Immunological role of sulfatide in the pathogenesis of multiple sclerosis
Mio Hamatani, Takayuki Kondo
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. A certain population of patients with MS present with a relapse-remitting disease course (RRMS) during the early phase and eventually advance to a progressive course (PMS). While immune modulation therapies have recently seen tremendous success in RRMS, our limited knowledge of pathogenesis hampers the development of effective treatments for PMS.
Myelin lipids and MS:Myelin is a unique highfat membrane composed of multiple lipids that ensures axon insulation and enables rapid transmission of electrical signals. MS is considered an autoimmune disease, and despite over 70% of the myelin sheath being comprised of lipids, most studies on antigen targets have focused on the myelin proteins. Autoreactive lymphocytes against myelin proteins have been highlighted by the numerous studies of experimental autoimmune encephalomyelitis (EAE), the prominent animal model for MS, and by epidemiologic, genetic, and histopathological human studies. However, there is growing interest in the roles of myelin glycolipids in the pathogenesis of MS.
Functionally, glycolipids and related secondary metabolites play prominent roles in oligodendrocyte differentiation and thus in remyelination. Moreover, glycolipids are crucial for the formation and stabilization of functional axonglia interactions and intracellular signaling (Halder et al., 2007; Nowack et al., 2021). An imbalance of glycolipid composition is a hallmark of neurological diseases, including MS, and likely causes the unhealthy state of neurons that leads to the axonal loss, as well as the demyelination, observed in the brains of patients with PMS.
The dynamics of sulfatide in MS pathogenesis:Sulfatide is one of the major components of myelin. Its isoforms feature a range of fatty acids that vary in chain length, hydroxylation, and degree of unsaturation (Blomqvist et al., 2017), and hence the composition of sulfatide differs between organs. The predominant isoforms in myelin are characterized by long-chain fatty acids (22–26 carbon atoms) (Nowack et al., 2021).
Several studies support the notion that sulfatide can be involved in the pathogenesis of MS and in the transition to a progressive disease course: higher sulfatide concentration in the serum and cerebrospinal fluid (CSF) has been reported in patients with PMS compared to those with RRMS (Halder et al., 2007), and sulfatide isoforms incorporating longer (C24, 26) and unsaturated fatty acids were found to be elevated in the CSF of patients with PMS compared with healthy donors and RRMS patients (Novakova et al., 2018). In addition, the sulfatide fraction is markedly reduced in remyelinated brain lesions in patients with MS and animal models of experimental demyelination (Illés et al., 2000; Halder et al., 2007), with particularly prominent reduction in sulfatide isoforms having longer (C24, 26) and unsaturated fatty acids. Relatively higher myelin degradation alongside slower or incomplete remyelination is one possible reason for the discrepancy between sulfatide components found in the CSF and in brain tissue (Kanhai et al., 2022). Changes in qualitative and quantitative sulfatide composition may be associated with pathophysiology of PMS, where sustained tissue damage and insufficient remyelination are the key features.
Immunological roles of sulfatide in MS pathogenesis:The immunological functions of sulfatide in MS pathogenesis are also noteworthy. For one, autoantibodies directed against sulfatide can be another player in MS pathogenesis. Some MS patients display increased sulfatide-reactive antibodies as well as higher fractions of sulfatidereactive T cells in peripheral blood (Illés et al., 2000; Halder et al., 2007). These reports support the existence of an immune response against sulfatide in MS.
Furthermore, administration of sulfatide was found to ameliorate experimental autoimmune encephalomyelitis, suggesting it to have an immune-regulatory role in MS (Halder et al., 2007). One possible mechanism of this sulfatidemediated amelioration could involve natural killer T (NKT) cells. NKT cells can be divided into two subsets according to the T cell receptor: one having a semi-invariant T cell receptor (type I) and the other expressing more diverse T cell receptors (type II). Type II NKT cells have been shown to mainly recognize sulfatide in the context of CD1 molecules on antigen-presenting cells. The sulfatide-mediated activation of type II NKT cells and suppression of type I NKT cells and dendritic cells are important elements in the sulfatidemediated regulation of experimental autoimmune encephalomyelitis (Maricic et al., 2014). Additionally, chronically active central nervous system lesions in MS increase CD1d expression (Illés et al., 2000). However, the mechanisms of sulfatide-induced suppression are not yet fully understood. We have recently found that sulfatide directly modifies the immune system, independently of CD1d molecules and NKT cells, and can be involved in the pathogenesis of MS (Hamatani et al., 2022).
MS progression diminishes the action of sulfatide as a direct modulator of autoreactive T cell activation:Besides its potential as an autoantigen, we recently showed that sulfatide directly suppresses the proliferation of polyclonally activated human peripheral T cells collected from healthy subjects (Hamatani et al., 2022). Specifically, sulfatide markedly reduced both CD4+and CD8+T cell proliferation and their interleukin 2 production induced by stimulating polyclonal T cell receptors. T cell viability did not differ with respect to the presence or absence of sulfatide, which indicates that sulfatide does not induce cell death but rather suppresses proliferation. This suppressive effect was unaffected by blocking of CD1a and CD1d and by depletion of CD56+cells or antigen-presenting cells, thus is independent of CD1 molecules and NKT cells. We also observed such sulfatide-mediated suppression in T cells from patients with MS bearing mild disability (expanded disability status scale scores of less than four). However, T cells from patients with higher disability (expanded disability status scale scores of four or more) instead exhibited enhanced proliferation in the presence of sulfatide. The observed difference in reactivity was not attributable to a difference in the proliferative capacity of T cellsper se, age, or medications.
This difference in reactivity implies sulfatide to be important in the process of transitioning to the progressive form of MS. Our results suggest that, in the healthy and physiological state, sulfatide inhibits the excessive immune reaction against myelin protein that can be triggered during myelin turnover. Similarly, sulfatide serves beneficial functions in milder MS cases when released at sites of demyelination, acting as an endogenous immunomodulator and promoting remission through its suppressive effect on T cells. In contrast, while the advanced stage of MS is suggested to feature higher concentration of and hence stronger or chronic exposure to sulfatide, escape of T cells from sulfatide-induced suppression or even enhanced activation of T cells could be involved in the chronic inflammation and thus disease progression (Figure 1).
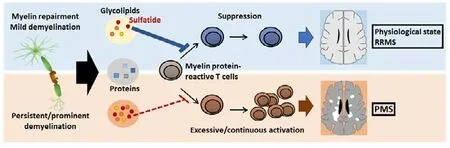
Figure 1| Sulfatide in the pathogenesis of MS.
Supposed mechanism of sulfatide-mediated T cell suppression:The mechanism of sulfatideinduced suppression and its abrogation in T cells from higher-disability MS patients warrants further study.
One possible approach is to search for the receptor for sulfatide and its downstream signaling pathways. Sulfatide has been identified as a ligand for some membranous proteins such as L-selectin and Galectin-4, and the interactions between sulfatide and these proteins expressed on lymphocytes have been shown to directly modulate lymphocyte function (Mycko et al., 2014).
Focusing on sulfatide molecular species is another potential approach. As mentioned above, sulfatide composition is altered in the CSF and likely in the brain tissue of PMS patients. This imbalance of sulfatide isoforms, along with the attenuation of sulfatide-induced immune-suppression, could be one reason for the intrathecal long-lasting inflammation and the dyshomeostasis in axon-glia interaction seen in patients with PMS.
Although the 3-sulfate group was reported as critical for sulfatide-induced inhibition of T cell proliferation (Mycko et al., 2014), an increase in the fraction of sulfatide incorporating a shortened fatty acid chain can explain the altered reaction of T cells in patients with higher disability. Further studies on the T cell response to sulfatide isoforms with different fatty acid moieties will hopefully produce novel insights into the mechanism of sulfatide-induced immune suppression.
To evaluate the difference in sulfatide composition between peripheral blood and CSF may provide further insights into its mode of action in the central nervous system diseases.
Although technical difficulties have been encountered in analyzing glycolipids and their molecular species and in detecting lipidlipid or lipid-protein interactions, continuous methodological advancement in areas such as mass-spectrometry imaging has helped reveal novel insights concerning the biological roles of glycolipids in the pathogenesis of central nervous system diseases.
Sulfatide in other neurological diseases:Sulfatide is also a potential target in Alzheimer’s disease (AD) pathogenesis (Blank and Hopf, 2021). Numerous studies have shown that sulfatide is specifically and dramatically reduced in both gray and white matter and in the CSF of patients with AD at an early clinical stage of cognitive impairment. Moreover, a recent report demonstrated using a mouse model that loss of sulfatide causes the activation of disease-associated microglia and astrocytes and increased expression of AD risk genes such asApoeandTrem2, leading to chronic neuroinflammation and mild cognitive impairment (Qiu et al., 2021). Additionally, a growing body of data emphasizes the involvement of the adaptive immune system in AD pathogenesis. Genome-wide association studies have identified several genetic risk factors among major histocompatibility complex alleles, the majority of which encode class II human leukocyte antigens. Increased T cell number was observed in the CSF and brain tissue of AD patients and clonal expansion of T cells and the autoreactive T cells against beta amyloid and tau protein further support these cells as playing roles in AD pathogenesis. An investigation of sulfatide-mediated immunomodulation of T cells in the context of AD pathogenesis would be promising.
Sulfatide as a therapeutic target:Glycolipids are attractive targets for new therapeutic approaches in PMS since they have potential roles in both immunomodulation and regeneration/neuroprotection.
The synthetic glycolipid OCH, an alphagalactosylceramide analogue, has been reported to prevent experimental autoimmune encephalomyelitis by inducing Th2 bias of autoimmune T cells, mediated through predominant production of interleukin 4 by NKT cells (Miyamoto et al., 2001). This glycolipid is now undergoing phase II clinical trial as a novel oral medication for patients with MS in Japan.
As our data indicates, attenuation of T cell reactivity to sulfatide-induced suppression may have an important role in PMS pathogenesis, suggesting that sulfatide can also be a therapeutic option in this challenging disease. Because sulfatide is ubiquitous throughout the body and fulfills various functions, it is critical to first establish the mechanism by which sulfatide suppresses T cell proliferation and identify corresponding target molecules. In addition, further research should elucidate how T cells from MS patients with higher disability become insensitive to immune suppression by sulfatide. Studies pursuing these questions will provide novel insights into the pathogenesis of MS, particularly its progression, along with therapeutic targets. With the technical advancements, sulfatide would also be a potential biomarker in evaluating the disease progression in MS. In addition, the findings of such studies can be applied to a wide range of challenging neurological diseases, such as AD.
This perspective has highlighted the immunomodulating roles of one myelin glycolipid, sulfatide, as well as its non-immunological functions. We hope that continuous interdisciplinary efforts will discover new insights into the pathogenesis of a wide range of presently intractable neurological diseases.
*Correspondence to:Takayuki Kondo, MD, PhD, takakon78@hotmail.com.
https://orcid.org/0000-0003-0331-6476(Mio Hamatani)
https://orcid.org/0000-0001-7048-9401 (Takayuki Kondo)
Date of submission:October 7, 2022
Date of decision:November 9, 2022
Date of acceptance:November 28, 2022
Date of web publication:January 30, 2023
https://doi.org/10.4103/1673-5374.366498
How to cite this article:Hamatani M, Kondo T (2023) Immunological role of sulfatide in the pathogenesis of multiple sclerosis. Neural Regen Res 18(9):1950-1951.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

