reversal, indicating that the spinal cord transection (SCT) model was successfully established (Zhang et al., 2017). Antibiotics (cefoxitin sodium) were locally administered to prevent postoperative infection, and the incision was disinfected and sutured. After SCT, body temperatures of rats were maintained and their bladders were expressed three times a day to assist in urination. Bedding was replaced as needed to maintain a clean and dry environment. The rats in the normal group were maintained under the same environmental conditions without surgery.
Construction of lentivirus of SRF overexpression and silencing
TheSRFgene sequence (gene serial No. NM_001109302) was acquired from the National Center for Biotechnology Information. Lentivirus of SRF overexpression (LV-srf) and silencing (srf-RNAi), and an empty vector (negative control, NC), all of which of expressed green fluorescent protein (GFP), were constructed by Jikai Genechem (Shanghai, China) according to the Lentiviral Vector Production Packaging protocol. Two potential small interfering RNA (siRNA) sequences (detailed information shown in Table 1) targeting the SRF mRNA were designed to silence SRF expression. The most effective one was identified by local injection into the spinal cord of rats with SCI.

Table 1 |siRNA sequences for SRF silencing
Determination of the optimal virus titer
PC12 cells (iCell Bioscience Inc., Shanghai, China, Cat# iCell-r024, identified by multiplex PCR system) were used to detect the optimal titer of lentivirus. Once the confluence of PC12 cells reached 60%, the medium (Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 [DMEM/F12], Gibco, Grand Island, NY, USA), was replaced with serum-free medium containing lentivirus and polybrene (5 µg/mL, Solaibao, Beijing, China, Cat# H8761) for approximately 24 hours at 37°C in an incubator with 5% CO2. Subsequently, the medium was exchanged with fresh DMEM/F12, and the appropriate time to change the medium was determined by the cell state dynamically observed under an inverted microscope (CKX41, Olympus, Tokyo, Japan). Seventy-two hours later, the cells were digested by trypsin (0.25%, Gibco, Cat# 15090046) and collected. Then, the cell suspension and trypan blue solution (0.4%, Gibco, Cat# 15250061) were mixed (9:1), and the ratio of living cells was counted under the inverted microscope. The transfection efficiency was determined by cell viability and fluorescence intensity with inverted fluorescence microscopy (LEICA DMIL LED3040105, Leica).
Animal grouping and lentivirus injection
Another 60 rats were randomly divided into four groups: normal (no treatment), NC (administered blank vector immediately after SCT), LV-srf (administered SRF-overexpressed lentivirus), and srf-RNAi (administered SRFsilenced lentivirus), with 15 rats in each group. All rats were evaluated before and after injury until the testing was completed.
Once the SCI model was successfully made, the broken end of the spinal cord was fully exposed for lentivirus injection. The injection site was located at 0.5 cm from the spinal cord cross section and 1 mm from both sides of the back central vein. Lentivirus (10 µL, titer 1 × 108TU/mL) was uniformly and slowly injected into the spinal cord. The needle was inserted vertically at the long axis of the spinal cord and to a depth of 1.5 mm. After the injection was complete, the needle was left in place for approximately 3 minutes, and then slowly removed to prevent loss of the lentivirus suspension.
Sample preparation
After normal and SCI rats were deeply anesthetized with 1% pentobarbital sodium (60 mg/kg, intraperitoneally), heart perfusion was performed with physiological saline (0.9%) to replace the blood until the liver was pale. Perfusion was then continued with 4% paraformaldehyde for approximately 30 minutes for preliminary fixation. Then, samples 0.5-cm distal from the injured section were prepared quickly on ice and further fixed in paraformaldehyde. Rats used for quantitative polymerase chain reaction (qPCR) and western blotting detection were perfused only with physiological saline after anesthesia. Finally, the spinal cord tissue distal from the cross section was quickly harvested on ice and stored at –80°C for further detection.
Immunohistochemical analysis
Spinal cord tissue fixed by paraformaldehyde was rinsed, dehydrated by alcohol gradient, cleared with xylene, immersed in wax, and embedded with paraffin. The paraffin sections used for immunohistochemical analysis were 4 µm thick. For the experiment, nine tissue sections per group were chosen, dewaxed with xylene, hydrated by alcohol gradient and microwaved for antigen retrieval. Endogenous peroxidase was inhibited by 3% hydrogen peroxide and 5% goat serum (Beyotime Biotechnology, Nanjiang, China, Cat# C0265) was used to block non-specific antigens. Then, anti-SRF antibody (mouse, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, Cat# sc-25290, RRID: AB_2239787) was added and the tissue slices were incubated at 4°C overnight. Subsequently, the sections were rinsed with phosphate buffered saline three times, and then secondary antibody labeled with horseradish peroxidase (goat anti-mouse IgG, 1:1000, Abcam, Cambridge, UK, Cat# ab205719, RRID:AB_2755049) was added to the sections for 30 minutes at 37°C. Diaminobenzidine was then added at room temperature (approximately 26°C), and the result was observed with a microscope (DM6000B, Leica, Wetzlar, Germany). The reaction was terminated with water. Finally, photographs were captured, and five fields in the gray matter selected under high magnification (40×) were used to count the number of SRF-positive cells in each section.
Quantitative polymerase chain reaction
Total RNA of frozen spinal cord tissue was isolated using Trizol reagent (Thermo Fisher Scientific) and reversed transcribed to complementary DNA with the reverse transcription kit (TaKaRa, Kyoto, Japan), according to the manufacturer’s instructions. All the primer sequences (Table 2) were synthetized by Sangon Biotech Co., Ltd (Shanghai, China). PCR was performed according to the following protocol: 95°C for 30 seconds; 40 cycles of 95°C for 5 seconds, 60°C for 30 seconds, and 73°C for 10 seconds. SRF mRNA relative expression was determined by normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values using the 2–ΔΔCtmethod (Yildiz Gulhan et al., 2022).

Table 2 |The detailed information of primers for qPCR
Western blotting
Total protein was extracted from rat spinal cord tissue, and the protein concentration was determined by the bicinchoninic acid method (Reichelt et al., 2016). Then, loading buffer was added to sufficiently denature the protein by boiling for 10 minutes. Total protein was resolved in 15% sodium dodecyl sulfate-polyacrylamide gel, using the electrophoresis buffer at 60 V for 30 minutes, followed by 100 V for 1.5 hours. After separation, the protein was transferred to polyvinylidene fluoride membranes at 200 mA for 4 hours, and then the membrane was blocked with 5% nonfat milk for 2 hours at room temperature. The polyvinylidene fluoride membranes were incubated with mouse anti-SRF antibody (1:1000, Santa Cruz Biotechnology, Cat# sc-25290, RRID: AB_2239787), rabbit anti-growth associated protein 43 (GAP43) antibody (1:2000, Abcam, Cat# ab75810, RRID: AB_1310252) and mouse anti-GAPDH (1:10,000, Proteintech, Wuhan, China, Cat# 60004-1-Ig, RRID: AB_2107436) in Tris-buffered saline overnight at 4°C. Then, the membranes were rinsed in Tris-buffered saline with Tween-20 three times, and further incubated for 2 hours at room temperature (approximately 25°C) with the appropriate secondary antibody (goat anti-mouse IgG, 1:10,000, Cat# ZB2305, RRID: AB_2747415; or goat anti-rabbit IgG, 1:10,000, Cat# ZB2301, RRID: AB_2747412, both ZSGB-BIO, Beijing, China). Finally, the membranes were rinsed again and developed with the chemiluminescence imaging system (Clinx, Shanghai, China) with enhanced chemiluminescence (KeyGEN BioTECH, Nanjing, China). The optical density ratio of SRF to GAPDH (an internal control) was analyzed by ImageJ software (version 1.42q, National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Behavioral assessment
Locomotor function was assessed using the Basso, Beattie, and Bresnahan (BBB) motor rating scale by two independent experimenters skilled in the BBB method for 4 weeks after SCI operation. First, the rats were placed in a circular basin (diameter 1.5 m) for 10 minutes to be familiarized with the surrounding environment, and then their locomotive function was observed after the basin wall was hit. Coordination of rats was evaluated, as well as the extent and range of hindlimbs, including the ankle, knee and hip joints. Rat BBB scores range from 0 (no visible hindlimb movement) to 21 (normal hindlimb movement) (Basso et al., 1995).
Transmission electron microscopy
After rats received heart perfusion with 4% paraformaldehyde following deep anesthesia and saline perfusion, caudal spinal cord tissue (T10) was isolated and excised, then further trimmed to 1 mm3and preserved in glutaraldehyde overnight. The proximal and distal ends were marked. The next day, the spinal cord tissue was further fixed in 1% OsO4for 2 hours, and then dehydrated by alcohol gradient and acetone before the tissue was embedded in resin. Neuronal axons and surrounding myelin were observed and captured by transmission electron microscope (JEM-1400, JEOL, Tokyo, Japan) after contrast-staining with uranyl acetate and lead citrate.
Biotinylated dextran amine anterograde tracing
At 14 days after SCI, anesthetized rats were placed on a head positioning instrument. After routine preparation, the skull was fully exposed and four injection points located at the cerebral cortex managing hindlimb movement (Zhao et al., 2011) were determined by crown seam and sagittal seam: 2 mm from the coronary seam and 2 mm lateral to the sagittal seam. Four holes (1.5 mm deep) were drilled with oral high-speed turbine diamonds, and 10% biotinylated dextran amine (BDA; 2 µL/hole, Invitrogen, Carlsbad, CA, USA, Cat# N7167) was slowly injected into the cortex through the hole by a microsyringe. The needle was taken out after 2 minutes, and the holes were filled with gelatin material. After that, the skin was sutured. Fourteen days later, the spinal cord tissue 0.5 cm below the cross section was excised to detect BDA expression by immunohistochemistry (ABC, Invitrogen) (Butler et al., 2019; Jiang et al., 2019).
Immunofluorescence staining
Caudal spinal cord tissue (T10) obtained 28 days after SCI and fixed by paraformaldehyde was successively immersed in 15% and 30% sucrose/paraformaldehyde solution until the tissue settled to the bottom. Then, the tissue was embedded with optimal cutting temperature compound (Sakura Finetek, Japan, Cat# 4583) and placed in a cryostat at –20°C for quick freezing approximately 10 minutes before sectioning. The thickness of frozen sections used for immunofluorescence staining was 5–10 µm. The tissue was circled by an immunohistochemical pen (Biosharp, Hefei, China, Cat# BC003) and rinsed with phosphate buffered saline, and then 5% goat serum (ZSGB-BIO, Beijing, China, ZLI-9022) was added (37°C for 30 minutes) to block non-specific binding. Subsequently, mouse anti-GAP43 (1:200, Santa Cruz Biotechnology, Cat# sc-17790, RRID: AB_627660) and rabbit anti-Nogo A (1:200, Invitrogen, Cat# 36-6600, RRID: AB_2533273) were added to the tissue sections and incubated at 4°C overnight. After the sections were rinsed with phosphate buffered saline three times, secondary antibody goat anti-mouse (Alexa Fluor 488, 1:100, Invitrogen, Cat# A-11094, RRID: AB_221544) and goat anti-rabbit (Alexa Fluor 594, 1:200, ZSGB-BIO, Cat# ZF-0516) were added to the sections (37°C for 2 hours). Then, after three washes with phosphate buffered saline, the slides were covered with coverslips. Finally, the sections were observed and photographed under a fluorescence microscope.
Statistical analysis
The number of animals (n= 3–10) was based on the sample sizes of relevant research (Liu et al., 2022). The evaluators were blinded to the experimental grouping. All data in the study are presented as mean ± standard deviation. Statistical analysis was performed with SPSS version 17.0 (SPSS, Chicago, IL, USA) and comparisons between groups were analyzed with one-way analysis of variance followed by Tukey’spost hoctest and independent samplest-test.P< 0.05 was considered statistically significant.
Results
SRF expression in normal and injured rat spinal cord
Immunohistochemistry was used to determine the cellular localization and expression of SRF in the spinal cord tissue of rats. SRF was mainly localized at the nucleus and cytoplasm of gray matter neurons in normal and injured spinal cord tissue, and varied at different time points after SCI. Compared with that of the normal group, the number of SRF-positive cells in the SCI group was increased on the 1stday after SCI, reached the maximum on the 7thday, then was decreased on the 14thday, and eventually reached an expression level similar to that of the normal group (Figure 1A and B).
qPCR and western blotting experiments were used to further verify SRF expression in the injured spinal cord, and the results were consistent with those acquired by immunohistochemistry. qPCR, western blotting and immunohistochemistry showed that SRF gradually increased after SCI, and reached the highest level on the 7thday, then gradually decreased, and finally returned to similar to the normal level on the 28thday after SCI (Figure 1C–E).
Construction and verification of lentiviral vector for SRF overexpression and silencing
To explore the function of SRF in SCI rats, human immunodeficiency virusbased vectors, the most popular and effective lentivirus-based expression system, were used. First, transfected PC12 cells were applied to screen the optimal titer of the lentivirus by considering the cell status and fluorescence intensity (Figure 2A and B). Then, verification for viral transfectionin vivowas performed. One week after injection of GFP-labeled LV-srf into the spinal cord tissue, a high expression of green fluorescence was detected under a fluorescence microscope (Figure 2C and D). qPCR and western blotting were used to determine the difference of SRF expression between the NC and normal groups, and showed that the empty vector had no significant effect on SRF expression level. Compared with the NC and normal groups, the SRF overexpressed and silenced groups had significant differences in SRF expression level (Figure 3).
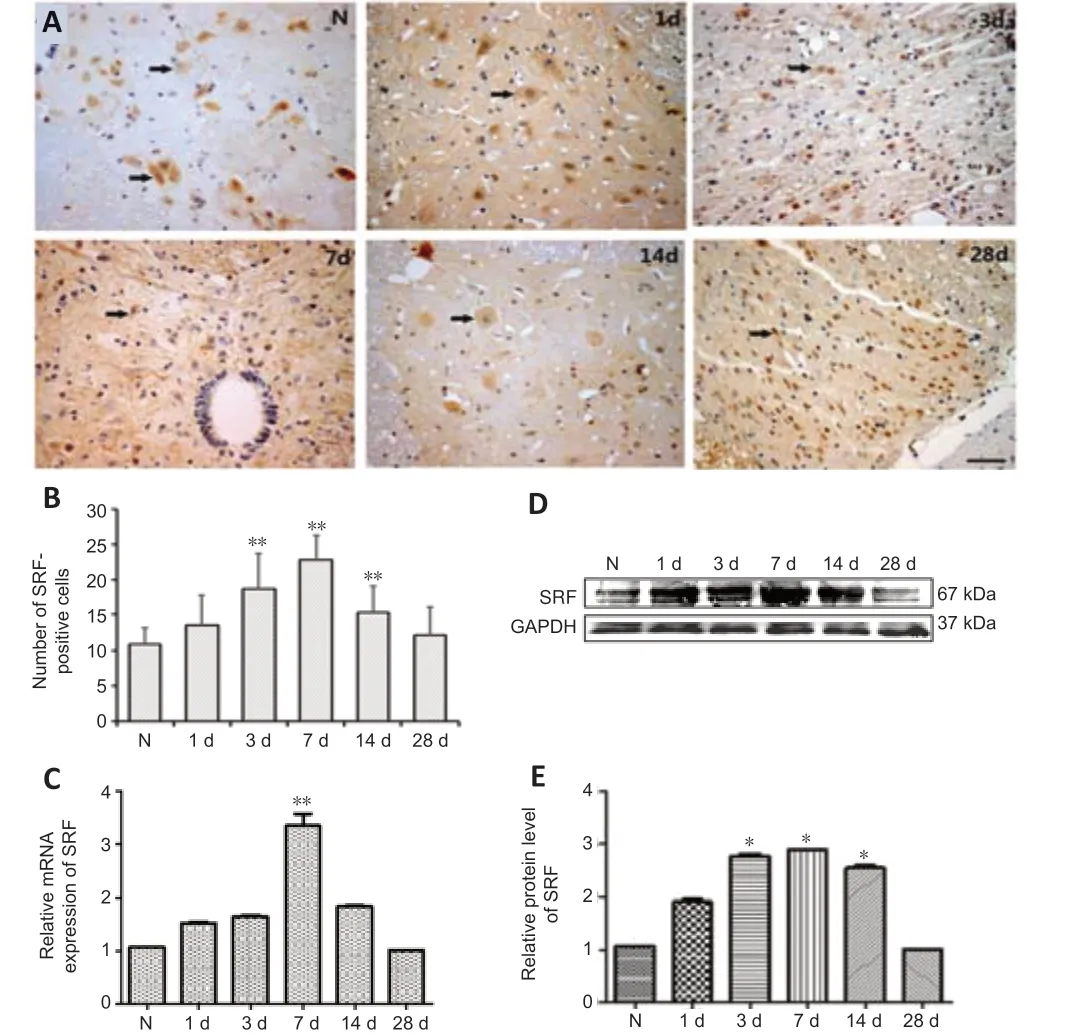
Figure 1|SRF expression in spinal cord tissue in normal and SCT rats.
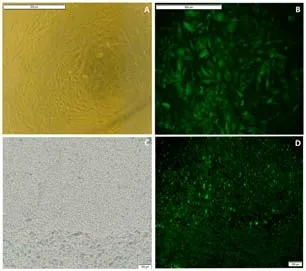
Figure 2|Identification of lentivirus transfected into PC12 cells and spinal cord tissue.
Effect of SRF on motor function recovery after SCI
Rats displayed deficient motor function in hindlimbs with poor recovery after SCI, which was assessed by BBB score. Compared with the NC and srf-RNAi groups, the LV-srf group had significantly higher BBB scores (P< 0.05) from 1 week after injection (Figure 4A and B). At the 4thweek after injury, the rats in the LV-srf group could bear some amount of weight on the hindlimb and slightly move two to three joints, whereas control rats relied solely on the drag force of the front limbs to complete the crawling action. In the srf-RNAi group, BBB scores were decreased markedly at 4 weeks compared with the LV-srf group (Figure 4C). These findings indicate that increasing SRF expression promoted motor function recovery in SCI rats.
BDA anterograde tracking of axonal growth after SCI
To investigate the growth of nerve fibers after SCT, BDA injection was used to anterogradely track the growth of corticospinal tract nerve fibers. Very few short and loose nerve fibers were observed in the spinal cord 14 days after BDA injection in the NC group. More nerve fibers with a certain length and density were observed in the LV-srf group, whereas no visible brown-yellow staining was observed in the srf-RNAi group, suggesting that an increase of regenerated nerve fibers was associated with increased SRF expression (Figure 5).
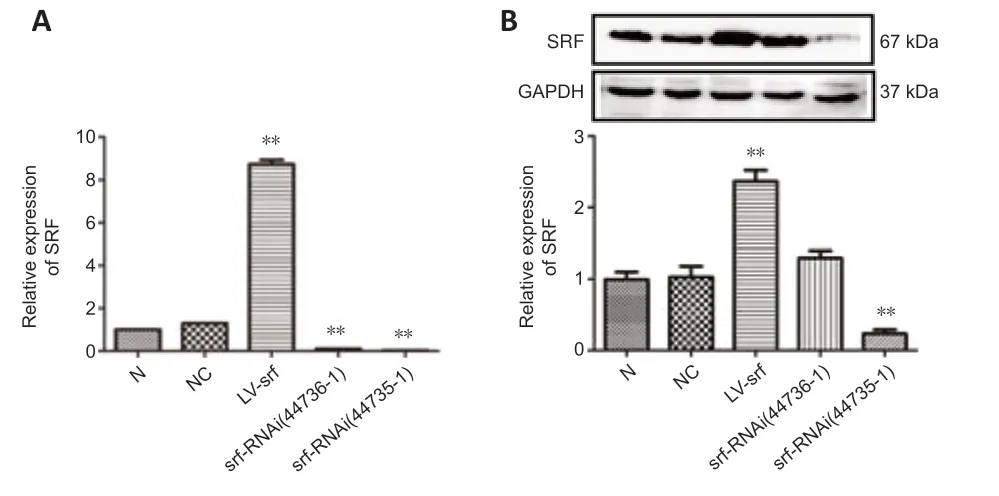
Figure 3|SRF expression after lentivirus injection into spinal cord tissue.
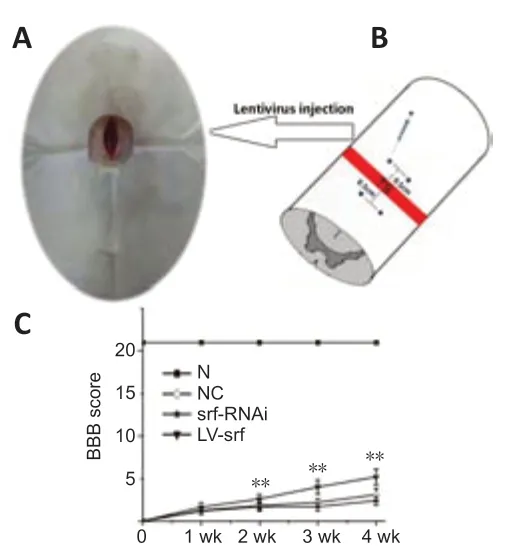
Figure 4|SRF improves motor function in rats with SCI
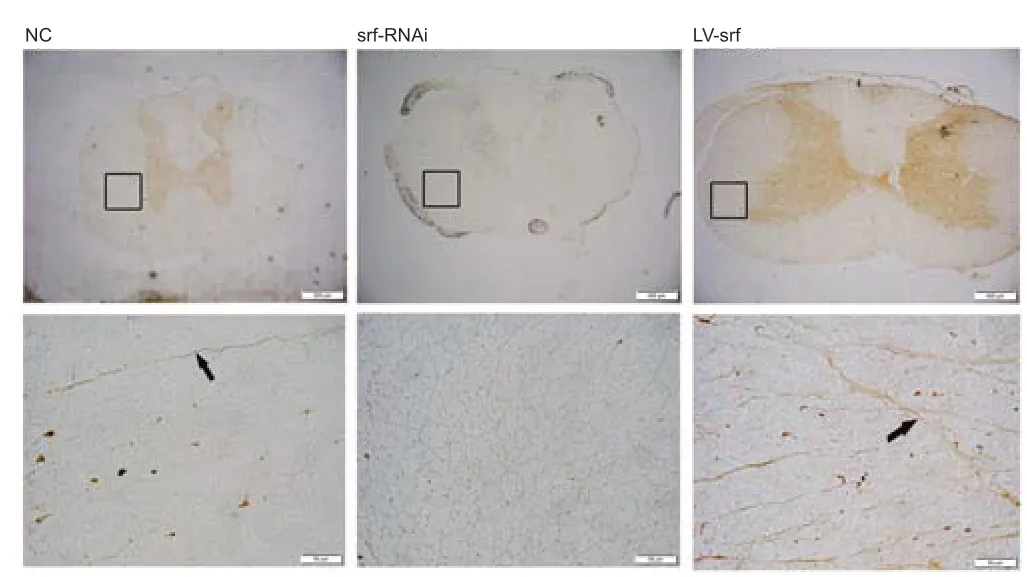
Figure 5|SRF regulates axonal regeneration after SCI.
SRF promotes axon regeneration after SCI
We also observed the myelin sheaths surrounding axons by electron microscope. Loose and disordered myelin sheaths surrounding nerve axons were observed in the NC group, and abundant extracellular matrix was observed in the white matter. Severe demyelination was found in the srf-RNAi group, whereas, in the LV-srf group, a higher density of myelin sheath was observed around axons, mitochondrial structure in neurons was relatively complete, and the cross sections of the myelin sheath were roughly consistent with the neuron axon (Figure 6). These results suggest that SRF may promote axon regeneration.
SRF regulates GAP43 expression in injured spinal cord
To further investigate the relationship between SRF overexpression and axon regeneration, we performed immunofluorescence to detect GAP43 expression after SCI. Compared with those in the NC group, axons in the LV-srf group had noticeably increased GAP43 expression (Figure 7A). This increase in GAP43 protein expression was quantified by western blot assay (Figure 7B and C). Additionally, axon growth inhibitor, Nogo A expression was displayed by double immunostainingwith GAP43 expression (Figure 7A). Taken together, the results suggest that newborn axons were present at the lower end of the injured spinal cord in SCI rats with SRF overexpression.
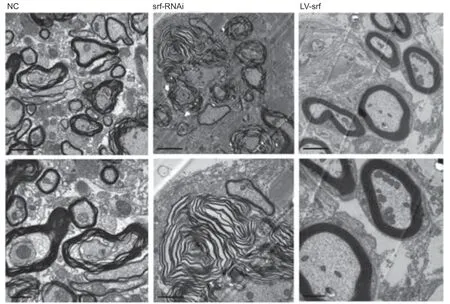
Figure 6|SRF promotes axon regeneration after SCI, shown by electron microscope.
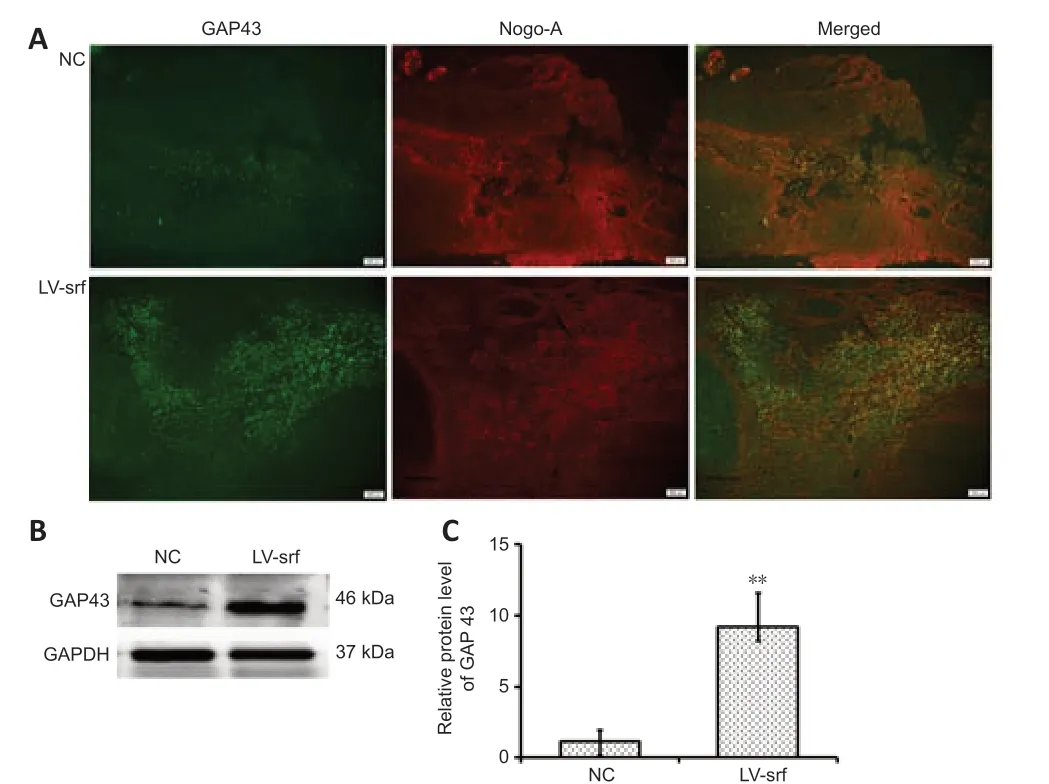
Figure 7|Overexpressed SRF accompanied by upregulated GAP43 after SCI.
Discussion
In the present study, our findings suggest that overexpression or knockdown of SRF affected the functional recovery of locomotion in rats subjected to SCT and regulated axon growth. The underlying mechanism may be associated with the expression of GAP43, a neuron-specific protein involved in nerve cell growth and synapse formation.
SRF, as a transcription factor, is mainly expressed in the nucleus and by binding to the serum response element of some genes (such as C-FOS), controls the cytoskeleton during development, morphogenesis and cell migration under physiological and pathological conditions (Knöll and Nordheim, 2009; Onuh and Qiu, 2021). In the nervous system, SRF also plays an important role in the development of oligodendrocytes and astrocytes (Jain et al., 2021). Previous studies reported that SRF overexpression promoted axonal growth in facial nerve injury (Knöll, 2011; Stern and Knöll, 2014). Axons in the peripheral nervous system readily regenerate, whereas axon regeneration in the central nervous system is short and local, and can only achieve partial functional recovery (Montani and Petrinovic, 2014; Curcio and Bradke, 2018; Gordon, 2020). In the present study, to investigate the effect of SRF in central nervous system injury, we initially observed that SRF was mainly expressed in neurons of spinal cord tissue in normal and SCT rats. SRF was observed at the nucleus and some portions of cytoplasm, shown by immunohistochemistry, and its expression gradually increased after SCI, reached the highest level on the 7thday, then gradually decreased and finally returned similar to normal level on the 28thday. Expression changes of SRF after SCI were verified by qPCR and western blot assay. Stern and Knöll (2014) reported that axon growth inhibitors (myelin, Nogo and chondroitin suhate proteoglycans) stimulated the expression of SRF and related genes in cultured neurons. The results suggested that SRF was involved in the intrinsic self-healing after nerve injury. The adverse factors surrounding injured central nervous system tissue and the poor intrinsic regenerative ability of adult neurons hinder the regeneration of injured axons to rebuild connections with their original targets, and eventually result in the permanent disabilities following SCI (van Niekerk et al., 2016; Mahar and Cavalli, 2018; Brown and Martinez, 2019). In this study, we found that upregulated SRF improved the motor function of SCI rats and may improve axonal regeneration, which is consistent with a previous report that SRF facilitated axon regeneration in the peripheral nervous system (Stern et al., 2013). Interestingly, Mason et al. (2022) reported that Jun, the first identified and verified regeneration-associated gene, played an important role in axonal regeneration after axotomy, whereas SRF, as an alternative plasticity-associated transcription factor, mainly increased the synaptic density in neurons that lacked Jun. Those findings are similar to the report that SRF controlled immediate early gene activation, which was associated with perception of synaptic activity, learning and memory (Knöll and Nordheim, 2009). Given the high expression of SRF in spinal cord tissue after SCI, we predict that SRF regulates the expression of many targets, which are potentially involved in axonal regeneration and function.
GAP43 is a neuron-specific protein that regulates axonal growth and accumulates along neonatal axons (Okada et al., 2021; Alibardi, 2022). In this study, we found that when SRF was upregulated in spinal cord tissue, GAP43 was substantially increased, which confirmed that GAP43 may be a downstream target of SRF. This is also supported by the previous finding that heterogeneous ribonucleoprotein Q, isoform 1 was involved in cortical axonal growth by regulating GAP43 expression (Williams et al., 2015, 2016). In the present study, we also found that the expression of Nogo A (a potent inhibitor of axonal growth) might be associated with GAP43 and SRF.
In the present study, we only showed the effect of SRF on spinal cord tissuein vivo. Validation and further research usingin vitromodels is needed. Additionally, in addition to qualitative observations, quantification of the axon regeneration measures should be supplemented. Because SRF is widely expressed in spinal cord tissue, it will also be important to confirm the cell type and conductin vitrostudies targeting a single cell type to explore the mechanism of SRF neuroprotection in the future. Together, our findings suggest that SRF overexpression promotes motor function recovery in transected spinal cord in rats by improving axonal regeneration. The findings also suggest that the role of SRF may be associated with GAP43 expression in the spinal cord of rats with SCT, but its specific mechanism needs to be further studied.
Acknowledgments:We gratefully thank Department of Electron Microscopy, Binzhou Medical University for assistance with ultra-structural observation and the funds support.
Author contributions:Experimental design and supervision: FH, NLZ, LPZ. Literature review, experimental operation, data analysis and collation: XWL, LXT, CLZ, SZ. Manuscript writing, editing and revewing: GYF, NLZ, LPZ, XWL. All authors read and approved the final manuscript.
Conflicts of interest:All authors declare that they have no conflict of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan; Zihui Wang, University of Maryland, Baltimore, USA.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

